Abstract
The karst hydrogeology systems of the Watuputih Hills region of Central Java, Indonesia, have many springs with varying discharge and are composed of formations with complex geological structures. This work characterized the karst hydrogeology by studying 50 hydrogeological features (caves, springs and wells) and by analyzing the chemical-physical properties of groundwater in the field (pH, temperature, EC, HCO3−, 222Rn) and the major ions and stable isotopes of the groundwater samples in the laboratory, along with the stable isotope content of rainwater sampled over 1 year. Hierarchical cluster analysis of the water samples identified three hydrochemical groups: groundwater flowing through carbonate rocks (groups C2 and C3), through quartz sandstones and volcanic rocks (group C4), and through carbonate rocks and the siliciclastic rocks (quartz sandstones) underneath them (group C1). Springs with large discharge, typified as artesian fault-guided springs, were categorized into group C1. These springs are Sumbersemen, Brubulan Tahunan, and Brubulan Pesucen, with mean discharges of 1,516, 165, and 95 L/s, respectively. Based on the results of the stable isotope analysis, the d-excess calculation and the 222Rn concentrations, groups C2, C3, and C4 associate with shallow groundwater systems that dominantly flow through pores, whereas group C1 associates with a deep groundwater system controlled by geological structure. The geological structure also determines the groundwater flow in the cave streams. The shallow groundwater system is sourced by local rainwater, while the deep groundwater system displays a relationship with the groundwater in the northern hills at an elevation >375 m above sea level.
Résumé
Les systèmes hydrogéologiques karstiques de la région des collines de Watuputih dans la partie centrale de Java en Indonésie, comprennent plusieurs sources avec des débits variables et sont composés de formations caractérisées par des structures géologiques complexes. Ce travail a concerné la caractérisation de l’hydrogéologie karstique en étudiant quelques 50 caractéristiques hydrogéologiques (cavités, sources et puits) et en analysant les propriétés physicochimiques des eaux souterraines sur le terrain (pH, température, conductivité électrique, HCO3−, 222Rn) et les ions majeurs et isotopes stables à partir d’échantillons d’eaux souterraines analysés au laboratoire, ainsi que les teneurs en isotope stable pour les eaux des précipitations collectées sur une période d’une année. L’analyse d’ensembles hiérarchisés des échantillons d’eau a permis d’identifier trois groupes hydrochimiques: l’eau souterraine qui s’écoule au sein des roches carbonatées (groupes C2 et C3), des roches de type grès riches en quartz et des roches volcaniques (groupe C4), et des roches carbonatées et silicilastiques (grès à quartz) sous-jacentes (groupe C1). Les sources avec des débits importants, de type sources artésiennes associées à une faille, ont été classées dans le groupe C1. Ces sources sont Sumbersemen, Brubulan Tahunan, et Brubulan Pesucen, avec des débits moyens de 1,516, 165 et 95 L/s, respectivement. Sur la base des résultats des analyses en isotopes stables, le calcul de l’excès en d et les concentrations en 222Rn, les groupes C2, C3 et C4 sont associés à des systèmes d’écoulements d’eau souterrain peu profonds dominés par des écoulements en milieu poreux, alors que le groupe C1 est associé à un système d’écoulement d’eau souterraine profond contrôlé par la structure géologique. La structure géologique détermine également l’écoulement d’eau souterraine dans les cours d’eau au sein des cavités. Le système d’écoulement d’eau souterraine peu profond est alimenté par les précipitations locales, alors que le système d’écoulement d’eau souterraine profond indique une relation avec les eaux souterraines dans les monts septentrionaux d’une altitude de recharge supérieure à 375 m (au-dessus du niveau de la mer).
Resumen
Los sistemas hidrogeológicos kársticos de la región de Watuputih Hills en Java Central, Indonesia, tienen muchos manantiales con descargas variables y están compuestos de formaciones con estructuras geológicas complejas. Este trabajo caracterizó la hidrogeología kárstica mediante el estudio de 50 rasgos hidrogeológicos (cuevas, manantiales y pozos) y el análisis de las propiedades químico-físicas de las aguas subterráneas en el campo (pH, temperatura, EC, HCO3−, 222Rn) y los principales iones e isótopos estables de las muestras en el laboratorio, junto con el contenido de isótopos estables del agua de lluvia muestreada durante un año. El análisis jerárquico de las muestras de agua identificó tres grupos hidroquímicos: agua subterránea que fluye a través de rocas carbonatadas (grupos C2 y C3), a través de areniscas de cuarzo y rocas volcánicas (grupo C4), y a través de rocas carbonatadas y rocas siliciclásticas (areniscas de cuarzo) debajo de ellas (grupo C1). Los manantiales con gran descarga, tipificados como manantiales artesianos guiados por fallas, fueron categorizados en el grupo C1. Estos manantiales son Sumbersemen, Brubulan Tahunan, y Brubulan Pesucen, con descargas medias de 1,516, 165, y 95 L/seg, respectivamente. En base a los resultados del análisis de isótopos estables, el cálculo del exceso de deuterio y las concentraciones de 222Rn, los grupos C2, C3 y C4 se asocian con sistemas de aguas subterráneas poco profundos que fluyen predominantemente a través de los poros, mientras que el grupo C1 se asocia con un sistema profundo controlado por la estructura geológica. La estructura geológica también determina el flujo de agua subterránea en las corrientes de las cuevas. El sistema poco profundo se alimenta del agua de lluvia local, mientras que el profundo muestra una relación con las aguas subterráneas de las serranías del norte a una altura >375 m sobre el nivel del mar.
摘要
印度尼西亚爪哇省中部的Watuputih山区的喀斯特水文地质系统有许多不同排泄量的泉水, 而且由具有复杂地质结构的地层构成。这项工作通过研究50个水文地质特征(洞穴,泉水和水井)并分析了场地地下水的化学物理特性(pH, 温度, EC, HCO3−, 222Rn)以及实验室中地下水样的主要离子和稳定同位素、一年内采集雨水样的稳定同位素含量,从而描述了喀斯特水文地质特征。水样的层次聚类分析确定了三个水化学组:地下水流经碳酸盐岩(C2和C3组), 流经石英砂岩和火山岩(C4组)以及流经其下方的碳酸盐岩和硅质碎屑岩(石英砂岩)(C1组)。大流量的泉水, 典型地如自流断层泉, 被分类为C1组。这些泉包括Sumbersemen,Brubulan Tahunan和Brubulan Pesucen泉, 平均流量分别为1,516、165和95 l / sec。根据稳定同位素分析的结果,d-过量计算和222Rn浓度,与浅层地下水系统相关的C2,C3和C4组主要流经孔隙水层, 而与深层地下水系统相关的C1组受地质结构控制。地质结构还决定了洞穴溪流的地下水流量。浅层地下水系统是由当地雨水补给, 而深层地下水系统则与海拔高于375 m的北部丘陵地区的地下水有一定的关系。
Resumo
Os sistemas hidrogeológicos cársticos da região de Watuputih Hills, no centro de Java, Indonésia, têm muitas nascentes com descargas variáveis que são compostas por formações com estruturas geológicas complexas. Esse trabalho caracterizou a hidrogeologia cárstica estudando 50 características hidrogeológicas (cavernas, nascentes e poços) e analisando as propriedades físico-químicas das águas subterrâneas no campo (pH, temperatura, CE, HCO3−, 222Rn), além dos íons maiores e isótopos estáveis de amostras de águas subterrâneas em laboratório, juntamente com o conteúdo de isótopos estáveis da água de chuva durante um ano. A análise hierárquica em grupos de amostras de água identificou três grupos hidroquímicos: águas subterrâneas fluindo através de rochas carbonáticas (grupos C2 e C3), através de arenitos quartzosos e rochas vulcânicas (grupo C4) e através de rochas carbonáticas e rochas siliciclásticas (arenitos quartzosos) debaixo deles (grupo C1). Nascentes com grandes vazões, tipificadas como nascentes artesianas guiadas por falhas, foram categorizadas no grupo C1. Essas nascentes são Sumbersemen, Brubulan Tahunan e Brubulan Pesucen, com descargas médias de 1,516, 165 e 95 L/s, respectivamente. Baseados nos resultados das análises de isótopos estáveis, do cálculo do excesso de d e das concentrações de 222Rn, os grupos C2, C3, e C4 associam-se a um sistema de água subterrânea rasa que flui dominantemente através de poros, enquanto o grupo C1 associa-se a um sistema de água subterrânea profunda controlado por estrutura geológica. A estrutura geológica também determina o fluxo de água subterrânea nos córregos das cavernas. O sistema de águas subterrâneas rasas é abastecido pela água da chuva local, enquanto o sistema de águas subterrâneas profundas possui uma relação com as águas subterrâneas nas colinas do norte, a uma altitude >375 m acima do nível do mar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Karst regions have a specific hydrogeological character (Milanovic 1981; Ford and Williams 1989, 2007) because the constituent rocks, like limestone and dolomite, are highly susceptible to chemical dissolution (Milanovic 1981; Goldscheider and Andreo 2007; Ford and Williams 1989, 2007). For karst regions, study of chemistry is essential (White 2015) because hydrochemical properties reflect the mechanism of groundwater flow in karstic rocks (Ford and Williams 1989; Ford and Williams 2007). Karst aquifers have heterogeneous characteristics owing to the three media-based systems through which groundwater flows, namely pores, fractures, and cavities (Goldscheider et al. 2007). In Indonesia, karst aquifers develop mainly in limestones and only occasionally in dolomite and marble, and these features have not been found in noncarbonate rocks (Haryono 2001).
