Abstract
Elevated fluoride (F) concentration in groundwater is posing a public health risk in the Manas River Basin (MRB), Northwest China. Based on the characterization of regional groundwater flow, 90 groundwater samples from aquifers were analyzed, along with top-soil leachate and pore-water samples from aquitards. Stable oxygen (δ18O) and hydrogen isotopes, radiocarbon and hydrochemical analyses of the groundwater and pore-water samples were conducted to trace groundwater hydrological and hydrochemical processes and thereby understand the distribution and migration mechanism of F. The groundwater is recharged by meteoric precipitation through vapor condensation processes in the Tianshan Mountains. The F concentration in groundwater samples from this basin ranged from 0.11 to 48.15 mg/L (mean 2.56 mg/L). In 37 of the 90 groundwater samples, the F concentrations were above the safe level for drinking water. The F concentrations progressively increased with the residence time and well depths in the northwest of the alluvial-fluvial plain, where groundwater is overexploited for agricultural and domestic use. Positive correlations between F and sodium (Na)/calcium (Ca) indicate that the enrichment and migration of F are influenced by cation exchange processes under high-Na and alkaline pH conditions. The relationships between δ18O and F and chloride (Cl) concentrations were nonlinear due to leaching and mixing processes. This shows that vertical leaching by irrigation return flow and mixing with pore water are the dominant processes driving the migration of F in the groundwater flow system of MRB, in addition to geochemical processes.
Résumé
Une concentration élevée en fluorures (F) dans l’eau souterraine constitue un risque de santé publique dans le Bassin de la Rivière Manas (BRM), en Chine du Nord-Ouest. Sur la base d’une caractérisation de l’écoulement régional des eaux souterraines, 90 échantillons d’eau souterraine provenant des aquifères ont été analysés, en même temps que le lixiviat de la couche arable et des échantillons d’eau interstitielle provenant des aquitards. Des analyses des isotopes de l’oxygène stable (δ18O) et de l’hydrogène, du radiocarbone et hydrochimiques ont été réalisées sur l’eau souterraine et des échantillons d’eau interstitielle, pour tracer les processus hydrologiques et hydrochimiques de l’eau souterraine et ainsi comprendre le mécanisme de la distribution et de la migration des fluorures (F). L’eau souterraine est rechargée par les précipitations météoriques grâce aux processus de condensation de la vapeur d’eau dans les Monts Tianshan. La concentration des fluorures (F) dans les échantillons d’eau souterraine provenant de ce bassin est comprise entre 0.11 et 48.15 mg/L (moyenne 2.56 mg/L). Dans 37 des 90 échantillons d’eau souterraine, les concentrations en fluorures (F) étaient au-dessus du seuil de sécurité pour une eau potable. Les concentrations en fluorures (F) augmentaient progressivement avec le temps de résidence et la profondeur des forages dans le Nord-Ouest de la plaine fluvio-alluviale, où l’eau souterraine est surexploitée du fait de son utilisation agricole et domestique. Des corrélations positives entre les fluorures (F) et sodium (Na)/ calcium (Ca) indiquent que l’enrichissement et la migration des fluorures (F) sont influencés par les processus d’échanges de cations sous des conditions de Na élevé et de pH alcalin. Les relations entre δ18O et les concentrations en fluorures (F) et en chlorures (Cl) sont non linéaires en raison des processus d’infiltration et de mélange. Cela montre que l’infiltration verticale générée par l’écoulement en retour d’irrigation et le mélange avec l’eau interstitielle sont les processus dominants qui contrôlent la migration des fluorures (F) dans le système d’écoulement souterrain BRM en sus des processus géochimiques.
Resumen
La concentración elevada de fluoruro (F) en el agua subterránea representa un riesgo para la salud pública en la cuenca del río Manas (MRB), en el noroeste de China. Con base en la caracterización del flujo regional del agua subterránea, se analizaron 90 muestras de los acuíferos, junto con muestras de lixiviación del suelo y del agua poral de los acuitardos. Se llevaron a cabo análisis de isótopos estables, oxígeno (δ18O) e hidrógeno, radiocarbono e hidroquímicos de muestras del agua subterránea y del agua intersticial para el seguimiento de los procesos hidrológicos e hidroquímicos del agua subterránea y comprender así el mecanismo de distribución y migración del F. El agua subterránea se recarga por precipitación meteórica a través de procesos de condensación de vapor en las montañas Tianshan. La concentración de F en muestras de agua subterránea de esta cuenca varió de 0.11 to 48.15 mg/L (media 2.56 mg/ L). En 37 de las 90 muestras de agua subterránea, las concentraciones de F estaban por encima del nivel recomendado para el agua potable. Las concentraciones de F aumentaron progresivamente con el tiempo de residencia y las profundidades del pozo en el noroeste de la planicie aluvial-fluvial, donde el agua subterránea está sobreexplotada para uso agrícola y doméstico. Las correlaciones positivas entre F y sodio (Na)/calcio (Ca) indican que el enriquecimiento y la migración del F están influenciados por los procesos de intercambio catiónico bajo condiciones de alto Na y pH alcalino. Las relaciones entre δ18O y F y las concentraciones de cloruro (Cl) no fueron lineales debido a procesos de lixiviación y mezcla. Esto muestra que la lixiviación vertical mediante el flujo de retorno de riego y la mezcla con agua intersticial son los procesos dominantes que impulsan la migración de F en el sistema de flujo de agua subterránea de MRB, además de los procesos geoquímicos.
