Abstract
Alpine karst aquifers control the availability and longevity of some water resources, but are not well understood. A conceptual model of the alpine karst aquifer system in the Bear River Range of northern Utah (USA) has been developed by geochemical analysis (major ions, δ18O, δ2H and δ13C values) of seasonal water samples from seven perennial springs, and residence-time assessment (3H and CFCs) of two low- and two high-discharge springs. All spring data can be explained by reaction paths dominated by the dissolution of calcian dolomite. The δ13C values align well with reaction paths for open-system dissolution. Saturation indices and low Ca:Mg molar ratios indicate that incongruent dissolution exerts a strong control on water–rock interactions, complicating interpretation of natural solute tracers. Values of δ18O and δ2H in springs follow the Utah meteoric water line. Snow δ18O values correlate with elevation, but not with increasing rainout distance, providing qualitative estimates of recharge elevation that generally align with previous dye-traces to five of the seven springs. Concentrations of 3H and CFCs likely are best described by binary mixing of subannual recharge with 60–65-year-old groundwater, suggesting that the alpine karst aquifer system in the Bear River Range is best represented by a double-porosity model. Subannual recharge documented by dye traces implies that caverns are the primary flowpaths to the springs, but the presence of decadal-age water may indicate that lower permeability flowpaths dominate during baseflow. No evidence was found for a longer-residing flow component, suggesting high sensitivity to future climate variability.
Résumé
Bien que les aquifères karstiques alpins contrôlent la disponibilité et la durabilité de certaines ressources en eau, ils ne sont pas bien compris. Un modèle conceptuel de l’aquifère karstique alpin de la chaîne de Bear River, dans le nord de l’Utah (États-Unis d’Amérique), a été développé grâce à l’analyse géochimique (ions majeurs, teneurs en δ18O, δ2H et δ13C) d’échantillons d’eau saisonniers provenant de sept sources pérennes, et à l’évaluation du temps de résidence (3H et CFC) de deux sources à faible débit et deux à fort débit. Toutes les données des sources peuvent être expliquées par des réactions dominées par la dissolution de la dolomite. Les teneurs en δ13C sont cohérents avec des réactions de dissolution en système ouvert. Les indices de saturation et les faibles rapports molaires Ca:Mg indiquent qu’une dissolution incongruente exerce un contrôle important sur les interactions eau-roche, compliquant ainsi l’interprétation du traçage naturel en phase dissoute. Les valeurs de δ18O et δ2H des sources s’alignent sur la droite météorique locale de l’Utah. Les teneurs en δ18O de la neige sont corrélées avec l’altitude, mais pas avec l’augmentation de la distance de la pluie, fournissant des estimations qualitatives de l’altitude de recharge qui s’alignent généralement sur les traçages artificiels antérieurs de cinq des sept sources. Les teneurs en 3H et CFC sont mieux décrites par un mélange binaire de la recharge infra-annuelle avec des eaux souterraines âgées de 60–65 ans, ce qui suggère qu’un modèle à double porosité permet de mieux représenter le fonctionnement du système aquifère karstique alpin du bassin versant de la rivière Bear. La recharge infra-annuelle, mise en évidence par des traçages artificiels, implique que les conduits souterrains constituent les voies d’écoulement principales jusqu’aux sources, bien que la présence d’eau d’âge décennal puisse indiquer que des trajets de perméabilité inférieure dominent le débit de base. Aucune donnée probante n’atteste d’une composante d’écoulement avec un temps de résidence important, suggérant une sensibilité élevée à la variabilité climatique future.
Resumen
Los acuíferos kársticos alpinos controlan la disponibilidad y la antigüedad de algunos recursos hídricos, pero no se conocen suficientemente. Se ha elaborado un modelo conceptual del sistema acuífero kárstico alpino en Bear River Range, en el norte de Utah (EEUU) mediante análisis geoquímicos (iones principales, valores de δ18O, δ2H y δ13C) de muestras estacionales de agua de siete manantiales perennes, y una evaluación del tiempo de residencia (3H y CFCs) de dos manantiales de baja y dos de alta descarga. Todos los datos de los manantiales pueden explicarse por las líneas de reacción dominadas por la disolución de la dolomita calcárea. Los valores de δ13C se alinean bien con las líneas de reacción para la disolución en sistema abierto. Los índices de saturación y las bajas proporciones molares de Ca:Mg indican que la disolución incongruente ejerce un fuerte control sobre las interacciones agua-roca, lo que complica la interpretación de los trazadores naturales de solutos. Los valores de δ18O y δ2H en los manantiales siguen la línea de aguas meteóricas de Utah. Los valores de δ18O en nieve se correlacionan con la altura, pero no con el aumento de la distancia a la lluvia, lo que proporciona estimaciones cualitativas de la altura de recarga que generalmente se alinean con los trazadores de colorantes de cinco de los siete manantiales. Es probable que las concentraciones de 3H y CFCs se describan mejor mediante la mezcla binaria de la recarga subanual con aguas subterráneas de 60–65 años, lo que sugiere que el sistema acuífero kárstico alpino de la Bear River Range está mejor representado por un modelo de doble porosidad. La recarga subanual documentada por trazadores de colorante implica que las cavernas son las vías de flujo primarias hacia los manantiales, pero la presencia de agua de edad decádica puede indicar que las vías de flujo de menor permeabilidad dominan durante el flujo de base. No se encontraron pruebas de un componente de flujo de mayor duración, lo que sugiere una alta sensibilidad a la futura variabilidad climática.