This report elaborates on hydrochemical characteristics using hierarchical group analysis, which is validated with the data of stable isotopes (18O, 2H) and radon (222Rn), to identify the karst hydrogeological system characteristics in Watuputih Hills and the surrounding areas. The area is located in Rembang Regency, Central Java Province, Indonesia, where active limestone mining of the Paciran Formation takes place. Since approximately 2014, the study area has been heavily discussed due to the growing concerns of the community about the sustainability of springs connected to the limestone mining.
Studies of karst hydrogeological systems using hydrochemical and stable isotope analyses (18O and 2H) have been carried out by, for instance, Ashjari and Raeisi (2006), Petitta et al. (2011), Krishnaraj et al. (2012), Dimitriou and Tsintza (2015), and Thilakerathne et al. (2015), while more specific study employing multivariate hydrochemical analysis has also been conducted by, e.g., Valdes et al. (2007), Narany et al. (2014), Chihi et al. (2015), and Yuan et al. (2017). These previous studies generally explain hydrochemical processes such as dissolution and precipitation of carbonate and silicate mineral and cation exchange, and identification of karst hydrogeological systems including: the process of karst groundwater recharge, flow, and discharge. In addition to explaining the hydrochemical processes, the studies that apply multivariate statistics can also unfold the relationships between hydrochemical facies and aquifer groups and between the hydraulic characteristics of faults and physical-chemical processes in aquifers.
The majority of karst hydrogeological studies take place in nontropical regions in Paleozoic-Mesozoic rocks whose groundwater has varying electrical conductivity (EC) or total dissolved solids (TDS), like studies conducted by Yidana et al. (2010), (2011), Belkhiri et al. (2011), Narany et al. (2014), Chihi et al. (2015), and Yuan et al. (2017); for these studies, grouping using multivariate analysis can adequately explain hydrochemical characteristics. Karst groundwater in the tropics in Cenozoic rocks has a low variability of EC. To produce a more convincing multivariate analysis using EC of low variability, a validation that relies on stable and unstable isotopes as tracer agents becomes necessary.
The stable isotopes and 222Rn have been widely applied as tracers in groundwater studies. The isotopes 18O and 2H are conservative (Falcone et al. 2008; Pu et al. 2013; Tillman et al. 2014; Murillo et al. 2015; and Sun et al. 2016), that is, not affected by water–rock interaction processes at temperatures lower than 200 °C (Marfia et al. 2004). These isotopes have been used in studies of groundwater recharge and flow (Marfia et al. 2004; Rodgers et al. 2005; Blasch and Bryson 2007; Mukherjee et al. 2007; Ryu et al. 2007; Al-Gamal 2011; Singh et al. 2013), the heterogeneity of aquifer hydraulic properties (Marfia et al. 2004; Leibundgut et al. 2009; Doveri et al. 2013), residence time of groundwater (Rademacher et al. 2003; Mahlknecht et al. 2006), and the mixing of groundwater from different sources (Coplen 1993). The isotope 222Rn can also be used as a tracer element (Burnett et al. 2001) because, in addition to having a short half-life of 3.8 days (Michel 1990; Clark and Fritz 1997), it is a noble gas that has the highest water solubility (Clever 1985) through diffusion. Moreover, it is conservative, meaning that it does not react with the surrounding environment (Clark and Fritz 1997). The study of fractured aquifers using 222Rn has successfully pinpointed areas affected by faults (Choubey and Ramola 1997; Villalobos et al. 2017) and regions with intensive fractures and active hydraulic characteristics (Cook et al. 1999; Hamada 1999; Skeppstrom 2005), to identify geological controls on water chemistry in geothermal systems (Iskandar et al. 2018), and to assist in groundwater studies in karst aquifers (Criss et al. 2007).
By conducting a validated hydrochemical study with stable isotopes and 222Rn data on a more detailed scale, a thorough comprehension of hydrogeological characteristics in relation to lithological control and geological structure, as well as the origin of groundwater in the study area, can be achieved and, therefore, used as the basis for spring management. This study hypothesizes that important springs (with large discharges) have a deep groundwater system that not only interacts with carbonate rocks but also with nonkarst (siliciclastic) rocks, which are controlled by geological structures.
Geological and hydrogeological setting
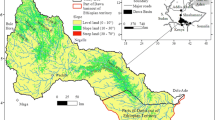
The study area is an anticlinorium zone with an east–west direction, forming a range of hills at an elevation averaging less than 500 m above sea level (masl) (Bemmelen 1949). Geomorphologically, it is dominated by structural hills, karst morphology, low to undulating hills, and a small portion of volcanic cones that are geologically composed of various rock formations such as carbonate rocks, siliciclastic sediments, and volcanic rocks (Luthfi et al. 2017; Novita et al. 2017). Karst morphology develops in limestones of the Bulu Formation with isolated karst cone patterns. The spatial variation of the lithological types in the study area is presented in Fig. 1.
a Map of the geology and hydrogeological sampling features in the study area (Modified from Luthfi et al. 2017; Novita et al. 2017). b Geological cross section along the A–B line, based on resistivity and gravity anomalies investigation (GAI, Geological Agency of Indonesia, Jakarta, Indonesia, unpublished report, 2017)
The stratigraphy of the study area is composed of rock formations from early Miocene to Pleistocene epoch (Luthfi et al. 2017; Novita et al. 2017). The limestone of the Tawun Formation (Nmtl), formed in the later early Miocene, is composed of sandy marl alternating with bioclastic limestone, with coarse grains at several sites. The quartz sandstone of the Ngrayong Formation (Nmns), formed in the middle Miocene, is composed of quartz sandstones with limestones and sandy limestone intercalations. The limestone of the Bulu Formation (Nmbl), formed in the later middle Miocene, is composed of layered clastic limestone (packstone-wackestone), which is solid, locally jointed, and porous. The sandstone of the Wonocolo Formation (Nmws), formed in the later middle Miocene, is composed of calcareous sandstone with sandy marl intercalations. The sandstone of the Ledok Formation (Nmls), formed in the late Miocene, is composed of glauconitic sandstones in layered structures of coarse to fine sand, and cemented by carbonates. The limestone of the Ledok Formation (Nmll), formed in the late Miocene, is composed of clastic limestone, of medium to fine sand. The marl of the Mundu Formation (Npmm), formed in the Pliocene, is distributed around the synclinal axis. The limestone of the Paciran Formation (Nppl), formed in the Pliocene-Pleistocene, is composed of layered clastic limestone, with fine-to-very-coarse grain size, and is distributed in Watuputih Hills surrounded by the limestones of Bulu Formation. The Gutak Volcanic Rocks (Qvg), formed in the Pleistocene, are composed of dacitic andesitic lava, andesitic breccia, and andesitic intrusions in the northern part of the study area.
The geological structures developed in the study area are folds (syncline, anticline), with west–east and northwest–southeast directions, and northwest–southeast-oriented faults. The anticlines in the south and north and the syncline in the middle of the study area plunge to both directions. This syncline is partially covered by the limestone of the Paciran Formation. In the north, there is another syncline thrusting from the east. Meanwhile, in the east and west, there is a northwest–southeast-oriented lateral or strike-slip fault (Luthfi et al. 2017; Novita et al. 2017). Such complex geological structures are apparent in the geological cross-sections, a product of geophysical investigations conducted by the Geological Agency of Indonesia-GAI (Geological Agency of Indonesia, Jakarta, Indonesia, unpublished report, 2017) using resistivity and gravity anomalies (Fig. 1). The cross-sections also show a downthrown block in the eastern lateral fault and a slightly upthrown wall on the western lateral fault. In the middle of the study area, the fault systems cut through the limestone of the Tawun Formation, the quartz sandstone of the Ngayong Formation, and the limestone of the Bulu Formation. These faults are not visible on the surface because they are covered by the limestone of the Paciran Formation that forms Watuputih Hills.