摘要
高氟地下水在玛纳斯河流域地区广泛分布,已严重威胁公众的用水安全。在区域水流系统分析的基础上,获取了研究区内90个含水层地下水样品,4组表层土壤淋滤液以及8组弱透水层粘性土孔隙水样品,测试其水化学成分、氘氧同位素以及部分样品14C同位素;分析水文地球化学与水动力条件,探讨研究区地下水中氟离子的运移机制。结果表明研究区地下水主要来源于天山山区的降水与冰川融水。研究区地下水中氟离子含量为0.11- 48.15 mg/L,其中1/3以上的地下水样品中氟离子浓度高于饮用水标准。高氟地下水主要分布于研究区西北部,此地区因大面积农田灌溉需要,地下水严重超采。地下水氟离子浓度随着滞留时间增长和井深加大而增高,说明地下水在径流过程中因含氟矿物溶解提高了氟离子浓度。氟与Na/Ca的正相关关系显示在高Na和弱碱性条件下,阳离子交换作用会影响氟的富集与迁移转化。因淋滤和混合作用,地下水中δ18O与F和Cl离子之间的关系是非线性的。通过三端元线性混合模型计算发现,除地球化学过程外,弱透水层孔隙水与灌溉回归水的垂向入渗补给是影响玛纳斯河流域地下水流系统氟离子迁移转化的重要过程。
Resumo
Concentrações elevadas de fluoreto (F) nas águas subterrâneas representam um risco para a saúde pública na Bacia do Rio Manas (BRM), noroeste da China. Baseado na caracterização regional do fluxo de águas subterrâneas, 90 amostras de águas subterrâneas dos aquíferos foram analisadas, juntamente com amostras de chorume e de água de poros no solo superior do aquitardo. Isótopos estáveis de hidrogênio e oxigênio (δ18O), radiocarbono e análises hidroquímicas das águas subterrâneas e amostras dos poros foram realizadas para rastrear os processos hidrológicos e hidroquímicos das águas e assim, compreender os mecanismos de distribuição e migração do F. A água subterrânea é recarregada pela precipitação meteórica, através de processos de condensação de vapor nas Montanhas Tianshan. A concentração de F nas amostras subterrâneas dessa bacia variou de 0.11 a 48.15 mg/L (média de 2.56 mg/L). Em 37 das 90 amostras de água subterrâneas, as concentrações de F estavam acima do nível permitido para água potável. As concentrações de F aumentaram progressivamente com o tempo de residência e as profundidades dos poços no noroeste da planície aluvial-fluvial, onde a água subterrânea é superexplotada para uso agrícola e doméstico. Uma correlação positiva entre F e sódio(Na)/cálcio(Ca) indicam enriquecimento e migração de F, que são influenciados por processos de troca de cátions sob condições de pH alto e alcalino. A correlação entre as concentrações de δ18O, F e cloreto (Cl) são não-lineares, devido ao processo de lixiviação e mistura. Isso mostra que a lixiviação vertical pelo retorno do fluxo de irrigação e mistura das águas dos poros são os processos dominantes que impulsionam a migração de F no sistema de fluxo subterrâneo da BRM, além dos processos geoquímicos.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Elevated fluoride (F) concentrations (>1.5 mg/L) have been observed in groundwater across the globe (Ali et al. 2016; Vithanage and Bhattacharya 2015), especially in arid and semiarid areas such as Mexico (Irigoyen et al. 1995), African countries (Tekle-Haimanot et al. 2006), Pakistan (Rafique et al. 2009), India (Handa 1975; Jacks et al. 2005; Meenakshi et al. 2004; Vikas et al. 2013), and Northwest China (Amini et al. 2008; Currell et al. 2011; Guo et al. 2007, 2012; He et al. 2013; Li et al. 2015). Groundwater resources are critical for meeting domestic, industrial and agricultural water demands in such areas (Rockström et al. 2009; Williams 1999). Soluble F is commonly present as a minor element in groundwater, soils and crops. The resultant F in food and drinking water can be easily adsorbed by humans and affects physical health (Amini et al. 2008; Ayoob and Gupta 2006; Irigoyen et al. 1995). Excessive intake of F in concentrations higher than the allowable limit set by the World Health Organization (WHO; 1.5 mg/L) causes various types of fluorosis depending on the concentration and exposure time, the most commonly observed are dental and skeletal fluorosis (WHO 2004; Rango et al. 2014).