摘要
高山岩溶含水层控制着水资源的可利用性和使用时间, 但人们对此知之甚少。通过来自七个持续性泉的季节性水样的地球化学分析(主要离子, δ18O, δ2H 和 δ13C值), 以及两个低流量和高流量的泉水(3H 和 CFCs)滞留时间, 开发了Utah北部Bear河山脉(美国)高山喀斯特含水层系统的概念模型。所有泉水数据都可由以钙质白云石溶解为主的反应路径来解释。δ13C值与开放系统溶解的反应路径完全吻合。饱和指数和低的Ca:Mg摩尔比表明, 非均匀溶蚀强烈控制水-岩相互作用, 使天然溶质示踪剂的解释复杂化。泉水的δ18O 和 δ2H值遵循犹他州的降水线。降雪δ18O值与海拔高度相关, 但与增加的降雨距离无关, 从而提供了补给高度的定性估计值, 该估计值通常与七个泉水中五个泉以前的染色剂示踪一致。3H 和 CFCs的浓度可通过将次年补给量与60至65年的地下水进行二元混合来最好地描述, 这表明Bear河山脉的高山喀斯特含水层系统最好用双孔隙度模型来表示。染色剂示踪记录的次年补给表明, 洞穴是通往泉水的主要流路, 但年代际水的存在表明在基流期间渗透率较低的流路占主导地位。没有证据表明更长的流动组分, 说明对未来的气候变化高度敏感。
Resumo
Aquíferos cársticos alpinos controlam a disponibilidade e longevidade de alguns recursos hídricos, mas não são bem compreendidos. Um modelo conceitual do sistema aquífero cárstico alpino na cordilheira do Rio Bear do norte de Utah (EUA) tem sido desenvolvido por analises geoquímicas (íons principais, δ18O, δ2H e δ13C) de amostras sazonais de águas de sete nascentes perenes, e análise do tempo de residência (3H e CFCs) de duas nascentes de baixa vazão e duas de alta vazão. Todos os dados das nascentes podem ser explicados por caminho de reação dominados por dissolução de calcário dolomitico. Os valores δ13C se alinham bem com o caminho de reação para sistemas abertos de dissolução. Índices de saturação e baixa razão molar Ca:Mg indica que a dissolução incongruente exerce um forte controle na interação água -rocha, complicando a interpretação dos traçadores naturais de soluto. Valores de δ18O e δ2H nas nascentes seguem a linha meteórica de Utah. Valores de δ18O da neve se correlacionam com a elevação, mas não com o aumento da distância da chuva, fornecendo estimativas qualitativas da elevação de recarga que geralmente se alinham com os traços de corantes anteriores de cinco das sete nascentes. Concentrações de 3H e CFCs provavelmente são melhores descritas pela mistura binária de recarga subanual com águas subterrâneas de 60–65 anos de idade, sugerindo que o sistema aquífero cárstico na cordilheira do Rio Bear é melhor representado por um modelo de dupla porosidade. A recarga subanual documentada por traçadores de corante indicam que cavernas são os caminhos de fluxo primários para as nascentes, mas a presença de água com idade decadal pode indicar que fluxos de baixa permeabilidade dominam o fluxo de base. Nenhuma evidencia encontrada para um componente de fluxo de longa duração, sugerindo alta sensibilidade para variabilidade climática futura.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Approximately 25% of the Earth’s population relies on karst-hosted groundwater (Ford and Williams 2007). In mountainous regions with extensive carbonate bedrock, “alpine karst aquifers” host the majority of groundwater, and are sensitive to changing recharge and precipitation patterns due to climate change (Ozyurt 2008; Moral et al. 2008; Goldscheider and Neukum 2010; Zeng et al. 2012; Frondini et al. 2014)—for example, approximately 50% of the population of Austria, including its capital, Vienna, relies on water from such aquifers (Savio et al. 2019). However, because of difficult accessibility, alpine karst aquifers have received little attention, and almost exclusively in the Alps (Chen and Goldscheider 2014; Filippini et al. 2018; Gremaud et al. 2009; Lauber and Goldscheider 2014). In fact, the only studies of alpine karst in North America were conducted in the Canadian Rocky Mountains in the 1980s (Ford 1983; Smart 1983, 1988). Furthermore, no studies of such aquifers have been conducted in arid to semiarid regions, such as the western United States, where water supplies in these aquifers may be adversely impacted by the effects of climate change. The Bear River Mountains of northern Utah are an excellent field laboratory to improve the understanding of groundwater recharge and movement in alpine karst aquifers.
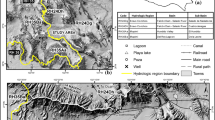
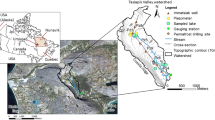
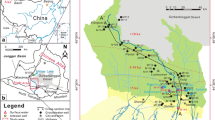
The north–south trending Bear River Range in northern Utah is located in the Rocky Mountain province of the western United States, bounded on the west by Cache Valley that hosts the city of Logan and other communities (Fig. 1). It contains an alpine karst aquifer system that sustains the baseflow of the Logan River (Gooseff et al. 2005; Kolesar et al. 2005), the largest stream in the range. A number of springs drain into the Logan River, one of which, Dewitt Spring, is the primary source of water for the city of Logan (Spangler 2001), the largest city in Cache Valley. The eastern margin of Cache Valley hosts a highly productive aquifer system (Bjorklund and McGreevy 1971), later termed the principal aquifer by Robinson (1999), that is recharged in part by the Logan River.
Nearly 7,600 m of Paleozoic marine limestones, dolostones and interbedded siliciclastic deposits comprise the bedrock geology of the Bear River Range (Williams 1948, 1958; Francis 1972). Quartzites and shales separate thick carbonate aquifer units and likely act as aquicludes (Dover 1995). The East Cache fault, a Basin-and-Range normal fault, cuts the west limb of the NNE-trending, south-plunging, Sevier-age Logan Peak syncline (Williams 1948; McCalpin 1989, 1994; Evans and Oaks 1996; Evans et al. 1996). The syncline (Fig. 2) funnels groundwater flow south to seven primary springs draining limestone- and dolostone-aquifer units adjacent to the Logan River (Fig. 2). The spring discharges range from 0.03 to 0.71 m3/s (Mundorff 1971; Spangler 2001).
Dissolution of carbonate minerals can enhance the permeability of fractures, bedding planes and pore space in karst aquifers, with most flow typically occurring in solution-enhanced conduits and most storage occurring interstitially if enhanced pore space is present (White 2003). The resulting flowpath architecture may adhere to one of three conceptual karst flow models (Fig. 3): (flowpath a) a single-porosity model assumes that all groundwater moves through conduits on the order of days to weeks; (flowpath b) a double-porosity model adds an intermediate velocity component (years to decades) that moves along less-enhanced fractures; and (flowpath c) a triple-porosity model adds a third component of slow velocity (hundreds to thousands of years) that moves through solution-enhanced pore space.
Schematic of single, double and triple porosity conceptual models (after White 2003). Subsurface white areas are void space
Dye traces in the Bear River Range indicate that groundwater flow along bedding planes and solution-enhanced fractures can cross topographic divides and drop up to 300 m, spending as little as 2–4 weeks in the subsurface (Spangler 2001, 2012; Bahr 2016). Bright (2009) used tritium data to identify possible decadal residence times for springs in the eastern Bear River Range. This bimodal age distribution aligns with that observed in a low-relief karst aquifer in Florida (Katz et al. 2009). Kolesar et al. (2005) and Neilson et al. (2018) both inferred the possibility for zones of slow karst development based on major-ion data of the Logan River and major tributary springs. Whereas matrix-dominated flowpaths of this type are documented in low-relief karst aquifers, geochemical lines of evidence used in alpine systems rarely address natural processes or anthropogenic solutes that confound interpretation. The presence or absence of groundwater with long (centennial to millennial) residence times has significant implications for water resource sensitivity to a variable climate.