The development of the hydrogeological system in the study area is indicated by the presence of around 39 springs and four cave streams around the limestones of the Paciran Formation (Fig. 1). Based on the results of measurements in the dry season (July–August 2017), the springs’ discharge varies from 0.01 to 1,516 L/s with a geometric average of 0.5 L/s. The springs are generally permanent and, based on lithological conditions, divided into two types, namely springs in karst rocks and springs in nonkarst rocks. The typology of the former refers to the classification of karst springs (Ford and Williams 2007), which includes artesian fault-guided springs and free-draining or contact springs. The artesian fault-guided springs emerge through a fault plane and have eroded impermeable layers in the limbs of the folds. The contact springs are formed on the cliffs of a valley by gravity or due to the presence of an impermeable layer under the karst rocks (Ford and Williams 2007).
Materials and methods
Field sampling
Samples, consisting of waters from 33 springs, four cave waters, and 13 dug wells, were taken in July–August 2017 for hydrochemical and stable isotope (18O and 2H) analysis. Some parameters like temperature, pH, electrical conductivity (EC), HCO3−, and radon (222Rn) were examined in the field. The temperature, EC, and pH were measured with a portable LaMotte water testing kit. Meanwhile, the HCO3− ion and 222Rn concentration were determined using an alkalinity test kit by titration and RAD7, respectively. The stable isotope analysis of rainwater was carried out every month for 1 year (2018) at three locations to determine the local meteoric water line (LMWL) equation.
The sampled waters were filtered using a 0.45-μm syringe filter and then inserted into two 200-ml polyethylene bottles, each for anion and cation analysis. For cation analysis, the water samples were acidified with 0.1 N HNO3 to prevent precipitation. All water samples were preserved at 4 °C for transport to the laboratory. The 222Rn concentrations in the field were measured with RAD7 connected to the RAD-H2O accessory. The water sample was poured into a 250-ml reagent bottle attached to the scintillator. Air was then circulated in a closed circuit for 5–10 min until the 222Rn was mixed homogeneously with the air, and the resulted 222Rn activity was recorded directly. Each sample was subjected to five measurement cycles.
Hydrochemical analysis
Analysis of major ions, namely Ca2+, Na+, Mg2+, K+, HCO3−, SO42−, Cl−, and NO3−, was carried out in the hydrochemical laboratory of the Center for Groundwater and Environmental Geology, the Geological Agency of Indonesia. Ions Ca2+, Na+, Mg2+, and K+ were analyzed using the Dionex ICS-1500 ion chromatography system. As for SO42− and NO3−, these ions were determined with a Varian Cary 100 UV-Vis spectrophotometer. Meanwhile, Cl− and HCO3− were measured with argentometric titration and alkalinity titration, respectively. The laboratory analysis results can be used only if the charge balance error (CBE) is <5% (Yidana et al. 2010, 2011, 2017; Al-Charideh 2011; Wu et al. 2013), which, in this study, was calculated using the following equation (Freeze and Cherry 1979):
The notation Z is ionic valence, mc is molality of cations, and ma is molality of anions.
Stable isotope analysis
The stable isotope analysis was performed in the hydrochemical laboratory of the Center for Groundwater and Environmental Geology, the Geological Agency of Indonesia. The δ 18O and δ2H values were measured relative to Vienna Standard Mean Ocean Water (VSMOW) using the Picarro L-2130-i analyzer. The allowed error in the analysis is ±0.2% for δ18O or ± 1.0% for δ2H. The LMWL equation was determined by considering the amount-weighted factor, i.e., monthly rainfall at each rainwater sampling location, which was calculated using the following equation (Zuppi 1981; Clark 2015):
The notation \( {P}_i^{\mathrm{m}} \) represents monthly rainfall (mm) and \( {\delta}_i^{\mathrm{m}} \) is the oxygen and hydrogen isotope ratios (‰ expressed as δ18O and δ2H).
Calculation of saturation index and partial pressure of CO2
The saturation index for minerals (SI) and partial pressure of CO2 (Pco2) are parameters that determine the characteristics of a karst aquifer system (White 2015). Pco2 serves as the basis for evaluating the level of interaction between water and CO2, which can be calculated using the following equation (Ford and Williams 2007):
The notation (HCO3−) is bicarbonate ions, (H+) is hydrogen ion activity, K1 is equilibrium constants of reactions at 25 °C, and Kco2 is equilibrium constants for CO2 in water at 25 °C.
The mineral saturation index (SI) can determine the degree of chemical equilibrium between water and minerals in aquifers (Domenico and Schwartz 1990; Ford and Williams 1989, 2007; Yuan et al. 2017), which, in this study, was calculated using the equation below:
The notation K is the equilibrium constant of the mineral and IAP is the ion activity product of the mineral. SI > 0 means that water is saturated with minerals, whereas SI < 0 signifies the opposite, i.e., water is not saturated with minerals. When SI = 0, it indicates an equilibrium water–rock interaction. This study calculated the saturation index of calcite (CaCO3; expressed as SIc) and dolomite (CaMg(CO3)2; expressed as SId). The SIc and SId values generally have an error of ±0.1–0.2 (Ford and Williams 1989, 2007). The mineral saturation and Pco2 values were calculated using the program PHREEQC (Parkhust and Appelo 1999).
Hierarchical clustering of hydrochemical data
Hierarchical cluster analysis is a multivariate statistical method that has been widely used in Earth sciences (Davis 1986), one of which is for hydrochemical studies (Swanson et al. 2001; Guler et al. 2002; Guler and Thyne 2004). This method has provided various benefits to explaining hydrological processes in nature (Yidana et al. 2010, 2011; Narany et al. 2014; Chihi et al. 2015; Yuan et al. 2017). This technique is useful for pinpointing similarity and dissimilarity in a group of data based on the Euclidean distance; the smaller the distance, the more similar the data group is (Yidana et al. 2010, 2011). The Euclidean distances are calculated, preferably, using parameters with the highest variant, instead of those with the lowest one (Guler et al. 2002). Parameters with the most similarity are grouped into one cluster and then linked to another cluster based on this similarity; therefore, clusters connected with shorter relationship distances share more similarities to each other than those with longer ones (Yidana et al. 2010, 2011). For this analysis, the required data included temperature, EC, pH, Ca2+, Mg2+, Na+, K+, HCO3−, SO42−, Cl−, Pco2, SIc, and SId. Major cations such as Ca2+, Mg2+, and HCO3− are autochthonous as the results of the dissolution of limestone, whereas the anions Cl, Na, K, SO4 are allochthonous (Valdes et al. 2007). This analysis was performed in SPSS Statistics v20.
Results
Hydrochemical characteristics
For the hydrochemistry and environmental isotopes in the study area, the water samples were divided into three types, namely spring (S), dug well (D), and cave water (C). The analysis results of major ions and groundwater type, according to the Szczukariew-Priklonski’s hydrochemical classification scheme (Jankowski 2001), are summarized in Table 1 and graphically depicted in a Piper diagram in Fig. 2. The order of cation abundance from the highest to the lowest is Ca2+ (average = 102.99 mg/L), Mg2+ (14.50 mg/L), Na+ (10.67 mg/L), and K+ (6.16 mg/L), while the order of the anion abundance is HCO3− (average = 357.75 mg/L), SO42− (26.29 mg/L), and Cl− (16.93 mg/L). The Piper diagram shows that the groundwater samples are dominantly bicarbonate types, and some of them indicate enrichment of Cl (D-26), SO4 (S-43), and both Cl and SO4 ions (S-49 and S-36).
The hierarchical group analysis based on the Euclidean distance is presented in a dendrogram (Fig. 3). For the Euclidean distance of 5, the water samples are divided into four hierarchical groups, namely group one (C1; 25 samples), group two (C2; 19 samples), group three (C3; two samples), and group four (C4; four samples; Table 2). Electrical conductivity (EC) is the most crucial factor that distinguishes one group from another. The EC and major ions composition in group C1 are lower than in C2. Group C4 has lower EC, pH, and compositions of Ca2+, Mg2+, HCO3− than C1− and C2. Since group C3 is characteristically similar to C2, the discussion is focused on three main groups, namely C1, C2, and C4. Based on the hierarchical division, the intensive mineral dissolution is the highest in water samples included in groups C2 and C3, then followed by group C1 and, the lowest, group C4.