In Northwest China, more than 60 million people live in areas where the risk of fluorosis is high. More than 1 million people have been diagnosed with dental and skeletal fluorosis in Xinjiang province (Ministry of Health of China 2015). Elevated F concentrations in groundwater, used as drinking and irrigation water sources, are responsible for this public health problem (Li et al. 2015; Rango et al. 2014). Groundwater in the Manas River Basin (MRB), one of the most productive areas for cash crops, has elevated F concentrations (Chen et al. 2009; Luan et al. 2016). Groundwater is a major water source for domestic and agricultural use in this area, resulting in high F exposure to humans. Luan et al. (2016) investigated groundwater fluoride concentrations in Shihezi City; however, the spatial distribution and migration mechanisms of F in the groundwater in the MRB have not been systematically reported so far. Soluble F ions in groundwater can be formed due to geological factors (Gaciri and Davies 1993; Jacks et al. 2005; Nordstrom and Jenne 1977) and anthropogenic activities (Chae et al. 2007; Rafique et al. 2009). The weathering of fluorine-bearing minerals in sediments can release F into the pore water in the aquitards and the groundwater in the aquifers (Currell et al. 2011; Kwong et al. 2015); therefore, longer water residence time in sediments is believed to help increase F concentration owing to efficient water–rock interactions. The residence time of the Quaternary groundwater in this basin has been reported to range from modern to more than 10,000 years (Liu et al. 2006; Tian et al. 2010). The quality of the groundwater is also degraded due to the irrigation return flow and enrichment by soil contaminants when there is a strong evapotranspiration effect on the soils and waters in such arid and semiarid basins (Chen et al. 2009; Negrel et al. 2011; Perrin et al. 2011). Irrigation, combined with the increasing use of phosphate fertilizer and fluorine pesticides, can increase F concentrations in waters and soils (Kim et al. 2011; Rao 2003). The variations in the hydrogeochemical characteristics of groundwater such as the calcium (Ca) and bicarbonate (HCO3) concentrations and pH conditions, also affect the F distribution and migration, through the precipitation and dissolution of F-bearing minerals (Jacks et al. 2005; Reddy et al. 2010). Therefore, local hydrological and hydrogeochemical conditions are important factors to control F concentration, distribution and migration in groundwater (He et al. 2013; Li et al. 2015).
The main objectives of this study are to: (1) investigate F abundance and spatial distribution in the groundwater of the MRB and (2) elucidate the hydrological and hydrogeochemical processes responsible for the enrichment and migration mechanisms of F in the groundwater. This study not only improves understanding of the hydrological and hydrogeochemical processes that control F concentration in groundwater but also formulates strategies for irrigation regimes and the sustainable utilization of groundwater resources to reduce the risk of F exposure.
Hydrogeological background
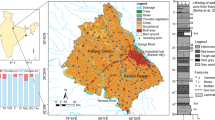
The MRB is an arid inland basin located in the south of the Junggar Basin in Xinjiang province in Northwest China. It covers an area of 17 × 103 km2 and has four major oases: Shihezi, Mosuowan, Jin’an and Xiayedi (Fig. 1). The Manas River, the largest inland river in this basin, originates by precipitation and melting of glaciers in the Tianshan Mountains. It flows through the basin area, supplies irrigation water, and reaches the terminal Manas Lake with an average flux of 1.27 × 109 m3/year. The influences of the westerly winds and Mongolian anticyclone result in an arid climate with hot summers and freezing winters (Bothe et al. 2012). Mean annual precipitation is 200 mm and the mean annual potential evapotranspiration rate is 1,500 mm.
The Quaternary sediments in the MRB comprise interstratified gravels, clay, and sand, and constitute a multiple aquifer/aquitard system for storing groundwater. Groundwater generally flows from the southern mountains to the northern Gurbantunggut Desert and finally into the terminal Manas Lake. However, anthropogenic activities, such as groundwater exploitation and interception by reservoirs for retaining surface water, have changed the Quaternary groundwater flow paths in the past 60 years (Chen et al. 2009); in particular, groundwater exploitation supplied 50% of the total water use in 2010. The regional groundwater levels declined 10–60 m from 1980 to 2003 and have been continuously declining since then, due to over-pumping; thus, groundwater depression cones have developed and are distributed at the margins of the desert area. As a result, the hydraulic gradients between the shallow and deep aquifers are inversed with respect to those natural conditions in some areas, where groundwater leaks from the upper shallow aquifers to deep aquifers and the inter-aquitards are consolidated to release pore water to adjacent aquifers (Huang et al. 2012; Zhou et al. 2013).
The main recharge sources for groundwater are the vertical infiltration from rivers, lateral inflow of groundwater at the mountain front, irrigation return flow, and channel leakage on the plain. The media characteristics, hydraulic gradients and the residence times shown in Fig. 2 demonstrate a decreasing groundwater velocity along the groundwater flow path. Low rainfall in this basin can hardly infiltrate into the shallow aquifers because of the intense evapotranspiration. Withdrawal of groundwater and evapotranspiration are the principle means of discharge in the inland basin (Chen et al. 2009).
Materials and methods
A total of 90 groundwater samples from MRB water supply wells in 2015 and 2016 (Fig. 1c) were collected. This included 32 groundwater samples that were collected and reported by Ma et al. (2017), while the remaining were sampled as part of this study. Most well depths range from 10 to 450 m. From these wells, 26 samples were collected from shallow wells (depth less than 50 m), 32 samples from middle-depth wells (50–150 m) and 32 samples from deep wells (150–450 m). During the sampling periods, no significant precipitation occurred. Each well was purged for more than 10 min before sampling. Electrical conductivity (EC), pH and temperature (T) were recorded using Hach instruments (Hach 40d, USA); CO3 and HCO3 concentrations were determined by the titration method. All collected water samples were filtered through 0.45-μm membranes and then stored in 50-ml polyethylene bottles; furthermore, samples for cation measurements were acidized with ultra-purified HNO3 to pH < 2 and those for anion analysis were stored directly. All samples for hydrogen and oxygen isotopes measurements were collected in polyethylene bottles and sealed with Para-film to avoid fractionations by evaporation and isotope exchange with air.