The purposes of this study are to develop a conceptual model of the alpine karst aquifer system in the Bear River Range, and to increase the understanding of alpine karst aquifers in general by quantifying water–rock interactions and residence times. The new data reported here include major ion chemistry, oxygen and hydrogen stable isotope ratios (δ18O and δ2H values), stable isotope ratios of dissolved inorganic carbon (δ13C values), and tritium (3H) and chlorofluorocarbon (CFC) concentrations from the seven springs, as well as the δ18O of snow cores from 17 locations. These data are used to: (1) determine if springs have geochemical signatures that relate to specific hydrogeologic flowpaths, and if average spring discharge magnitudes indicate open-system (high discharge) or closed-system (low discharge) dissolution of calcite and dolomite; (2) calculate carbonate mineral saturation indices to ascertain whether geochemical signatures are controlled by dissolution processes or instead impart flowpath-specific fingerprints; (3) assess whether springs drain groundwater in equilibrium with contemporary atmospheric values reflective of short (weeks to months) or indicate longer (decadal or older) residence times; and (4) determine if snowpack isotopic values can be used to estimate groundwater recharge elevation for these springs. The data reported here were collected in March, May and November of 2017.
Materials and methods
Field chemical parameters
Temperature, pH, electrical conductivity (EC), dissolved oxygen (DO) and carbonate alkalinity were measured in the field at all seven springs during all three chemical sampling excursions. A YSI-30 m was used to measure temperature and EC during the first two excursions, but was replaced by a YSI EcoSense EC 300A meter for the third excursion. An Orion 2013A pH meter, a Hanna HI 9142 dissolved-oxygen meter, and a Hach model AL-AP MG-L alkalinity test kit were used to measure pH, DO and alkalinity, respectively, during all three field excursions. Total alkalinity also was measured by laboratory colorimetric titration to reduce error (±0.05 mg/L as HCO3) over the Hach field test kit (±20 mg/L as HCO3). Only the results from the laboratory titrations are reported here.
Major ion analysis
Samples for major ion analysis were collected from each spring during low-flow (4 March 2017) and high-flow (31 May 2017) conditions. Samples were collected in November 2017 only at Benchmark, China Row, Spring Hollow (2 November) and Dewitt (3 November) Springs. Chemically clean polyethylene bottles were prerinsed, filled with filtered (0.45 μm) sample water, and acidified with 1:1 trace-metal-grade nitric acid to a pH less than 2 (Wilde 2008). Samples for anion (chloride and sulfate) analysis were collected in glass bottles without acidification or filtering. All samples were refrigerated prior to analysis. Cation and anion analyses were performed at Utah State University (USU) Geochemistry Lab and the USU Water Research Lab using a Thermo X Series 2 Quadrupole inductively-coupled plasma mass spectrometer (ICP-MS) and a Dionex ion chromatograph (IC) to ±0.1 and 1.1 mg/L accuracy, respectively. The unacidified, unfiltered samples were also titrated for alkalinity as bicarbonate with 0.02 N sulfuric acid and Bromo Blue indicator to ±0.05 mg/L accuracy at the USU Geochemistry Lab.
O, H and C stable isotope ratios
Samples for O and H stable-isotope analysis were collected on 4 March and 31 May 2017 in chemically clean, clear glass bottles and refrigerated prior to analysis. Samples for C stable-isotope analysis were collected at the same time and in the same manner as O and H samples, but in amber glass bottles with no headspace to minimize aquatic biological-fractionation due to microbial activity (Mook 2001).
Snow samples for O and H stable isotopes were collected in March 2017 with a custom 10-ft (3.05 m) PVC toothed snow coring tool. The tool was driven through the snowpack until the soil was reached. The core then was cleaned of soil, ejected into a 1-gal plastic bag until melted, transferred to a chemically clean, clear glass bottle and refrigerated until analysis. Cores were collected over a range of elevations (1,543–2,393 m asl) where the snowpack was pristine and unaltered by remobilization via wind or anthropogenic activity.
Analyses for O, H and C stable isotope ratios were performed at the USU Stable Isotope Lab using a Thermo Scientific Delta V Advantage isotope-ratio mass spectrometer (IRMS) with a Gasbench II peripheral. The stable-isotope values were corrected for temporal drift and linearity effects as appropriate using in-house standards and calibrated to international standards and are reported using delta notation in per mille (‰). The δ18O and δ2H values of water and snow are reported +0.06 and 2.0‰, respectively, versus Vienna Standard Mean Ocean Water (VSMOW). The δ13C values of dissolved inorganic carbon (DIC) are reported +0.1‰ versus Vienna Pee Dee Belemnite (VPDB). To quantify the statistical relationship of δ18O values to elevation and location (“rainout” distance), bulk-snowpack stable-isotope values were assessed with analysis-of-variance. Linear regression was used to estimate a local elevation gradient, in ‰ per 100 m, after James et al. (2000). Precision for a data-dense δ18O elevation gradient is on the order of ±0.5‰ (Brooks et al. 2012).
Tritium and chlorofluorocarbon sampling and analyses
Two low-discharge springs (Benchmark, China Row) and two high-discharge springs (Dewitt, Spring Hollow) on either side of the Logan River were sampled for 3H and CFCs on 2 November (Benchmark, China Row and Spring Hollow) and 3 November (Dewitt) 2017. Tritium samples were collected in chemically clean 1-L Nalgene bottles such that no bubbles remained after pre-rinsing with spring water (Solomon 2017). Samples were transported overnight to Brigham Young University (BYU) and analyzed to a precision of ±0.1 TU and a detection limit of 0.3 TU using a Quantulus ultra-low-level liquid-scintillation counter after electrolytic enrichment (S. Nelson, Brigham Young University, written communication, 2017).
Chlorofluorocarbon samples were collected in accordance with USGS GDL (2017), using a peristaltic pump with Viton and copper tubing extended to the base of a 4-oz Boston-type glass bottle within a 1-gal stainless-steel container. The container was allowed to overflow such that a minimum of one gallon flushed through the sample bottle. The bottle then was capped (foil-lined) underwater with no visible air bubbles, inverted, and stored at room temperature until delivery to the University of Utah Dissolved and Noble Gas Lab the next day. The samples were analyzed to a precision of ±5%, and a detection limit of 1 pg/kg for CFC-113 and 0.5 pg/kg for CFC-11 and CFC-12.
Geochemical modeling
Reaction paths for water-chemistry evolution were created with the PHREEQC geochemical model using the llnl database (Parkhurst and Appelo 2013; after Langmuir 1971). The enthalpies of reaction for dissolution of stoichiometric calcite and stoichiometric dolomite were obtained from the PHREEQC llnl database. The enthalpy of reaction for dissolution of magnesian calcite was selected from Bischoff (1998). The enthalpy of reaction for dissolution of calcian dolomite was set to a default value of zero, which is standard in the absence of a thermodynamic estimate.
These models simulate simple end-member conditions for equilibrium water–rock–CO2 interactions in open- or closed-systems. Equilibrium speciation, mineral saturation indices and pCO2 were calculated for equilibration with dolomite or calcite to assess similarity of the models to field data. Carbon mass-balance modeling after Frondini et al. (2014) was performed using δ13C as a response variable to distinguish more readily open- and closed-system dissolution.
Lumped parameter modeling
Tracers such as 3H and CFCs are used to estimate groundwater ages by lumped-parameter modeling (Solder et al. 2016). The standard suite of lumped-parameter models, TracerLPM, is maintained by the US Geological Survey (USGS). Choosing the best model requires an understanding of the input functions for atmospheric concentrations of each tracer.