Group C1 is mainly scattered in the geological structure zone (faults, folds) and the contact between limestones and two types of rocks, namely calcareous sandstones and quartz sandstones (Fig. 4). The groundwater in group C1 has types Ca-HCO3 (64%) and Ca-Mg-HCO3 (36%), most of which comes from springs that are controlled by the underlying geological structure (artesian fault-guided spring) and cave waters, and some are from contact springs and dug wells. Springs with large discharge are included in this group, for instance, Sumbersemen (S2), Brubulan Tahunan (S1), and Brubulan Pesucen (S10) with instantaneous discharges of 1,516, 165, and 95 L/s., respectively. Group C2 is spread mainly in layered limestones, calcareous sandstones, fault zones, and the places of contact between limestones and quartz sandstones. The groundwater in this group has types Ca-HCO3 (58%) and Ca-Mg-HCO3 (42%), most of which comes from dug wells and contact springs, and some are from springs in the fault zone and one cave water. The groundwater in group C3 has types Ca-Na-HCO3-Cl and Ca-HCO3-SO4, which is found in calcareous sandstones (dug wells) and the sites of contact between limestones and quartz sandstones (contact springs). Group C4 has varied groundwater types, namely Ca-HCO3, Ca-Na-Mg-HCO3-Cl, Ca-Cl-HCO3-SO4, and Ca-K-Na-Cl-SO4. These groundwater types come from nonkarst springs, namely three springs in quartz sandstones and one spring in volcanic rocks.
The study area is primarily composed of carbonate rocks. Accordingly, one of the important hydrochemical processes is chemical weathering, which refers to a chemical reaction between water and the constituent minerals of rocks at low temperatures (Kehew 2001). The Mg2+/Ca2+ ratio reflects the intensity of carbonate rock dissolution. The ratio for dolomite dissolution is approximately 1 (El-Fiky 2010), while 0.5–1 indicates calcite dissolution (Mayo and Loucks 1995; Rajmohan and Elango 2004). The Mg2+ vs. Ca2+ plots show that most of the groundwater samples are below the line Mg2+/Ca2+ = 0.5 or Mg2+/Ca2+ < 0.5 (Fig. 5), indicating the effects of silicate dissolution along with the dominant calcite dissolution (Narany et al. 2014; Katz et al. 1997; Thilakerathne et al. 2015). The Mg2+/Ca2+ratios range from 0.03 to 0.56 (average = 0.20) in group C1, 0.04–0.65 (average = 0.30) in C2, 0.20 and 0.25 in group C3, and 0.10–0.57 (average = 0.29) in group C4. The Mg2+/Ca2+ratios of the water samples in the study area are mainly in the range of 0.1–0.33, and the enrichment of Ca2+ and Mg2+ ions in group C2 implies greater water-carbonate rock interaction.
The interaction between water and carbonate rocks is the primary determinant in the hydrochemistry of karst aquifers. Such interaction is expressed as the saturation index for dolomite (SId) and calcite (SIc; Fig. 6). Most of the water samples in group C1 are saturated with calcite (SIc = −0.16–0.52, average = 0.24) but not with dolomite (SId = −0.7–0.35, average = −0.18). The majority of water samples in group C2 are saturated both with calcite (SIc = 0.02–0.58, average = 0.32) and dolomite (SId = −0.58–0.71, average = 0.16). In group C3, the values of the SIc are −0.03 and 0.51, while the values of the SId are −0.60 and 0.57. In group C4, all water samples are not saturated with either calcite (SIc = −1.80 to −0.95, average = −1.37) or dolomite (SId = −4.43 to −2.46, average = −3.21). With this distribution of index scores, group C2 has relatively more intensive groundwater–carbonate rock interaction (longer residence time) than group C1.
The interaction between CO2 and water is observable by comparing Pco2 with atmospheric conditions, which is around 0.04% (Clark 2015). The Pco2-pH plots show that all water samples are supersaturated with CO2 in atmospheric conditions, meaning that the groundwater has experienced CO2 diffusion in the soil zone (Fig. 7). The Pco2 ranges from 1.07 to 5.37% (average = 2.37%) in group C1, 2.34–4.68% (average = 3.33%) in group C2, 2.24 and 2.40% in group C3, and 1.2–2.45% (average = 1.88%) in group C4. The analysis results revealed that the water samples in group C2 had the highest Pco2 and, therefore, the most intensive CO2-H2O interaction among the groups. A high Pco2 also indicates that the groundwater comes from an aquifer system located close to the epikarst zone (Savoy 2007).
In addition to chemical dissolution, cation exchange is also an important process in hydrochemical evolution (Charfi et al. 2013). The process is demonstrated by the decrease in Ca2+ and Mg2+ levels and the enrichment of Na+ ions (Schoeller 1977; Hem 1992). The cation exchange process in water samples is represented by the decrease in Ca2+/Na+ molar ratio along with the increase in salinity (Cl−; Fig. 8). This pattern was identified in three samples of springs appearing in quartz sandstones (group C4) with very strong correlations (R2 = 0.96). Group C2 has a moderate correlation (R2 = 0.43), while group C1 has a low correlation (R2 = 0.17). Based on the Ca2+/Na+ vs. Cl− graph, the process of cation exchange between Ca2+ and Na+ occurs mainly in group C4 and less intensive in group C2. Cation exchange, i.e., the exchange of Ca2+ and Mg2+ ions by Na+, is a common process in natural groundwater systems. It involves the potential absorption of Ca2+-rich water by the surface of clay minerals and the release of Na+, causing an increase in Na+ concentration in groundwater (Kehew 2001).
Stable isotope characteristics
The results of the stable isotope analysis are summarized in Table 3, while the numerical ranges, averages, standard deviations (SD), and coefficient of variance (CV) based on the type of hydrogeological sampling source (caves, springs and wells) are listed in Table 4. The stable isotope composition of the samples of cave water and artesian fault-guided spring water has low standard deviation and coefficient of variance, while the composition of samples from contact springs, nonkarst springs, and dug wells has low to high standard deviation and a high coefficient of variance. These findings show that the range of the stable isotope composition in artesian fault-guided springs and cave water is relatively more homogeneous than the other water sources.
The stable isotope analysis of the local meteoric water was carried out during 1 year (2018) at three locations, and the results are presented in Table 5. The amount-weighted annual isotopic values (Table 6) were calculated by considering the intensity of the precipitation (I). The analysis of the stable isotope composition in rainwater produced a local meteoric water line (LMWL) equation δ2H = 7.45δ18O + 6.45.
The origin of groundwater can be determined by plotting its δ18O and δ2H values relative to the Global Meteoric Water Line (GMWL) and the LMWL. The equation of GMWL is δ2H = 8δ18O + 10 (Craig 1961). The plots of the δ2H and δ18O values in the water samples and their association with LMWL and GMWL are presented in Fig. 9. These plots show the relative positions of the groundwater samples relative to GMWL and LMWL, which indicates that they come from modern rainwater (Li et al. 2013; Fig. 9). Most groundwater samples from the dug wells and contact springs, as well as some samples from the artesian fault-guided springs, are closer to the LMWL. Deviations from this line represent the effect of evaporation, as seen in some dug wells and one nonkarst spring, e.g., D-21, D-25, D-27, D-32, and S-36. Evaporation can occur during the process of rainwater infiltration into groundwater (Gibson et al. 2008; Li et al. 2013). The majority of the samples from artesian fault-guided springs and cave waters, and some contact springs and dug wells, have a lower isotopic composition with a relative position above the LMWL, signifying the nonexistence of the secondary evaporation process.
The effect of evaporation is also observable from the deuterium excess (d-excess), which can be calculated using the formula d = δD - 8 δ18O (Dansgaard 1964; Gat 1980). The d-excess is useful for inferring any secondary processes that shape the composition of atmospheric vapor in the evaporation-condensation cycle in nature (Craig 1961; Merlivat and Jouzel 1979; Gat et al. 1994; Machavaram and Krishnamurthy 1995). These processes affect the δ18O and δ2H values in groundwater (Dansgaard 1964). If the d-excess is lower than 10, that suggests that this isotopic composition is generally attributable to a secondary evaporation process (Dansgaard 1964). The d-excess values of the water samples of the artesian fault-guided springs and cave waters are >10, whereas in some contact springs, nonkarst springs, and most dug wells, these values are <10 (Table 7).