Soil samples at four sites were collected at 50-cm intervals along the profile (shown in Fig. 1c), and each sample was stored in a zip-lock bag. All soil samples were dried, grinded and sieved through a 1-mm screen for further analyses in the laboratory at room temperature. The soluble ions in soil samples were leached by deionized water (soil:water = 1:5 by weight ratio) after shaking and centrifuge, and subsequently filtered through 0.45-μm membranes.
A borehole (ZK-01) with a depth of 103 m was drilled without fluids in August 2016 in the Xiayedi Oasis (for location see Fig. 1c). Sediment samples for particle-size analysis were collected at 0.5–2-m intervals along the borehole depth, and tested by a laser diffraction particle size analyzer (LS I3 320, Beckman Coulter, California, USA). Eight undisturbed core samples were collected from the aquitards at 10–20-m depth intervals, packed in polyethylene film and stored in a cooler box. Pore water was then extracted from the core sample by squeezing in the laboratory (Li et al. 2013; Kwong and Jiao 2016).
For all groundwater, leachates of soils and pore-water samples, cations (all acidized) were measured using inductively coupled plasma optical emission spectrometry (IRIS Intrepid II XSP, Thermo Elemental, MA and USA), and anions were analyzed by ion chromatography (Metrohm 761 Compact IC, Switzerland). Hydrogen and oxygen isotopes were measured by a Finnigan MAT-253 mass spectrometer (Thermo Fisher, USA, manufactured in Bremen, Germany) through a temperature conversion elemental analysis (TC/EA) method and normalized to the Vienna Standard Mean Ocean Water (VSMOW) standards. Instrument precisions for δD and δ18O were ± 0.5 and ± 0.1‰, respectively. 14C contents of some groundwater samples had been measured through an ultra-low level liquid scintillation spectrometer (1220 Quantulus, PerkinElmer, USA). The hydrogeochemistry and isotope measurements were all performed at China University of Geosciences, Wuhan.
Results
Hydrogeochemistry in the Manas River Basin
Groundwater with total dissolved solids (TDS) >1,000 mg/L is called saline groundwater and is widely distributed in the MRB (13 out of 26 shallow groundwater samples, 13 out of 32 middle ones, and 6 out of 32 deep ones are classified as saline). A trend of increasing TDS concentration for the deep groundwater has been observed in the past few decades (Chen et al. 2009).
Groundwater pH is usually near neutral and slightly alkaline (7.3–11.2) in the MRB. Chloride (Cl) and sulfate (SO4), and sodium (Na), are the predominant anions and cation, respectively, in the saline groundwater (TDS >1 g/L; Fig. 3). HCO3 and Ca are the predominant anion and cation, respectively, in most MRB fresh groundwater samples. The HCO3 concentrations of groundwater samples are between 54.92 and 900.00 mg/L, Cl concentrations between 8.07 and 3,773.97 mg/L, Na concentrations between 10.6 and 4,185.95 mg/L, and Ca concentrations between 1.91 and 519.82 mg/L. The regression line of the Cl/Na ratios is approximately 0.5 (Fig. 4a, 0.52, R2 = 0.86), implying that the dissolution of halite contributes to the formation of the Cl anions, in addition to the weak evapotranspiration of groundwater and mixing processes. The positive relationship between Na and SO4 indicates that SO4 may be balanced by Na from the dissolution of sodium sulfate (Fig. 4b). The groundwaters are generally Na•Ca-HCO3, Na-SO4 and Na-Cl types in the MRB, from the recharge area to discharge area.
Groundwater hydrogen (δD) and oxygen (δ18O) isotope values range from −27.21 to −114.94‰ and −4.24 to −12.72‰, respectively. The δ18O vs. δD diagram in Fig. 5 shows that most groundwater samples locate along the local meteoric water line (LMWL) of Urumqi (about 150 km east of the study area, data from the International Atomic Energy Agency/World Meteorological Organization 2002). The groundwater regression line (GWL) slope is 6.68 (R2 = 0.85; Fig. 5), lower than the slope of the LMWL (7.23). Most samples are distributed to the left of the global meteoric water line (GMWL). This shows that the groundwater is mainly formed by meteoric precipitation through condensation of the evaporated water in the mountains and slightly affected by the evaporation around the MRB (Pang et al. 2017).