Tritium atmospheric concentrations were monitored at the IAEA Ottawa, Canada station until 2012 (IAEA 2014), after which records were extended with the known decay rate. It is the most complete atmospheric record in North America, and is commonly correlated with incomplete records at other locations to estimate the local atmospheric history (Michel 1989). The 3H record for Ottawa was correlated with the incomplete record from Salt Lake City (1963–1984) to produce a local estimate of atmospheric concentrations of 3H. Chlorofluorocarbon atmospheric concentrations are documented historically, and currently monitored at the Niwot Ridge facility in Colorado (Niwot Ridge LTER 2018).
Results
Field chemical parameters
Water with the lowest temperatures emerged from Dewitt and Spring Hollow springs (6.4 °C) in May and March, respectively, and the warmest water emerged from Benchmark Spring (11.0 °C) in May. All springs are near neutral, with pH values ranging from 7.1 to 7.8. Electrical conductivities ranged from 193 μS/cm at Woodcamp Hollow Spring in March to 446 μS/cm at China Row Spring in November. Dissolved-oxygen concentrations are relatively invariant across space and time, although concentrations in large springs (10.3–11.6 mg/L) exceeded concentrations in Benchmark and China Row Springs (5.4–8.8 mg/L). Alkalinity via laboratory colorimetric titration ranged from 208 and 305 mg/L (Table 1).
Major ion concentrations
Major-ion concentrations are summarized in Table 1. Major ions are dominated by Ca2+, Mg2+ and HCO3− across seasons. Anomalously high levels of Na+ and Cl− are present in China Row Spring in March and May, but were similar to other springs when resampled in November. Concentrations in the original two samples likely reflect contamination by road salt used during the winter months. These anomalous Na+ and Cl− results do not adversely impact interpretations presented in this paper.
Mineral saturation index and pCO2 calculations in PHREEQC
Mineral saturate states were determined using PHREEQC and are reported as the saturation index (SI):
where Ksp is the solubility product (Table 2). Langmuir (1971) estimated equilibrium with respect to calcite and dolomite to be ±0.1 SI units (SI = 0 indicates equilibrium saturation), but propagation of error in this study (pH = ±0.1, Ca2+ and Mg2+ = ±1 mg/L) indicates that a value of ±0.3 is more appropriate. Saturation indices with respect to stoichiometric calcite and stoichiometric dolomite range from undersaturated to saturated and saturated to supersaturated, respectively. However, Spring Hollow Spring was undersaturated with respect to both calcite and dolomite in March. Carbon dioxide log partial pressures are shown in Table 2. Logan Cave and China Row Springs exhibit the highest partial pressures (log pCO2 = −2.1 atm). Benchmark and Woodcamp Hollow Springs exhibit the lowest (−2.5 atm).
Stable isotope ratios
The δ18O, δ2H and δ13C (DIC) values for spring waters are reported in Table 3. The δ18O values for springs in 2017 range from −18.2 to −16.5‰ during high- and low-flow conditions, respectively. Corresponding spring δ2H values range from −135‰ during high-flow conditions up to −124‰ during low-flow conditions. The δ18O and δ2H values of snow core samples collected in March 2017 (Table 4) range from −18.9 and −145‰ to −16.0 and −125‰, respectively. During low-flow conditions spring water DIC δ13C values ranged from −11.8‰ (China Row Spring) to −9.9‰ (Ricks Spring). The range increased during high-flow conditions, from −12.3‰ (Ricks Spring) to −8.5‰ (Spring Hollow Spring).
High-flow compositions in the springs represent a mixture of new seasonal snowmelt transported in surface water, shallow groundwater or both with preexisting groundwater. Thus, the high-flow isotope values for the snowmelt addition (Table 3) have been corrected using a simple volume-balance equation (after Kendall and Doctor 2003) that shift the high-flow δ18O values to −18.3‰ to −17.4‰. The δ2H and δ13C value adjustments are within analytical error (<2‰ and <0.1‰, respectively).
Tritium and CFCs
Tritium concentrations reported in tritium units (TU) and chlorofluorocarbon-11, -12, and - 113 concentrations in picomoles per kilogram (pmol/kg) are summarized in Table 5. In order to estimate the probable equivalent atmospheric concentration of CFCs at the time of recharge in parts-per-thousand by volume (pptv), a correction factor using estimates of recharge temperature and elevation was applied (Plummer et al. 2006). Air temperature in a thick unsaturated zone is typically close to the mean annual temperature (Busenberg and Plummer 1992). In Logan Canyon, the mean annual air temperature at SNOTEL sites from multiple elevations is approximately 4.5 °C (USDA 2018). Recharge elevations were estimated from highest-elevation dye-traced source locations (Spangler 2001) for Benchmark and Dewitt Springs. China Row and Spring Hollow springs were not dye traced. Their recharge elevations were estimated from the δ18O-elevation gradient determined in this study, and are presented in the following section.
A local record of 3H atmospheric concentrations was estimated by correlation of existing records from the Salt Lake City, Utah, station (1963–1984) with records from the Ottawa, Canada, station (1953–2012) following the linear regression procedure after Michel (1989):
where SL is the atmospheric 3H concentration (in TU) at the Salt Lake City station, and OT is the concentration (in TU) at the Ottawa station. The reconstructed tritium curve for Salt Lake City is shown in Fig. 4. All spring concentrations lie within the range of estimated atmospheric values for Salt Lake City (Table 5), and hence may be assessed via lumped-parameter models (Solder et al. 2016).
Estimated time-series of atmospheric tritium concentrations (USGS GDL 2017) based on correlation with the Ottawa, Ontario IAEA station
The CFC-12 concentration in China Row Spring is high enough to indicate some form of contamination (W. Mace, University of Utah Noble Gas Lab, written communication, 2018). The CFC-11 concentration in China Row Spring, if taken alone, is high enough to confound whether the recharge is contemporary or stems from the 1980s. However, the CFC-113 concentration is low enough to unambiguously attribute a pre-1990 recharge age. Values for all other springs are low enough to reasonably infer recharge ages.
Discussion
Water–rock interactions
PHREEQC water–rock reaction-path models
PHREEQC simulations of open and closed reaction paths of water with stoichiometric dolomite and calcite are explored for pCO2 conditions from 10–3.4 to 10–1.5 (Fig. 5). Measured spring values of pH and total dissolved inorganic carbon (TDIC) and calculated SI fall on open system simulations for pCO2 conditions between 10–2.5 and 10–2.0, consistent with the pCO2 for the spring waters (Table 2). Note that closed system reaction at a pCO2 of approximately 10–1.5 also matches the water compositions for the dolomite and calcite scenarios; however, this pCO2 is significantly higher than observed. Thus, these reaction-path models indicate that open-system water–rock reaction with calcite and dolomite are most likely.