The high d-excess values in cave waters and artesian fault-guided springs and their relative positions above the LMWL indicate that during the recharge process, the rainwater is relatively less subjected to secondary evaporation. This condition suggests that the relatively deep groundwater flow system has a recharge area with lower temperature and higher relative humidity than the study area, as well as a relatively fast infiltration process (Harvey 2001; Gibson et al. 2008; Li et al. 2013). Values of d-excess in contact springs and nonkarst springs vary, with an average approaching 10. In other words, there is no consistent evidence for the secondary evaporation process, except at some locations. It also represents a relatively fast infiltration process. The average d-excess values of the dug wells are <10, indicating that the water receives supply from local rainwater, and some of the water sources such as D-21, D-25, D-27, and S-36, are affected by a secondary evaporation process during infiltration. However, some dug wells such as D-8, D-18, and D-26, and contact springs have high d-excess values, and the waters thereby originate from a deeper groundwater system. The same case applies to artesian fault-guided springs and cave waters.
Radon characteristics
Monitoring of the 51 hydrogeological features (caves, springs and wells) produced a range of 222Rn concentrations from 441 to 19,300 Bq/m3 with an arithmetic average of 10,322 Bq/m3, a standard deviation (SD) of 5,038.85 Bq/m3, and coefficient of variance (CV) of 48.82. Based on the classification of radon concentration proposed by Przylibski (2005), these water sources fall into the categories of radon-poor water to low-radon water. The spatial distribution and the measurement results of 222Rn concentrations based on the geological characteristics of the hydrogeological features (caves, springs, wells) can be seen in Fig. 10 and Table 8.
The 222Rn concentration in the quartz sandstones of Ngrayong Formation has a relatively high value, i.e., an average 13,563 Bq/m3 (CV = 31.31; Table 8). The high 222Rn concentrations are also distributed in artesian fault-guided springs (average = 11,851 Bq/m3; CV = 41.95), springs in the contact zone of the quartz sandstones of Ngrayong Formation (average = 11,694 Bq/m3; CV = 19.39), and in cave waters (average = 10,315 Bq/m3; CV = 76.10). The 222Rn concentrations in dug wells average at 9367 Bq/m3 (CV = 60.25). In the contact springs appearing in the limestones of Bulu Formation, the 222Rn concentrations vary with an average of 7,521 Bq/m3 (CV = 50.86). One spring emerging from volcanic rocks has a low 222Rn concentration, that is, 1,470 Bq/m3.
The 222Rn concentration ranges in the different types of hydrogeological feature indicate correlation with lithology and geological structure. The high 222Rn levels in springs emerging from the quartz sandstones of Ngrayong Formation and the sites of contact between limestones and quartz sandstones suggest that the quartz sandstone naturally has a relatively high 222Rn concentration. In the springs and dug wells with the lithology of limestone and calcareous sandstones, the levels of 222Rn tend to be high around the faults or folds. This finding is in line with Skeppstrom (2005), Kendall and McDonnell (1998), and Cook et al. (1999), who state that high radon concentrations are caused by rock types and rock permeability factors such as grain size and fracture intensity.
Discussion
Hydrochemical analysis that has been validated with environmental isotope data can provide a clear overview of the characteristics of a complex karst hydrogeological system. This analysis identified three processes controlling the hydrochemical parameters in the study area, namely dissolution of carbonate rocks, dissolution of siliciclastic rocks, and cation exchange. The geological structure also plays an essential role in shaping the groundwater flow system in the study area, as indicated by both stable (18O and 2H) and unstable (222Rn) isotope data.
Spatial variation and hydrochemical processes
The hydrochemical characteristics of the study area show that the main processes in groups C2 and C3 are the dissolution of carbonate rocks, cation exchange, and slight dissolution of siliciclastic rocks, while in group C1, carbonate and siliciclastic rocks dissolution with limited cation exchange dominates. For the nonkarst aquifer system (group C4), the primary processes are the dissolution of siliciclastic rocks and cation exchange. The hydrochemical processes are represented by the Mg2+/Ca2+ and Ca2+/Na+ molar ratios, and the spatial distribution is shown in Fig. 11.
Water samples that reflect the dissolution of not only carbonate rocks but also silicate minerals are indicated by low Mg2+/Ca2+ ratio (Narany et al. 2014; Katz et al. 1997; Thilakerathne et al. 2015). These water samples are mainly located in the west to the northwest of the study area, namely in the sandstone of the Wonocolo Formation, the quartz sandstone of the Ngrayong Formation, and the sites of contact between limestones and the quarts sandstones of the Ngrayong Formation. This condition is plausible because the sandstones of the Wonocolo Formation, which has calcareous properties, and the quartz sandstones of the Ngrayong Formation are both siliciclastic rocks (Luthfi et al. 2017; Novita et al. 2017). The low Mg2+/Ca2+ ratio of springs emerging from the limestones of the Bulu Formation and the limestones of the Ledok Formation in the north and east of the study area reflects the effects of groundwater from the underlying siliciclastic rock formation. For this reason, three artesian fault-guided springs that yield the largest discharge (S-1, S-2, and S-10) and most of the other water sources are included in groups C1 and C4, which have low Mg2+/Ca2+ ratios (< 0.4). The Mg2+/Ca2+ ratio is around 0.5, which represents the dominance of carbonate rock dissolution (Mayo and Loucks 1995; Rajmohan and Elango 2004), particularly in springs appearing in the upper slope of the faulted hills whose lithology is composed of limestones from the Bulu Formation (S-40, S-44, and S-45) and at the bottom of the anticlinal axis at the limestones of the Tawun Formation (S-48, S-51, and S-53). Most of these springs are included in group C2. The water samples at these locations also show Mg2+ enrichment because they are saturated not only with calcite but also dolomite. This condition is probably related to the thickness of the limestone formation through which the groundwater in the faulted hills flows. Meanwhile, in the plunging anticlinal hills, which are the oldest rock in the study area, the influence of groundwater from the quartz sandstones of the Ngrayong Formation is relatively nonexistent. Mg2+ enrichment is also present in water samples in the north area, namely a spring in volcanic rocks (S-16) and two nearby springs that are located close to the limestones of the Bulu Formation (S-55 and S-56). The Mg2+ enrichment in these three springs marks the influence of volcanic rocks.
The cation exchange process that is associated with Na+ enrichment can be identified from the low Ca2+/Na+ ratio (Schoeller 1977; Hem 1992). Low Ca/Na ratio (<5) characterizes springs formed in the quartz sandstones of the Ngrayong Formation (group C4) and most of the dug wells whose lithology is composed of sandstones of the Wonocolo Formation (group C2). The Na+ enrichment in these water sources is the consequence of intensive cation exchange between Ca2+ and Na+ ions in a groundwater flow system involving slow movement through the pores. This condition occurs because the quartz sandstones of the Ngrayong Formation have many claystone intercalations, whereas the sandstone of the Wonocolo Formation has many sandy marl intercalations. A high Ca2+/Na+ ratio (>10) is exhibited in the water sources around the faults and folds (group C1), except for the springs in the southwest part of the study area, on the slopes of the faulted hills (S-44 and S-45). The high Ca2+/Na+ ratio in these water samples indicates that the cation exchange is not intensive because the water flows through fractures (geological structure). Based on the Ca2+/Na+ ratio, the cation exchange process is dominant in water sources with the lithology of the quartz sandstone of the Ngrayong Formation and the sandstone of the Wonocolo Formation.
Based on the information acquired, there are three groundwater flow systems characterized in the study area, namely the groundwater system that flows predominantly through carbonate rocks (groups C2 and C3), through siliciclastic (quartz sandstone) and volcanic rocks (group C4), and through not only carbonate rocks but also the siliciclastic rocks (quartz sandstones) below them (group C1). The high interaction between groundwater and carbonate rocks can also explain why groups C2 and C3 have higher EC, Ca2+, Mg2+, and HCO3− values and are more saturated with respect to calcite and dolomite (SIc and SId) than group C1.