Hydrogeochemical and isotopic characteristics of the groundwater in the shallow, middle, and deep wells are presented in Table 1. The temperature (T) of groundwater samples increases from the shallow to the deep wells, whereas the mean and median salinity, HCO3, Ca, Mg, δD and δ18O show decreasing trends with well depth. Ca concentrations are low in the deep groundwater with high F concentrations. Furthermore, 39.4% of all samples, especially those from the shallow wells, have elevated nitrate (NO3) concentrations (>10 mg/L, WHO standard). The mean NO3 values also show a decreasing trend with increasing well depth. The Pearson’s correlation matrix (Table 2) shows that Na and Na/Ca are significantly correlated with the F concentrations (p < 0.05), then following with Ca and depth. Percent modern carbon (pMC) values of 14C in some groundwater samples vary from 23.5 to 86.9% (Table 3), with corresponding apparent ages increasing from 1.16 to 11.96 ka before present (BP). The adjacent samples M127 and M132, with nearly the same depths (260 and 280 m), show different pMC values, 53.2 and 86.9%, respectively. The relative high pMC of M132 could be attributed to mixing with younger recharge water, as it is closer to Manas River.
Distribution of elevated fluoride concentrations in groundwater
In this basin area, F concentrations in groundwater range from 0.11 to 48.15 mg/L (mean of 2.05 mg/L, excluding the highest value 48.15 mg/L), of which 41.1% are higher than the WHO standard for drinking water (1.5 mg/L) and 51.1% higher than the Chinese standard (1 mg/L). The F concentrations increase systematically from the south piedmont to the northwest of the alluvial-diluvial plain. Groundwater F concentrations are less than 1 mg/L for most wells in the Shihezi Oasis, the piedmont along the Manas River (Fig. 1c). Groundwater flow velocity and renewal rate calculated based on the 3H concentrations—determined using the methodology proposed by Salle et al. (2001)—decrease along the groundwater flow path depicted in Fig. 2 (Tian et al. 2010). Therefore, F concentrations increase due to the water–rock interactions over a prolonged residence time, potentially exceeding the safe level for drinking in the alluvial-fluvial plains, where the topography is generally flat and the deposits of fine sand and silty clay have low permeability. High F concentrations are predominantly found in groundwater along the marginal area between the alluvial-fluvial plain and the desert land. In summary, according to the contour maps of F concentration (Fig. 6), the shallow groundwater regions with elevated F concentrations (>1.5 mg/L) are distributed in the northern and western parts (the Xiayedi and Jin’an oases) and northeastern part (the Mosuowan Oasis) of the MRB. As to the middle and deep groundwater, the F concentrations in the northwestern part are slightly elevated, and higher than those in the southern and eastern parts (i.e., the Shihezi and Mosuowan oases). On the basin scale, the distribution of elevated F in the shallow groundwater are consistently associated with the arable land, where channel and river water are scarce and groundwater is highly exploited for irrigation (Fig. 1c).
Contour maps of fluoride concentrations in groundwater in the study area interpolated using the Kriging method: a Shallow (0–3.4 mg/L), b Middle (0–10.5 mg/L), and c Deep (0–11.5 mg/L) groundwater. Most elevated-F groundwater samples are distributed in the mid-west and north part of Manas River Basin
Vertically, groundwater F concentrations >1.5 mg/L are distributed in the shallow, middle, and deep wells. Figure 7 shows that the F concentrations increase with well depth in the Xiayedi Oasis; however, most groundwater samples in the Shihezi Oasis have low F concentrations (<1 mg/L, Chinese standard) that are not related to well depth, because the Manas River water, with low F concentration of 0.13 mg/L, is intercepted and recharged into groundwater in this area. In the Mosuowan Oasis, the F concentrations in the shallow wells range from 0.85 to 3.2 mg/L, with a mean value of 1.68 mg/L; however, the concentrations range from 0.62 to 2.77 mg/L in the middle and deep wells, with a mean value of 1.20 mg/L (15 samples). Elevated F concentrations are observed in most groundwater samples from the shallow to the deep wells in the Xiayedi Oasis (Fig. 7). In this oasis, three groundwater samples associated with water levels for different aquifers from a multilevel wells cluster (ZK-01) were tested: F concentration is 2.82 mg/L for the M-I aquifer (depth 14–20 m) with a water level depth of 4.28 m, 2.23 mg/L for the M-II aquifer (60–72 m) with a water level depth of 2.84 m, and 1.70 mg/L for the M-III aquifer (100–124 m) with a water level depth of 53.1 m (Fig. 8). A downward vertical hydraulic gradient is calculated as −1.8 between the M-II and M-III aquifers in this discharging area; nonetheless, F concentrations >4 mg/L in groundwater samples mostly occur in the middle and deep wells.