Carbon stable isotope evolution models
Mass-balance calculations after Wigley (1972) were applied to test whether water–rock interactions likely occur in an open or closed system. For these calculations, the δ13C values of CO2 for precipitation in equilibrium with atmospheric CO2 was set to the current regional average (−7.3‰; Vogel et al. 1970), the δ13C for soil CO2 was set to the average value in the proximal Wasatch Mountains (−23.3‰; Cerling et al. 1991), and the mean δ13C value for the Garden City Limestone (−0.95‰; Davis 2017) was used to represent the carbonate bedrock.
Open- and closed-system water–rock reaction paths are explored in Fig. 6. The measured δ13C values and TDIC concentrations best match open-system evolution at pCO2 between 10–2.5 and 10–2.0 to equilibrium with calcite and dolomite. Closed-system paths at these pCO2 conditions can produce δ13C values similar to those observed, but at far lower TDIC concentrations. These simulations support evolution in an open system, implying that dissolution occurs mostly in conduit-dominated flowpaths that quickly transmit water. As this is the case across seasons for all observed springs, irrespective of discharge, it is likely that karst development is relatively uniform across the Bear River Range, and that flowpaths to each spring have similar hydrogeologic properties.
Congruent versus incongruent dissolution along flowpaths
The geochemical modeling presented above assumes that the aquifer materials dissolve congruently, with no coincident mineral precipitation. If so, Ca:Mg molar ratios for stoichiometric dolomite (1.0), calcian dolomite (1.1–1.3), and calcite (44–89) in the Bear River Range (Kaliser 1972) should be reflected in spring compositions from flowpaths within different geologic units. Ratios cluster around one in all springs during low-flow conditions (Table 6).
Lower water temperatures during low-flow conditions (interpreted as baseflow) indicate the possibility for incongruent dissolution, driven by increased solubility of dolomite over calcite in colder water (Williams et al. 2007). Langmuir (1971) and Wigley (1972) document the incongruent dissolution of dolomite in low-temperature water; hence, winter baseflow conditions may drive water–rock interactions to achieve Ca:Mg molar ratios of less than one.
To test if low Ca:Mg molar ratios reflect incongruent dissolution, PHREEQC was used to simulate dissolution of stoichiometric or calcian dolomite (Fig. 7). Thermodynamic data from Wigley (1972) were used to represent calcian dolomite in the model (Ca:Mg molar ratio = 1.17:1). The results show that dissolution will proceed incongruently after water reaches supersaturation or saturation, respectively, with stoichiometric or calcian dolomite. Reaction paths for congruent dissolution of calcian dolomite, fixed at pCO2 values of 10–3.0 and 10–2.0 atm, respectively, explain the observed concentrations only for Spring Hollow and Ricks Springs. Similarly, reaction paths for congruent dissolution of stoichiometric dolomite at the same fixed pCO2 values explain the observed concentrations for Benchmark, Dewitt and Logan Cave springs, and possibly China Row Spring. However, reaction paths for incongruent dissolution of calcian dolomite at these fixed pCO2 values explain the observed concentrations for all seven springs, including Woodcamp Hollow Spring. This supports the notion that at least some incongruent dissolution occurs in the Bear River Range karst aquifer system. Hence, it is unlikely that Ca:Mg molar ratios are a reliable metric for fingerprinting particular hydrogeologic units in alpine karst.
As with the Ca:Mg molar ratios, the saturation indices may yield insight into the type of dissolution. Saturation indices for calcian or stoichiometric dolomite and magnesian calcite of the March 2017 samples are positively correlated and adhere to a linear trend (Fig. 8a). Indices from subsequent sampling excursions cluster near slight dolomite supersaturation and calcite undersaturation. Calcite saturation is never exceeded, even though dolomite saturation indices exceed a value of one. This is a hallmark of incongruence (Langmuir 1971).
a Modeled saturation indices for dissolution of stoichiometric or calcian dolomite and magnesian calcite, along with calculated values for Bear River Range springs, shown in terms of saturation with respect to calcite and dolomite. b Modeled saturation indices for dissolution of stoichiometric or calcian dolomite and magnesian calcite, along with calculated values for Bear River Range springs, shown in terms of saturation with respect to calcite and calcian dolomite
All springs except Spring Hollow Spring discharge groundwater supersaturated with respect to stoichiometric dolomite (Fig. 8a), but groundwater never exceeds saturation with respect to calcian dolomite (Fig. 8b). Undersaturation with respect to stoichiometric dolomite in Spring Hollow Spring waters may stem from mixing of groundwater from two different flowpaths. This likely is the case, as Spring Hollow Spring bears a δ13C value and a TDIC concentration of water supersaturated with respect to dolomite (Fig. 6).
Dissolution of calcian dolomite inherently produces one degree of supersaturation with respect to stoichiometric dolomite, removing the need for additional mechanisms. All springs adhere closely to the simulated reaction path of dissolving calcian dolomite, and never reach supersaturation with respect to it or calcite (Fig. 8b). Hence, it is likely that incongruent dissolution of calcian dolomite, where present, exerts a strong control on water–rock interactions.
Oxygen and hydrogen stable isotopes
Meteoric water isotopic characteristics
The δ18O and δ2H values for spring waters are similar to those for precipitation in Utah (Fig. 9; Bowen 2017). Most spring samples collected in March appear to fall below (to the right of) the global and local meteoric water lines (Craig 1961; Kendall and Coplen 2001). Snow samples lie along a significant linear trend (shown in blue on Fig. 9) with a slope similar to the Utah meteoric water line (Kendall and Coplen 2001) and given by the formula:
On average, the spring isotope values measured in May are lower than those during March. This may reflect a seasonal difference where May conditions include winter precipitation in the form of snowmelt recharge and March reflects the isotopic value of late-fall recharge or a longer-term mean groundwater value. However, it seems unlikely that this represents an evaporative trend because all of the values lie within the range of observed values for precipitation in Utah (Bowen 2017). The range of snow isotope values encompasses the values measured in spring water, but also include much lower values. The lower values recorded by the snow is consistent with winter precipitation (Earman et al. 2006; Sharp 2007).
Stable-isotope elevation gradients
Oxygen stable isotope ratios for snow at different elevations are reported in Table 4. The δ18O values were assessed by analysis-of-variance (ANOVA) to correlate statistically the dominant geographic controls on fractionation. This tests the hypothesis proposed by Bright (2009) that progressive rainout (or precipitation as snow) of air masses traversing the Bear River Range is more significant than elevation on the δ18O values of precipitation. ANOVA ranks the significance of possible controlling factors (e.g., longitude and elevation), and informs the choice of variables for subsequent linear regression (Berthoux and Brown 2002).
The results (Table 7) suggest that elevation is the primary influence on fractionation in the Bear River Range. Although the F-statistic indicates a possible effect of longitude on δ18O values, iterative adjustment shows that it is not significant in conjunction with elevation, and decreases in significance when assessed separately. Elevation increases in significance to greater than 99.9% when used as the only predictor, which is evidence for a better empirical link. Hence, elevation (m asl) was chosen as the sole predictor variable for δ18O values in linear regression:
The elevation isotope gradient (line slope) is −0.28‰ per 100 m. This gradient is similar to others for rain in the United States, but contradicts other interpretations that such gradients do not exist in snow or away from coasts (Abbott et al. 2000; James et al. 2000; Blasch and Bryson 2007; Brooks et al. 2012).