Aquifer system
Referring to the concept of the Chebotarev sequence (Domenico 1972), the dominant HCO3− (anion) classifies the groundwater flow into an “upper zone”. However, based on the δ18O and δ2H values, the groundwater flows can be divided relatively into two groups, namely shallow and deep. The aquifer systems in the study area can be categorized from the spatial variation of the stable isotope compositions using the standard equal interval method and producing a map (Fig. 12). As illustrated in the map, the δ18O and δ2H values of the water in the fault and fold zones are relatively low, representing a deep groundwater flow system. Meanwhile, the water samples of the quartz sandstones of the Ngrayong Formation and the calcareous sandstones of the Wonocolo Formation have relatively medium–to–high isotope contents, indicating a shallow groundwater flow system with a relatively slow flow and, accordingly, show the effects of the evaporation process. The result of evaporation is also manifested in the low values of d-excess, as previously explained. In addition to fault and fold zones, water samples that have low δ18O and δ2H values are mainly situated in the north, which is the highest point of the study area (above 375 masl), including S-16, S-17, S-55, and D-18. This finding shows that the groundwater system in that area is the same as that of the springs that appear in the fault and fold zones; in other words, the groundwater flow is controlled by the hydraulic gradient and geological structures.
The role of geological structure in controlling the groundwater flow system can be seen from the correlation between δ18O and EC, as well as between 222Rn and Cl−, in each hierarchical group of hydrochemistry (Fig. 13). In group C1, there is no correlation between δ18O and EC (R2 = 0.005). In group C2, 15 out of 19 water samples (not D26, D27, S45, and C54) show a tendency of negative correlation (R2 = 0.45); the higher the EC, the lower the δ18O will be. In contrast, a positive correlation between the two parameters is present in group C4 (R2 = 0.96). These findings show that there is almost no relationship between δ18O and the duration of water–rock interaction in group C1, meaning that the geological structures control the groundwater flow system through high permeability zones. In group C2, the longer the groundwater interacts with rocks (the higher the EC value), the lower the δ18O, indicating that the water dominantly flows through pores. The positive correlation between EC and the δ18O in group C4 is possibly attributable to the effects of the evaporation process on the shallow groundwater flow system.
Group C1 also does not show any correlation between 222Rn and Cl− in each hierarchical group of hydrochemical (R2 = 0.035). In group C2, 18 out of 19 groundwater samples (not S-55) show that these two parameters have a negative correlation (R2 = 0.34); in other words, higher Cl− is associated with lower 222Rn concentration. Relationships with stronger values are present in three spring water samples in quartz sandstones of the Ngrayong Formation, i.e., in group C4 (R2 = 0.82). The correlation indicates that the low concentrations of 222Rn in groups C2 and C4 are influenced by evaporation that is associated with the relatively poor groundwater circulation associated with the springs. The significant effect of evaporation, especially in group C4 (water samples S-36 and S-49), is also shown by Cl and SO4 enrichment; these two ions exhibit conservative behavior toward the evaporation process (Sahli et al. 2013). The absence of correlation between 222Rn and Cl− ion in group C1 suggests that the springs have active groundwater circulation because of the evolved geological structure. This condition suggests that 222Rn can provide more explanation regarding the study area when applied as a tracer in fractured systems with active hydraulic character, as performed by Choubey and Ramola (1997), Cook et al. (1999), Hamada (1999), and Skeppstrom (2005).
The spatial variations of stable isotope composition and 222Rn concentration in the different sampling features (caves, springs and wells) can also explain the complexity of the groundwater flow systems in the study area. The spring S-10, which has large discharge (95 L/s) and is located close to the west synclinal axis, has lower δ 18O and δ2H values than the water sources to the east, such as D-30, D-32, D-28, and D- 25. This condition indicates that S-10 is a deep groundwater flow system that is controlled by the syncline, while the hydrogeological sampling features in the east are associated with a shallow groundwater flow system. The artesian spring S-2, with the largest discharge (1,500 L/s) and located in the east synclinal limb, also has low δ 18O and δ2H values. Meanwhile, the artesian spring with a small discharge in the south (S-4) shows a mixing with shallow groundwater because it is located in rice fields. S-10 has a lower 222Rn concentration (4,550 Bq/m3) than the springs S-2 and S-4 (14,400 and 11,600 Bq/m3; Fig. 10). The low concentration of 222Rn in the spring S-10 is possibly attributable to the development of a cavity system in the spring outlet. The high discharge of the spring S-2 is caused by its location (i.e., the synclinal limb and at a lower elevation than the other springs) and the east–west fault system that can be identified from gravity and resistivity investigation (GAI, Geological Agency of Indonesia, Jakarta, Indonesia, unpublished report, 2017).
The δ 18O and δ2H values of the springs at the top of the plunging anticline hills such as S-38, S-47, S-52, and S-53, are relatively low, while those at the bottom of the hills such as S-48 and S-51, are relatively moderate. These conditions indicate that the springs at the top of the anticline hills originate from a deep groundwater system, whereas the ones at the bottom are mixed with shallow groundwater. The springs (S-38, S-47, and S-48) in the anticline hills whose lithology is composed of the limestones of the Tawun Formation (the oldest rock) have lower concentration of 222Rn (4,490; 8,450; and 4,970 Bq/m3) than the springs (S-51 and S-52) that are in contact with the quartz sandstones of the Ngrayong Formation (12,100 and 13,000 Bq/m3). One spring at the peak of the faulted anticline (S-53) has a high radon concentration, that is, 18,700 Bq/m3, due to contact with the quartz sandstone of the Ngrayong Formation (Fig. 10).
The spring S-1, which has a flow discharge of 165 L/s and is located in the normal strike-slip fault in the east, along with several of the hydrogeological sampling features in the west of the fault (e.g., S-5, S-7, and D-8), have low δ 18O and δ2H values, but a high 222Rn concentration is present in S-1 and S-5 (14,100 and 14,400 Bq/m3). All springs in the part of the thrust strike-slip fault in the west (S-37, S-39, S-40, S-45, and S-44) also have low δ 18O and δ2H values with high levels of 222Rn (11,400; 17,500; 13,400; 17,900; and 12,600 Bq/m3, respectively; Fig. 10). In addition to the lower elevation, the spring discharge system in the east strike-slip fault is also more relatively centralized than in the west strike-slip fault. This condition is likely to yield more significant discharge in S-1 compared with the springs emerging from both fault zones even though they have one deep groundwater flow system.
Aside from the springs found around the faults and folds, the lower δ18O and δ2H values are also present in the sampled cave waters. The 222Rn concentrations in four cave waters (C-3, C-34, C-54, and C-57) vary, i.e., 12,800; 441; 19,200; and 8820 Bq/m3, respectively. The low 222Rn concentration in C-34 is probably related to water that is stagnant or retained in the cave. In contrast, the waters are flowing in the caves C-3 and C-54, representing active water circulation. For this reason, the geological structure controls not only the groundwater system in the springs that appear in fault and fold zones but also the deep groundwater flow system connected to the cave waters. From the various descriptions above, the hydrogeological system of the study area can be simplified in a conceptual model, as depicted in Fig. 14. The subsurface conditions are reconstructed based on a geophysical survey using gravity and resistivity methods (GAI, Geological Agency of Indonesia, Jakarta, Indonesia, unpublished report, 2017).
Conclusions
The integration of hierarchical cluster analysis of hydrochemical data and environmental isotopes (18O, 2H, and 222Rn) data has successfully identified a more comprehensive set of hydrogeological system characteristics for the study area. There are generally three groups of groundwater system that can be hydrochemically characterized, namely groundwater that flows predominantly through carbonate rocks (group C2 and C3), through siliciclastic rocks (quartz sandstone) and volcanic rocks (group C4), and through not only carbonate rocks but also the siliciclastic rocks (quartz sandstones) below them (group C1). Springs with large discharge, classified as artesian fault-guided springs, are included in group C1 such as Sumbersemen (S2), Brubulan Tahunan (S1), and Brubulan Pesucen (S10) with instantaneous discharges of 1516, 165, and 95 L/s, respectively. Based on the stable isotopes analysis, d-excess calculation, and the measurement of 222Rn concentrations, the groundwater flow systems in groups C2, C3, and C4 are identified as shallow groundwater flow systems in which the groundwater flows through the pores (relatively slow). Meanwhile, group C1 is a deep groundwater flow system that is controlled by the geological structure (relatively fast flow). The geological structure not only determines the groundwater systems in springs that appear in fault and fold zones but also in cave streams. The shallow groundwater system is indicated by water that originates from local rainwater, some of which is affected by secondary evaporation processes, whereas the deep groundwater system is associated with the groundwater in the hills located in the north of the study area whose elevation is above 375 masl.
Change history
01 May 2020
Fig. 3 in the original article contains an error in labelling. The correct figure is given here.