δ18O, Cl and F concentration profiles of groundwater in aquifers and pore water in the aquitards of bore ZK-01 in the Xiayedi Oasis. The F concentrations show an increasing trend, while the δ18O and Cl concentrations are nearly stable with depth >20 m from the surface, which indicates that the pore waters are nearly independent of evaporation effects and the chemical migration mechanism is a diffusion process, deduced from the characteristics of advective groundwater flow in aquifers (Hendry and Wassenaar 2011; Kuang et al. 2016)
Fluoride concentrations of the pore water in aquitards
Pore water in aquitards, an important component of groundwater, can release into adjacent aquifers and influence the hydrogeochemical characteristics of the aquifer groundwater when the aquifers are highly exploited (Ortega-Guerrero et al. 1993). Elevated F concentrations are observed in the pore water along the borehole ZK-01 profile. The F concentrations in pore-water range from 3.92 to 14.04 mg/L when the samples are from depths 41.5 to 100 m (Fig. 8). F concentrations in the groundwater are far lower than those in the pore water of adjacent aquitards, but the groundwater δ18O values in aquifers are more positive than those in pore water. This can be attributed to the different migration mechanisms in the aquifer and aquitards. It indicates that the migration of chemicals in pore water may be diffusion-dominated in the aquitards due to the hydrogeochemical gradients (Hendry et al. 2004; Hendry and Wassenaar 2011). Below 20 m in the profile, the nearly stable δ18O values close to −13.8‰ in the precipitating vapor (Wang et al. 2016) indicate that there are no intense evaporation effects. Therefore, the elevated F concentrations in the pore water are believed to mainly originate from the dissolution of fluorite and prolonged water–rock interactions with abundant clay and silt contents, due to the migration mechanism involving diffusion over a long residence time (Kwong et al. 2015; Misra and Mishra 2007).
Fluoride abundance in soils
Leachates of the saline soils near the marginal area between the alluvial-fluvial plain and desert were extracted using deionized water for analyzing the soluble F content (Table 4). Total soluble F ranged from 99.9 to 1,390.75 mg/kg, with a median of 388.5 mg/kg, which is slightly higher than the global soil mean value of 320 mg/kg (Wenzel and Blum 1992). The major ions (Cl, Ca, Mg, and NO3) accumulated at the surface (0–10 cm), mostly due to the intense evaporation effect in this arid basin. The decreasing Ca/Na ratios also suggest that the evaporation effect causes evaporite minerals to precipitate at the surface; however, there is a weak positive relationship between the F content and soil depth, in contrast to the Ca contents in the vadose zone as shown in Fig. 9. Elevated F concentration commonly coexists with a low concentration of soluble Ca due to the low solubility of CaF2 and the relatively high Na contents in deeper soil.
Discussion
Correlations between the major ions and F concentrations in groundwater, along with the hydrogeochemical characteristics of pore water in aquitards and soil leachates, could provide a deeper understanding of the spatial distribution and migration mechanisms of F in the MRB.
Development of elevated fluoride concentrations in groundwater
Elevated F concentrations exist in both saline and fresh groundwater samples in the MRB (Fig. 10a). High TDS (>1 g/L) coexisting with elevated F concentrations (>1.5 mg/L) is mainly observed in shallow and middle groundwater, whereas most samples with low TDS (<1 g/L) and elevated F concentrations are from deep wells. It indicates complex sources and migration mechanisms of F in aquifers at different depths. Hydrogeochemical and hydrological processes are responsible for the increases in the F concentrations and migration in groundwater. Groundwater with elevated F concentrations generally has low Ca and high Na concentrations and high Na/Ca ratios (Fig. 10b,c); this is similar to the findings of previous studies in arid and semiarid areas (Jacks et al. 2005; Rafique et al. 2009; Vithanage and Bhattacharya 2015). In this study, elevated F concentrations show a weak, positive linear relationship with HCO3 in the shallow and deep wells but negative in middle wells (Fig. 10d); this contradiction and low R2 indicate that F ions may be derived from other sources such as fluoride-bearing fertilizers and mixing with elevated fluoride sources in addition to the geochemical processes (Currell et al. 2011; Guo et al. 2007; Li et al. 2015; Su et al. 2015). However, the high HCO3/Ca ratios >1 (Fig. 10e) in most groundwater samples with elevated F concentrations demonstrate the competing effect of HCO3 on the dissolution of fluorine minerals. Meanwhile, most groundwater samples with elevated F concentrations are calcite saturated (SIcalcite value from −0.97 to +1.54 with a mean value +0.15) which helps remove Ca from groundwater, and F concentrations show a roughly positive relationship with the SIcalcite. In all, 80 out of the 90 groundwater samples are undersaturated with respect to the fluorite (SIfluorite from −3 to +2.11, mean value −1.29), and the SIfluorite values increase with the F concentrations (Fig. 11). Accordingly, these imply that fluorite dissolution contributes to the F concentrations and the high F concentration might be enhanced by calcite precipitation processes (Su et al. 2015); however, some groundwater samples with elevated F concentrations are oversaturated with respect to fluorite but undersaturated with respect to calcite, as shown in group A in Fig. 11, indicating that anthropogenic activities possibly affect F enrichment.
Correlations between the F concentrations and salinity, Ca, Na/Ca (meq/L), HCO3, HCO3/Ca (meq/L), F/Cl (meq/L) and NO3 for groundwater in shallow, middle and deep wells. Most elevated-F groundwaters have higher Na/Ca ratios than 1, and the presence of high NO3 concentrations in some elevated-F groundwater indicates that agricultural activities may affect the groundwater hydrochemistry and F concentrations and migration
The oxygen and hydrogen isotopic compositions of groundwater with elevated F concentrations show a regression line slope of 7.34 (R2 = 0.86); however, for the groundwater samples with low F concentrations, the regression line slope is only 5.50 (R2 = 0.89), owing to the evaporation effects (Fig. 5). Most groundwater samples have similar δ18O and δD composition characteristics in both the low and elevated F groundwater samples and the δ18O values are between −12 and −10‰. Most elevated-F groundwater is distributed to the left of the GMWL due to the origins of precipitation through vapor condensation processes in Tianshan Mountains, which are independent of evaporation effects. Some low-F groundwater distributes to the right of the LMWL and the regression line has a low slope, revealing that the evaporation effect could slightly increase F enrichment in groundwater. Furthermore, the existence of low F/Cl ratios (≤0.02) indicates the influence of evaporation effects on the groundwater in most middle wells (Fig. 10f).