Groundwater recharge elevations often are estimated from elevation-gradient regression of δ18O snow values (James et al. 2000; Poage and Chamberlain 2001). Mean recharge elevations estimated from the linear relationship with δ18O values are summarized in Table 8. The δ18O-predicted mean recharge elevations qualitatively align with dye-trace estimates, except for Ricks and Benchmark springs. The mean δ18O-predicted recharge elevation for Ricks Spring is lower than the two far higher dye-traced sources (Fig. 2), Tony Grove (640 m) and Bunchgrass Hollow (805 m). The δ18O value for Ricks Spring (−17.4‰; Table 8) likely represents mixing of high-elevation recharge with a significant amount of Logan River water (δ18O = −17.6‰; Spangler 2001). In contrast, the δ18O-predicted mean recharge elevation for Benchmark Spring is higher than the dye-trace estimate (Table 8). Because Benchmark Spring’s stable-isotope signature is consistently more negative than Logan Cave Spring’s (Table 3), it is likely that the former’s recharge area is higher than the latter’s dye-traced source, despite dye traces that link both springs to a shared surface catchment (Fig. 2).
Groundwater residence times
Groundwater age estimates from lumped-parameter models (LPMs) of 3H and CFC data are presented in this section. Chlorofluorocarbon concentrations in pmol/kg were corrected for temperature and recharge elevation, then converted to atmospheric units of pptv after Busenberg et al. (2006). The corrected values are less than contemporary atmospheric concentrations except for CFC-12 in China Row Spring, which is likely contaminated (W. Mace, University of Utah Noble Gas Lab, written communication, 2018). Accordingly, China Row Spring was excluded from LPMs that involved CFC-12.
Binary-mixing model
Dye traces document the ability of some baseflow to move through Bear River Range karst with subannual transit times (Spangler 2002). This suggests that a binary-mixing model fixed at modern concentrations may be more accurate than a simple piston-flow model of older water.
The applicable age range for a binary-mixing model is limited to the date that each tracer was first detected in the atmosphere. The earliest detection at Niwot Ridge occurred in 1940 for CFC-11 and CFC-12, and in 1943 for CFC-113 (Jurgens et al. 2012). Earliest detection of tritium above background levels occurred in 1945 at the Ottawa, Ontario station. Hence, age estimates by this method are only possible if groundwater is younger than 75–80 years.
Binary mixing models of 3H and each CFC support the notion that groundwater is a mixture of subannual and decadal recharge, in this case 60–65 years old (Fig. 10; Table 9). The estimated percentages of decadal recharge present in baseflow using 3H (0–80% decadal) vary more than those predicted using CFCs (40–85% decadal).
It is likely that zones of lesser karstification exist in each spring’s groundwater basin, yet only inhibit flow by several decades; however, the results of the binary-mixing model do not rule out the possibility that a small percentage of much older (centennial to millennial) recharge may be mixing with subannual and decadal recharge.
Residence time of this magnitude was documented using 3H concentrations in dripwater recharge to a cave system in Israel (Even et al. 1986). The spread for the decadal-age component is similar to that of probability-density functions (PDFs) of age simulated for a karst aquifer in South Dakota (Long and Putnam 2009). If this is the case, extended droughts could significantly affect the baseflow of critical water resources decades later. Furthermore, the possible absence of matrix-dominated flow in alpine karst indicates that groundwater resources would be highly responsive to long-term climate shifts.
Conclusions
Observed spring δ13C DIC values range from −11.8 to −9.9‰, which are intermediate between carbonate bedrock (~ −1‰) and local soil CO2 (~ −23‰). Modeled reaction paths for dissolution in an open system align well with data presented in this study. Saturation indices and low Ca:Mg molar ratios indicate that incongruent dissolution exerts a strong control on water–rock interactions. Reaction path modeling indicates that dissolution of calcian dolomite (Ca:Mg molar ratio = 1.17:1), a primary aquifer material in the Bear River Range, exerts strong control on water geochemistry. These results support Bright’s (2009) conclusion that incongruent dissolution of calcian dolomite controls groundwater chemistry in the Bear River Range, and contrast with previous inferences of a less solution-enhanced, matrix-dominated flowpath that would be more resilient to climate change (Kolesar et al. 2005; Neilson et al. 2018).
Spring water δ18O and δ2H values are similar to Utah meteoric water. The δ18O values in the alpine snowpack display a significant statistical relationship with elevation (−0.28‰ per 100 m), suggesting that it may be a useful tracer for groundwater recharge elevation. Predicted recharge elevations from the δ18O gradient qualitatively align with dye-trace estimates. However, Ricks and Benchmark Springs have δ18O values that do not align well with dye-traced recharge elevations. It is likely that Ricks Spring represents a mix of alpine snowmelt and Logan River water. If Benchmark Spring, like Logan Cave Spring nearby, draws recharge from higher elevations, both should have similar isotopic values from snowmelt runoff during high-flow conditions. Benchmark Spring’s stable-isotope signature is clearly more negative across seasons (Table 3); hence, it is likely that a higher recharge zone supplies water to Benchmark Spring.
Tritium and chlorofluorocarbon concentrations in representative springs are likely best described by binary mixing of subannual recharge with 60- to 65-year-old groundwater. This suggests that the alpine karst aquifer system in the Bear River Range is best represented by a double-porosity, rather than a triple-porosity, model (Fig. 3b). The subannual recharge documented by dye traces implies that caverns are the primary flowpath to each spring, but the possible presence of decadal-age water may indicate that a slightly lower permeability flowpath dominates during baseflow. Nonetheless, the binary-mixing model results do not rule out the possibility that a relatively small proportion of matrix flow occurs, and that a triple-porosity system exists.
Neilson et al. (2018) concluded that matrix-dominated flow plays a role in the karst hydrology of the Bear River Range; however, the results of the present investigation suggest that such a role is negligible. The documented subannual recharge indicates the importance of conduit flow. This combined with the strong likelihood that open-system conditions dominate supports the conclusion that the alpine karst aquifer system can be described best by a double-porosity model. If this is indeed the case, the aquifer system may not provide drought resilience for water resources linked to it. Further study of this aquifer system, as well as other alpine karst systems in arid to semiarid settings, with an enhanced suite of geochemical tracers, including carbon-14 and helium-3, will help refine the understanding of potential matrix-dominated flow, and improve management of alpine-karst water resources in light of climate change.