References
Al-Charideh A (2011) Geochemical and isotopic characterization of groundwater from shallow and deep limestone aquifers system of Aleppo basin (North Syria). Environ Earth Sci 65:1157–1168. https://doi.org/10.1007/s12665-011-1364-6
Al-Gamal SA (2011) An assessment of recharge possibility to North-Western Sahara aquifer system (NWSAS) using environmental isotopes. J Hydrol 398:184–190
Ashjari J, Raeisi E (2006) Lithological control on water chemistry in karst aquifers of the Zagros range, Iran. Cave Karst Sci 33:111
Belkhiri L, Mouni L, Tiri A (2011) Water-rock interaction and geochemistry of groundwater from the Ain Azel aquifer, Algeria. Environmental geochemistry and health 34:1–13. https://doi.org/10.1007/s10653-011-9376-4
Bemmelen RW (1949) The geology of Indonesia. Martinous Nijhoff, Leiden, The Netherlands
Blasch KW, Bryson JR (2007) Distinguishing sources of groundwater recharge by using δ2H and δ18O. Ground Water 45:294–308
Burnett WC, Kim G, Lane-Smith D (2001) A continuous monitor for assessment of 222Rn in the coastal ocean. J Radioanal Nucl Chem 249:167–172
Charfi S, Zouari K, Feki S, Mami E (2013) Study of variation in groundwater quality in a coastal aquifer in North-Eastern Tunisia using multivariate factor analysis. Quat Int 302:199–209
Chihi H, de Marsily G, Belayouni H, Yahyaoui H (2015) Relationship between tectonic structures and hydrogeochemical compartmentalization in aquifers: example of the “Jeffara de Medenine” system, south-east Tunisia. J Hydrol Reg Stud 4:410–430
Choubey VM, Ramola RC (1997) Correlation between geology and radon levels in groundwater, soil and indoor air in Bhilangana Valley, Garhwal Himalaya, India. Environ Geol 32:258–262. https://doi.org/10.1007/s002540050215
Clark I (2015) Groundwater geochemistry and isotopes. CRC, Boca Raton, FL
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. CRC, Boca Raton, FL, 185 pp
Clever HL (1985) Solubility data series, vol 2, krypton, xenon and radon. Pergamon, Oxford
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1702–1703
Cook PG, Love AJ, Dighton JC (1999) Inferring groundwater flow in fractured rock from dissolved radon. Ground Water 37(4):606–610
Coplen T (1993) Use of environmental isotopes. In: Alley WM (ed) Regional groundwater quality. Reinhold, New York, pp 227–254
Criss R, Davisson L, Surbeck H, Winston W (2007) Isotopic methods. In: Goldscheider N, Drew D (eds) Methods in karst hydrogeology. Taylor and Francis, London
Dansgaard W (1964) Stable isotopes in precipitation. Tellus 16:436–468
Davis JC (1986) Statistics and data analysis in geology. Wiley, New York
Dimitriou E, Tsintza P (2015) Hydrogeologic investigations in western Crete by using isotopic analyses and GIS techniques. J Water Resour Prot 07:923–937. https://doi.org/10.4236/jwarp.2015.712076
Domenico PA (1972) Concepts and models in groundwater hydrology. McGraw-Hill, New York
Domenico PA, Schwartz FW (1990) Physical and chemical hydrogeology. Wiley, New York
Doveri M, Menichini M, Cerrina Feroni A (2013) Stable water isotopes as fundamental tool in karst aquifer studies: some results from isotopic applications in the Apuan Alps carbonatic complexes (NW Tuscany). Ital J Eng Geol Environ 1:33–50
El-Fiky A (2010) Hydrogeochemical characteristics and evolution of groundwater at the Ras Sudr-Abu Zenima area, Southwest Sinai, Egypt. J King Abdulaziz Univ Earth Sci 21:79–109. https://doi.org/10.4197/Ear.21-1.4
Falcone RA, Falgiani A, Parisse B, Petitta M, Spizzico M, Tallini M (2008) Chemical and isotopic (18O‰, 2H‰, 13C‰, 222Rn) multi-tracing for groundwater conceptual model of carbonate aquifer (Gran Sasso INFN Underground Laboratory, central Italy). J Hydrol 357:368–388. https://doi.org/10.1016/j.jhydrol.2008.05.016
Ford D, Williams PW (1989) Karst geomorphology and hydrology. Chapman and Hall, London
Ford D, Williams PW (2007) Karst hydrogeology and geomorphology, revised edn. Wiley, Chichester, UK
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall, Upper Saddle River, NJ
Gat JR (1980) The isotopes of hydrogen and oxygen in precipitation. In: Fritz P, Fontes JC (eds) Handbook of environmental isotope geochemistry. Springer, Berlin, pp 21–47
Gat JR, Bowser CJ, Kendall C (1994) The contribution of evaporation from the Great Lakes of North America to the continental atmospheric moisture: detection by means of the stable isotope signature of the evaporated waters. Geophys Res Lett 20:557–560
Gibson J, Briks S, Edwards T (2008) Global prediction of δA and δ2H–δ18O evaporation slopes for lakes and soil water for seasonality. Glob Biogeochem Cycles 22:1–12
Goldscheider N, Andreo B (2007) The geological and geomorphological framework. In: Goldscheider N, Drew D (eds) Methods in karst hydrogeology. Taylor and Francis, London
Goldscheider N, Drew D, Worthington S (2007) Introduction. In: Goldscheider N, Drew D (ed) Methods in karst hydrogeology. Taylor and Francis, London
Guler C, Thyne DG (2004) Hydrologic and geologic factors controlling surface and groundwater chemistry in Indian Wells-Owens Valley area, southeastern California. USA J Hydrol 285:177–198
Guler C, Thyne GD, McCray JE, Turner AK (2002) Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeol J 10:455–474
Hamada (1999) Estimation of groundwater flow rate using the decay of 222Rn in a well. J Environ Radioact 47:1–13
Harvey F (2001) Use of NADP archive samples to determine isotope composition of precipitation: characterizing the meteoric input function for use in ground water studies. Ground Water 39(3):380–390
Haryono E (2001) Values of the karst hills. Paper presented at The National Seminar of Eco-Hydraulics, Gadjah Mada University, Yogyakarta, Indonesia, 28–29 March 2001
Hem JD (1992) Study and interpretation of the chemical characteristics of natural waters. US Geol Surv Water Suppl Pap 1473, pp 269
Iskandar I, Dermawan FA, Sianipar JY, Suryantini NS (2018) Characteristic and mixing mechanisms of thermal fluid at the Tampomas volcano, West Java, using hydrogeochemistry, stable isotope and 222Rn analyses. Geosciences 2018(8):103. https://doi.org/10.3390/geosciences8040103
Jankowski J (2001) Groundwater environment. Short course note, School of Geology, University of New South Wales, Sydney
Katz BG, Coplen TB, Bullen TD, Davis JH (1997) Use of chemical and isotopic tracers to characterize the interactions between ground water and surface water in mantled karst. Ground Water 35:1014–1028. https://doi.org/10.1111/j.1745-6584.1997.tb00174.x
Kehew AE (2001) Applied chemical hydrogeology. Prentice Hall, Upper Saddle River, NJ
Kendall C, McDonnell JJ (1998) Isotopes tracers in catchment hydrology. Elsevier, Amsterdam
Krishnaraj S, Murugesan VKV, Sabarathinam C, Paluchamy A, Ramachandran M (2012) Use of hydrochemistry and stable isotopes as tools for groundwater evolution and contamination investigations. J Geosci 1:16–25. https://doi.org/10.5923/j.geo.20110101.02
Leibundgut C, Maloszewski P, Külls C (2009) Tracers in hydrology. Wiley-Blackwell, Hoboken, NJ
Li J, Liu J, Pang Z, Wang X (2013) Characteristics of chemistry and stable isotopes in groundwater of the Chaobai River catchment, Beijing. Procedia Earth Planet Sci 7:487–490. https://doi.org/10.1016/j.proeps.2013.03.092
Luthfi M, Kudsji DK, Maryanto S, Supriyono (2017) Geological map of the Jatirogo quadrangle, scale of 1:50000. Center for Geological Survey, Geological Agency of Indonesia, Bandung, Indonesia
Machavaram MV, Krishnamurthy RV (1995) Earth surface evaporative process: a case study from the Great Lakes region of the United States based on deuterium excess in precipitation. Geochim Cosmochim Acta 59:4279–4283
Mahlknecht J, Garfias-Solis J, Aravena R, Tesch R (2006) Geochemical and isotopic investigations on groundwater residence time and flow in the Independence Basin, Mexico. J Hydrol 324:283–300