Source and migration of fluoride in groundwater
The high prevalence of elevated F concentrations in groundwater with varying residence time at all depths suggests that comprehensive geochemical and other processes influence the enrichment and migration of F in the groundwater in the MRB (e.g., sample M121 with a F concentration of 48.15 mg/L in Table 3). Soluble F ions can be formed by the dissolution of fluorite (CaF2) and weathering of hydroxyl minerals such as biotite and muscovite in aquifers (Guo et al. 2007; Jacks et al. 2005). In the MRB, the natural groundwater flow path has changed due to groundwater overexploitation; thus, the distribution of F concentration varies spatially, corresponding to the different groundwater residence times, as illustrated by the relationship between the F concentrations and 14C apparent ages (Table 3; Fig. 12). In the Shihezi Oasis, groundwater apparent age shows great variance from 1.16 to 6.89 ka BP, and all samples have low F concentrations owing to the low fluorite content in the gravels and coarse sand as well as the dilution by the Manas River. Thus, it shows low correlation between F concentrations (R2 = 0.13) and 14C apparent ages. Some deep groundwater samples in Xiayedi (e.g. M113 with 3.60 ka BP) are apparently younger than those in the Shihezi Oasis (e.g. M123 with 6.89 ka BP); however, the F concentrations are elevated. It indicates that the groundwater mixed with younger water, such as the irrigation return flow and the leakage from upper shallow aquifers. In the Xiayedi Oasis, groundwater F concentrations generally increase (R2 = 0.71) with increasing residence time, indicating that efficient dissolution of fluorine minerals and the weathering of hydroxyl minerals partially contribute to the increase in the F content during the groundwater migration process.
Fluorite release F during weathering of the F-bearing minerals and precipitation of calcite:
F/Cl ratios in the middle and deep groundwater are higher than those in the unpolluted rainfall (0.02; Saether et al. 1995) or the Manas River (0.03), and the relationship between F/Cl ratios and F concentrations indicate that the increase in F is influenced not only by evapotranspiration but also by the water–rock interactions (Currell et al. 2011). The sediments of the aquifers and aquitards could react with the groundwater and pore water, providing sources of soluble F ions (Currell et al. 2011; Ortega-Guerrero et al. 1993). The F/Cl ratios of the sediments along borehole ZK-01 are relatively high (up to 0.5 between 10 and 100 m) because the sediments may contain several metal oxides that can adsorb the F ions (e.g., Al, Fe), as indicated by the light red color (Vithanage and Bhattacharya 2015; Vithanage et al. 2012). In this study, most groundwater samples have high HCO3/Ca ratios (Fig. 10e) with an alkaline pH; this is a favorable geochemical condition for the dissolution of fluorite (Saxena and Ahmed 2003). The alkaline pH, larger than the point of zero charge of most minerals, could lead to the solid surface having negative charge and resisting the F adsorption processes, and thus promote F desorption when fluorine minerals react with the groundwater (Kumar et al. 2009; Sujana et al. 2009). Most groundwaters are undersaturated with fluorite, according to the negative fluorite saturation indices (Fig. 11), suggesting that the groundwater allows for the dissolution of additional fluorite; therefore, F in pore water and groundwater is originally and primarily derived from the sediments owing to water–rock interactions.
The Ca concentration or Na/Ca ratio in groundwater is considered as one of the major mechanisms driving F ion migration in this arid inland basin with terrestrial sediments. This mechanism was demonstrated by Currell et al. (2011) through a sediment-solution experiment, because low Ca concentrations can facilitate the dissolution of fluorite, while high Ca concentrations can easily help reach the CaF2 solubility point and cause fluorite precipitation in the aqueous phase (Guo et al. 2012). It shows that cation exchange between Na and Ca is critical for improving geochemical conditions for the dissolution of F-bearing minerals and F migration. Most of the extremely elevated F groundwater samples (>4 mg/L) with low Ca concentrations in this study and other similar studies (Guo et al. 2012; Nordstrom and Jenne 1977) demonstrate this fact owing to the low fluorite solubility. The cation exchange between Ca (or Mg) and Na (or potassium) can be evaluated by the two chloro-alkaline indices CAI 1 and CAI 2. When cation exchange processes occur between the dissolved Na in groundwater and the adsorbed Ca in sediments, the CAI 1 and CAI 2 both have positive values; when it occurs in reverse order, the indices are both negative.