References
Abbott MD, Lini A, Bierman PR (2000) δ18O, δD, and 3H measurements constrain groundwater recharge patterns in an upland fractured bedrock aquifer, Vermont, USA. J Hydrol 228:101–112
Bahr K (2016) Structural and lithological influences on the Tony Grove alpine karst system, Bear River range, north-central Utah. MSc Thesis, Utah State University, Logan, UT, 212 pp
Berthoux PM, Brown LC (2002) Statistics for environmental engineers. CRC, Boca Raton, FL, 512 pp
Bischoff WD (1998) Dissolution enthalpies of magnesian calcites. Aquat Geochem 4:321–336
Bjorklund LJ, McGreevy LJ (1971) Groundwater resources of Cache Valley, Utah and Idaho. Technical Publication 36, Utah Department of Natural Resources, Salt Lake City, 72 pp
Blasch KW, Bryson JR (2007) Distinguishing sources of ground water recharge by using δ2H and δ18O. Ground Water 45(3):294–308
Bowen G (2017) water isotopes database. http://waterisotopes.org. Accessed October 2018
Bright J (2009) Isotope and major-ion chemistry of groundwater in bear Lake Valley, Utah and Idaho, with emphasis on the Bear River range. In: Rosenbaum JG, Kaufman DS (eds) Paleoenvironments of Bear Lake, Utah and Idaho, and its catchment. Geol Soc Am Spec Pap 450:105–132
Brooks JR, Wiginton PJ, Phillips DL, Comeleo R, Coulombe R (2012) Willamette River basin surface water isoscape (δ18O and δ 2H): temporal changes of source water within the river. Ecosphere 3(5):1–39
Busenberg E, Plummer LN (1992) Use of chlorofluorocarbons (CCl3F and CCL2F2) as hydrologic tracers and age-dating tools: the alluvium and terrace system of central Oklahoma. Water Resour Res 28(9):2257–2283
Busenberg E, Plummer LN, Cook PG, Solomon DK, Han LF, Groning M, Oster H (2006) Sampling and analytical methods. In: Use of chlorofluorocarbons in hydrology: a guidebook. International Atomic Energy Agency, Vienna, pp 199–220
Cerling TE, Solomon DK, Quade JA, Bowman JR (1991) On the isotopic composition of carbon in soil carbon dioxide. Geochim Cosmochim Acta 55:3403–3405
Chen Z, Goldscheider N (2014) Modeling spatially and temporally varied hydraulic behavior of a folded karst system with dominant conduit drainage at catchment scale, Hochifen-Gottesacker, Alps. J Hydrol 514:41–52
Craig H (1961) Isotopic variations in meteoric waters. Science 133(3465):1702–1703
Davis CR (2017) Sequence stratigraphy, chemostratigraphy, and biostratigraphy of lower Ordovician units in northeastern and western Central Utah: regional implications. MSc Thesis, Utah State University, Logan, UT, 244 pp
Dover JH (1995) Geologic map of the Logan 30′ × 60′ quadrangle, Cache and Rich counties, Utah, and Lincoln and Uinta counties, Wyoming 1:100,000. Utah Geological Survey Miscell Publ MP06–8 DM, Utah Geological Survey, Salt Lake City, UT
Earman S, Campbell AR, Phillips FM, Newman BD (2006) Isotopic exchange between snow and atmospheric water vapor: estimation of the snowmelt component of groundwater recharge in the southwestern united state. J Geophys Res 111:1–18
Evans JP, Oaks RQ Jr (1996) Three-dimensional variations in extensional fault shape and basin form: the Cache Valley basin, eastern basin and range province, United States. Geol Soc Am Bull 108:1580–1593
Evans JP, McCalpin JP, Holmes DC (1996) Geologic map of the Logan quadrangle, Cache County, Utah 1:24,000. Utah Geological Survey Map 96–1, Utah Geological Survey, Salt Lake City, UT
Even H, Carmi I, Magaritz M, Gerson R (1986) Timing the transport of water through the upper vadose zone in a karstic system above a cave in Israel. Earth Surf Process Landf 11(2):181–191
Filippini M, Squarzoni G, De Waele J, Fiorucci A, Vigna B, Grillo B, Riva A, Rossetti S, Zini L, Casagrande G, Stumpp C, Gargini A (2018) Differentiated spring behavior under changing hydrological conditions in an alpine karst aquifer. J Hydrol 556:572–584
Ford DC (1983) Effects of glaciations upon karst aquifers in Canada. J Hydrol 61:149–158
Ford D, Williams PD (2007) Karst hydrogeology and geomorphology. Wiley, West Sussex, England, 576 pp
Francis GG (1972) Stratigraphy and environmental analysis of the swan peak formation and Eureka quartzite, northern Utah. MSc Thesis, Utah State University, Logan, UT, 125 pp
Frondini F, Zucchini A, Comodi P (2014) Water–rock interactions and trace element distribution in dolomite aquifers: the Sassolungo and Sella systems (northern Italy). Geochem J 48:231–246
Goldscheider N, Neukum C (2010) Fold and fault control on the drainage pattern of a double-karst-aquifer system, Winterstaude, Austrian Alps. Acta Carsol 39(2):173–186
Gooseff MN, Evans JP, Kolesar P, Lachmar TE, Payn R (2005) Hydrologic contributions of springs to the Logan River, Utah. Abstract H51C-02, AGU Spring Meeting, New Orleans, LA, May 2005
Gremaud V, Goldscheider N, Savoy L, Favre S, Masson H (2009) Geological structure, recharge processes and underground drainage of a glacierised karst aquifer system, Tsanfleuron-Sanetsch, Swiss Alps. Hydrogeol J 17:1833–1848
International Atomic Energy Agency (IAEA) (2014) Global network of isotopes in precipitation, the GNIP database. http://www.iaes.org/water. Accessed August 2017
James ER, Manga M, Rose TP, Hudston GB (2000) The use of temperature and the isotopes of O, H, C, and noble gases to determine the pattern and spatial extent of groundwater flow. J Hydrol 237:100–112
Jurgens BC, Bohlke JK, Eberts SM (2012) TracerLPM (version 1): an Excel® workbook for interpreting groundwater age distributions from environmental tracer data. US Geol Surv Tech Methods Rep 4-F3, 60 pp
Kaliser BN (1972) Environmental geology of Bear Lake area, Rich County, Utah. Utah Geol Mineral Surv Bull 96, 32 pp
Katz BG, Sepulveda AA, Verdi RJ (2009) Estimating nitrogen loading to ground water and assessing vulnerability to nitrate contamination in a large karstic springs basin, Florida. J Am Water Resour Assoc 45(3):607–627
Kendall C, Coplen TB (2001) Distribution of oxygen-18 and deuterium in river waters across the United States. Hydrol Process 15:1363–1393
Kendall C, Doctor DH (2003) Stable isotope applications in hydrologic studies. In: Drever J (ed) Treatise on geochemistry, vol 5. Elsevier, Amsterdam, The Netherlands, pp 319–364
Kolesar PT, Evans JP, Gooseff MN, Lachmar TE, Payn R (2005) A tale of two (or more) karsts, Bear River Range, Cache National Forest. Utah Geol Soc Am Abstr Programs 37(7):177
Langmuir D (1971) Geochemistry of some carbonate ground waters in central Pennsylvania. Geochim Cosmochim Acta 35(10):1023–1045