Marfia AM, Krishnamurthy RV, Atekwana EA, Panton WF (2004) Isotopic and geochemical evolution of groundwater and surface waters in a karst-dominated geological setting: a case study from Belize, Central America. Appl Geochem 19:937–946. https://doi.org/10.1016/j.apgeochem.2003.10.013
Mayo AL, Loucks MD (1995) Solute and isotopic geochemistry and ground water flow in the Central Wasatch Range, Utah. J Hydrol 172:31–59. https://doi.org/10.1016/0022-1694(95)02748-e
Merlivat L, Jouzel J (1979) Global climatic interpretation of deuterium-oxygen relationship for precipitation. J Geophys Res 84:5029–5033
Michel J (1990) Relationship of radium and radon with geological formations. In: Cothern CR, Rebers PA (eds) Radon, radium, and uranium in drinking water. Lewis, Chelsea, MI, 286 pp
Milanovic PT (1981) Karst hydrogeology. Water Resources Publ., Littleton, CO
Mukherjee A, Fryar AE, Rowe HD (2007) Regional-scale stable isotopic signatures of recharge and deep groundwater in the arsenic affected areas of West Bengal, India. J Hydrol 334:151–161
Murillo RS, Brooks E, Elliot JW, Bolla J (2015) Isotope hydrology and baseflow geochemistry in natural and human-altered watersheds in the inland Pacific Northwest, USA. Isot Environ Health Stud 51:231–254. https://doi.org/10.1080/10256016.2015.1008468
Narany ST, Ramli MF, Aris AZ, Sulaiman WNA, Juahir H, Fakharian K (2014) Identification of the hydrogeochemical processes in groundwater using classic integrated geochemical methods and geostatistical techniques in Amol-Babol plain, Iran. Sci World J 2014:1–15. https://doi.org/10.1155/2014/419058
Novita D, Margono U, Sanjaya I, Rijani S (2017) Geological map of the Blora quadrangle, scale of 1:50000. Center for Geological Survey, Geological Agency of Indonesia, Bandung, Indonesia
Parkhust DL, Appelo CAJ (1999) User’s guide to Phreeqc (version 2) – a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US Geological Survey, Reston, VA
Petitta M, Primavera P, Tuccimei P, Aravena R (2011) Interaction between deep and shallow groundwater systems in areas affected by Quaternary tectonics (central Italy): a geochemical and isotope approach. Environ Earth Sci 63:11–30. https://doi.org/10.1007/s12665-010-0663-7
Pu T, He Y, Zhang T, Wu J, Zhu G, Chang L (2013) Isotopic and geochemical evolution of ground and river waters in a karst dominated geological setting: a case study from Lijiang basin, South-Asia monsoon region. Appl Geochem 33:199–212
Przylibski TA (2005) Radon, specific component of medicinal waters in the Sudety Mountains. Oficyna Wydawnicza Politechniki Wrocławskiej, Wrocław, Poland
Rademacher LK, Clark JF, Boles JR (2003) Groundwater residence times and flow paths in fractured rock determined using environmental tracers in the Mission tunnel: Santa Barbara County, California, USA. Environ Geol 43:557–567
Rajmohan N, Elango L (2004) Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River basins, South India. Environ Geol 46(1):47–61
Rodgers P, Soulsby C, Waldron S, Tetzlaff D (2005) Using stable isotope tracers to assess hydrological flow paths, residence times and landscape influences in a nested mesoscale catchment. Hydrol Earth Syst Sci 9:139–155
Ryu JS, Lee KS, Chang HW (2007) Hydrogeochemical and isotopic investigations of the Han River basin, South Korea. J Hydrol 345:50–60
Sahli H, Tagorti MA, Tlig S (2013) Groundwater hydrochemistry and mass transfer in a stratified aquifer system (Jefara–Gabes Basin, Tunisia). Larhyss J 12:95–108
Savoy L (2007) Storage, transport and biodegradation of solute contaminants in the unsaturated zone of karst systems. PhD Thesis, University of Neuchâtel, Switzerland
Schoeller H (1977) Geochemistry of groundwaters. In: Ground-water studies: an international guide for research and practice. UNESCO, Paris, pp 1–18
Singh M, Kumar S, Kumar B, Singh S, Singh IB (2013) Investigation on the hydrodynamics of ganga alluvial plain using environmental isotopes: a case study of the Gomati River basin, northern India. Hydrogeol J 21:687–700
Skeppstrom K (2005) Radon in groundwater- influencing factors and prediction methodology for a Swedish environment. Licentiate Thesis, Universitetsservice US AB, Stockholm
Sun Z, Ma R, Wang Y, Ma T, Liu Y (2016) Using isotopic, hydrogeochemical-tracer and temperature data to characterize recharge and flow paths in a complex karst groundwater flow system in northern China. Hydrogeol J 24:1393–1412. https://doi.org/10.1007/s10040-016-1390-2
Swanson SK, Bahr JM, Schwar MT, Potter KW (2001) Two-way cluster analysis of geochemical data to constrain spring source waters. Chem Geol 179:73–91
Thilakerathne A, Schüth C, Chandrajith R (2015) The impact of hydrogeological settings on geochemical evolution of groundwater in karstified limestone aquifer basin in Northwest Sri Lanka. Environ Earth Sci 73:8061–8073. https://doi.org/10.1007/s12665-014-3962-6
Tillman FD, Oki DS, Johnson AG, Barber LB, Beisner KR (2014) Investigation of geochemical indicators to evaluate the connection between inland and coastal groundwater systems near Kaloko-Honoko-hau National Historical Park, Hawai‘i. Appl Geochem 51:278–292. https://doi.org/10.1016/j.apgeochem.2014.10.003
Valdes D, Dupont JP, Laignel B, Ogier S, Leboulanger T, Mahler BJ (2007) A spatial analysis of structural controls on karst groundwater geochemistry at a regional scale. J Hydrol 340:244–255. https://doi.org/10.1016/j.jhydrol.2007.04.014
Villalobos MR, Muños AC, Álvarez AP, Collado GM (2017) Hydrochemistry and 222Rn concentrations in spring waters in the arid zone El Granero, Chihuahua, Mexico. Geosciences 7:12
White WB (2015) Chemistry and karst. Acta Carsol 44:349
Wu J, Li P, Qian H, Duan Z, Zhang X (2013) Using correlation and multivariate statistical analysis to identify hydrogeochemical processes affecting the major ion chemistry of waters: a case study in Laoheba phosphorite mine in Sichuan, China. Arab J Geosci. https://doi.org/10.1007/s12517-013-1057-4
Yidana SM, Banoeng-Yakubo B, Akabzaa TM (2010) Analysis of groundwater quality using multivariate and spatial analyses in the Keta basin, Ghana. J Afr Earth Sci 58:220–234. https://doi.org/10.1016/j.jafrearsci.2010.03.003
Yidana SM, Banoeng-Yakubo B, Akabzaa TM, Asiedu D (2011) Characterization of the groundwater flow regime and hydrochemistry of groundwater from the Buem formation, eastern Ghana. Hydrol Process 25:2288–2301. https://doi.org/10.1002/hyp.7992
Yuan J, Xu F, Deng G, Tang Y, Li P (2017) Hydrogeochemistry of shallow groundwater in a karst aquifer system of Bijie City, Guizhou Province. Water 9:625. https://doi.org/10.3390/w9080625
Zuppi GM (1981) Statistical treatment of environmental isotope data in precipitation. Technical Reports Series, vol 206, IAEA, Vienna
Acknowledgements
Authors would like to express their deepest gratitude to the Head of the Center for Groundwater and Environmental Geology and the Head of the Geological Agency the Ministry of Energy and Mineral Resources of Indonesia for their data and laboratory facilities assistance during the research activity. Authors would also like to thank the Head of the Office of Energy and Mineral Resources Assessment and Monitoring in Kendeng Selatan for facilitating the field activities, the academic community of the Faculty of Geological Engineering UNPAD for their invaluable critiques during the writing of this report, and Ahmad Taufiq, Ph.D. for the additional discussion.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised: Figure 3 in the original article contains an error in labelling.
Rights and permissions
About this article
Cite this article
Setiawan, T., Syah Alam, B.Y.C.S.S., Haryono, E. et al. Hydrochemical and environmental isotopes analysis for characterizing a complex karst hydrogeological system of Watuputih area, Rembang, Central Java, Indonesia. Hydrogeol J 28, 1635–1659 (2020). https://doi.org/10.1007/s10040-020-02128-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10040-020-02128-8