Lauber U, Goldscheider N (2014) Use of artificial and natural tracers to assess groundwater transit-time distribution and flow systems in a high-alpine karst system (Wetterstein Mountains, Germany). Hydrogeol J 22:1807–1824
Long AJ, Putnam LD (2009) Age-distribution estimation for karst groundwater: issues of parameterization and complexity in inverse modeling by convolution. J Hydrol 376:579–588
McCalpin JP (1989) Surficial geologic map of the East Cache fault zone, Cache County, Utah 1:50,000. US Geol Surv Miscell Field Studies Map MF-2107
McCalpin JP (1994) Neotectonic deformation along the east cache fault zone, Cache County, Utah. Utah Geol Surv Spec Study 83, 37 pp
Michel RL (1989) Tritium deposition in the continental United States, 1953-83. US Geol Surv Water Resour Invest Rep 89-4072, 46 pp
Mook W (2001) Environmental isotopes in the hydrological: introduction—theory, methods, review. Tech Doc Hydrol vol 1, 39. UNESCO/Int Atomic Energy Agency, Paris, 164 pp
Moral F, Cruz-Sanjulian JJ, Olias M (2008) Geochemical evolution of groundwater in the carbonate aquifers of Sierra de Segura (Betic Cordillera, southern Spain). J Hydrol 360:281–296
Mundorff JC (1971) Nonthermal springs of Utah. Utah Geol Mineral Surv Water Resour Bull 16, 70 pp
Neilson BT, Tennant H, Stout TL, Miller MP, Gabor RS, Jameel Y, Millington M, Gelderloow A, Bowen GJ, Brooks PD (2018) Stream centric methods for determining groundwater contributions in karst mountain watersheds. Water Resour Res 54(9):6708–6724
Niwot Ridge LTER (2018) Niwot Ridge LTER long-term ecological research dataset. http://niwot.colorado.edu/data. Accessed January 2018
Ozyurt NN (2008) Residence time distribution in the Kirkgoz karst springs (Anntalya-Turkey) as a tool for contamination vulnerability assessment. Environ Geol 53:1571–1583
Parkhurst DL, Appelo CA (2013) Description of input and examples for PHREEQC version 3: a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US Geol Surv Tech Methods, Book 6, chapter A43, US Geological Survey, Reston, VA, 497 pp
Plummer LN, Busenberg E, Cook PG (2006) Principles of chlorofluorocarbon dating. In: Groning M, Han LF, Aggarwal P (eds) Use of chlorofluorocarbons in hydrology: a guidebook. International Atomic Energy Agency, Vienna, pp 17–29
Poage MA, Chamberlain CP (2001) Empirical relationships between elevation and the stable isotope composition of precipitation and surface waters: considerations for studies of paleoelevation change. Am J Sci 301:1–15
Robinson JM (1999) Chemical and hydrostratigraphic characterization of ground water and surface water interaction in Cache Valley, Utah. MSc Thesis, Utah State University, Logan, UT, 184 pp
Savio D, Stadler P, Reischer GH, Demeter K, Linke RB, Blaschke AP, Mach RL, Kirschner AKT, Stadler H, Farnleitner AH (2019) Spring water of an alpine karst aquifer is dominated by a taxonomically stable but discharge-responsive bacterial community. Frontiers Microbiol. https://doi.org/10.3389/fmicb.2019.00028
Sharp Z (2007) Stable sotope geochemistry. Pearson prentice hall. Upper Saddle River, New Jersey, p 344
Solder JE, Stolp BJ, Heilweil VM, Susong DD (2016) Characterization of mean transit time at large springs in the upper Colorado River basin, USA: a tool for assessing groundwater discharge variability. Hydrogeol J 24(8):2017–2033
Solomon DK (2017) University of Utah dissolved and noble gas lab tritium collection guide. http://www.noblegaslab.utah.edu/pdfs/tritium_collection.pdf. Accessed May 2017
Smart CC (1983) The hydrology of the Castleguard Karst, Columbia Icefields, Alberta, Canada. Arct Alp Res 15(4):471–486
Smart CC (1988) Artificial tracer techniques for the determination of the structure of conduit aquifers. Ground Water 26(4):445–453
Spangler LE (2001) Delineation of recharge areas for karst springs in Logan canyon, Bear River Range, northern Utah. US Geol Surv Water Resour Invest Rep 01-4011:186–193
Spangler LE (2002) Use of dye tracing to determine conduit flow paths within source-protection areas of a karst spring and wells in the Bear River Range, northern Utah. US Geol Surv Water Resour Invest Rep 2-4174. 6 pp
Spangler LE (2012) Karst hydrogeology of the Bear River Range in the vicinity of Logan canyon, northern Utah, Uinta-Wasatch-Cache National Forest. Beneath For 5(1):12–20
USDA (2018) Snow telemetry (SNOTEL) and snow course data and products. US Department of Agriculture, Natural Resources Conservation Service. https://www.wcc.nrcs.usda.gov/snow/. Accessed January 2018
USGS GDL (2017) Tracer input functions. US Geological Survey Groundwater Dating Laboratory, Reston, VA. http://water.usgs.gov/lab/software/air_curve/. Accessed November 2017
Vogel JC, Grootes PM, Mook WG (1970) Isotopic fractionation between gaseous and dissolved carbon dioxide. Zeitschrift Physik 230(3):225–238
White WB (2003) Conceptual models for karstic aquifers. Speleogenesis Evol Karst Aquifers 1(1):11–16
Wigley TM (1972) The incongruent solution of dolomite. Geochim Cosmochim Acta 37:1397–1402
Wilde FD (2008) Guidelines for field-measured water-quality properties (ver. 2.0). US Geological Survey Techniques of Water-Resources Investigations, book 9, chapter A6, section 6.0, USGS, Reston, VA, pp 3–29
Williams EL, Szramek KJ, Jin L, Ku TC, Walter LM (2007) The carbonate system geochemistry of shallow groundwater-surface water systems in temperate glaciated watersheds (Michigan, USA): significance of open-system dolomite weathering. Geol Soc Am Bull 119(516):515–528
Williams JS (1948) Geology of the Paleozoic rocks, Logan quadrangle, Utah. Geol Soc Am Bull 59:1121–1184
Williams JS (1958) Geologic atlas of Utah, Cache County Utah Geol Mineral Survey Bull 64, 98 pp
Zeng C, Gremaud V, Zeng H, Liu Z, Goldscheider N (2012) Temperature-driven meltwater production and hydrochemical variations at a glaciated alpine karst aquifer: implication for the atmospheric CO2 sink under global warming. Environ Earth Sci 65:2285–2297
Acknowledgements
The authors thank reviewers Brad Esser and Stephane Binet, as well as associate editor Hervé Jourde and editor Prof. Rui Ma, whose insights and suggestions greatly improved the final version of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lachmar, T., Sorsby, S. & Newell, D. Geochemical insights into groundwater movement in alpine karst, Bear River Range, Utah, USA. Hydrogeol J 29, 687–701 (2021). https://doi.org/10.1007/s10040-020-02256-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10040-020-02256-1