Negative CAI 1 and CAI 2 values in most of the groundwater samples (Fig. 13) indicate that the sediments can adsorb Ca from the groundwater and release Na into the groundwater in the presence of HCO3 (Schoeller 1965), enhancing CaF2 dissolution. The increasing monovalent Na concentrations relative to the divalent Ca concentrations can further reduce the repulsive potential between the positive hydrous metal oxide surfaces and negative ions with the help of alkaline conditions, and thus promote F desorption following the counterion effects (Li et al. 2015; Scanlon et al. 2009). In this study, Na/Ca ratios are consistently high with elevated F concentrations in most groundwater from the piedmont to the alluvial–fluvial plain. The relationship between F concentrations and Na/Ca ratios is not strongly positive in shallow (R2 = 0.12), middle (R2 = 0.15), or deep wells (R2 = 0.13; Fig. 10d); it indicates that the distribution and migration of F is partially influenced by the Na/Ca ratios in the groundwater. The low R2 values could attribute to the heterogeneous fluorite distributions in the aquifers and/or mixing with other high F sources at different depths across the basin.
Evapotranspiration is another process that could increase F concentrations in shallow groundwater under such an extreme arid climate. Two mechanisms are considered to explain the effects of evaporation on F enrichment. Intense evaporation and transpiration could concentrate the groundwater and increase the concentrations of all stable ions or reduced F/Cl and Na/Cl ratios; simultaneously, evaporation, but not transpiration, could cause the fractionation of hydrogen and oxygen isotopes. In some groundwater samples, positive relationships observed between δ18O values and the Cl (Fig. 14a) and δ18O values and F (Fig. 14b) concentrations indicate the evaporation effect. Evaporation also could cause the oversaturation of calcites and the prior precipitation can lower Ca concentrations and then promote the dissolution of fluorite as discussed already (Guo et al. 2007).
Plots of a δ18O vs Cl and b δ18O vs F concentration in groundwater, indicating that irrigation return flow and pore water in the aquitards contaminate groundwater, and leaching and vertical recharge are important processes for F mobilization and enrichment. M stands for Mosuowan, J for Jin’an, X for Xiayedi and S for Shihezi in the legend of part a
F ions in phreatic water can be transported, precipitate and accumulate in the vadose zone due to intense evaporation or transpiration effects. As reported in Table 4, high soluble F contents are distributed along the soil profiles (S1–S4) above the water table. As a result of the processes involved in agricultural activities, such as salt flushing and irrigation, soluble F contents in arable land can enter the groundwater during return flow (Han et al. 2011). Therefore, in addition to water–rock interactions and evaporation, agricultural activities in oases may provide other sources of F and drive F migration through irrigation and the use of fertilizer and pesticides, as indicated by the elevated NO3 in groundwater. The high NO3 concentrations (>5 mg/L), with high F content in shallow, middle and deep wells (Fig. 10g), indicate that the agricultural activities affect the groundwater F migration in the MRB, mostly owing to fertilizer as well as animal and human wastes (Li et al. 2015). However, most groundwater samples with relatively high F concentrations (>4 mg/L) have low to medium NO3 concentrations (0–14.1 mg/L). This indicates that input from agricultural activities affect the F enrichment only in the shallow and middle groundwater but do not act as a major source of F enrichment in the deep groundwater.
Influence of irrigation practices and groundwater exploitation
Groundwater from the deep aquifers has been exploited for flood irrigation and for flushing salt from the soil in the MRB for decades. This practice has made irrigation return flow and channel leakage important recharging sources of groundwater (Chen et al. 2009). The groundwater resources meet up to 50% of the total water demand, in particular for irrigation. Exploitation of the deep fresh groundwater causes reversal of the vertical hydraulic gradient between the shallow and deep aquifers. Shallow groundwater then can leak vertically into deep aquifers and contaminates the deep groundwater (Liu et al. 2015a).
The δ18O and δD compositions of most groundwaters with high F concentrations are aligned with or slightly apart from the LMWL in Urumqi (Fig. 5). Low significance differences between F concentrations and major ion concentrations indicate that the groundwater mixes with other high-F sources, in addition to F being derived from geochemical processes. These high-F sources can be attributed to the local vertical recharge and mixing with the pore water in the aquitards (Mazor 2004; Xie et al. 2012). The vertical recharge, e.g. irrigation return flow and channel leakage, can also flush the salts, and simultaneously contribute to the groundwater hydrogeochemistry in this basin (Chen et al. 2009; Han et al. 2011; Ma et al. 2017). Slight increases in the Cl concentration with depleted δ18O values in group B in Fig. 14a, i.e. mainly the middle and deep groundwater samples, indicate the presence of an additional recharge source in addition to the chemicals dissolution and evaporation that enrich the water with F (Li et al. 2015). Samples in group B have depleted δ18O values to lower than those in Manas River and in the mountains, but with significant increases in Cl. This can likely be attributed to mixing with the paleo-water and to salts dissolution. In Fig. 14b, the F concentrations are plotted against the δ18O values, and the groundwater samples occupy two triangular shapes (Tri 1 and Tri 2), identified by four end members: (1) the freshwater sample in the Manas River with low F concentration and low δ18O value, considered as the original water (EOri); (2) a spring sample in the mountain area with enriched δ18O and low F concentration, mainly the result of evaporation (EEva); (3) groundwater with extremely elevated F concentration and low δ18O value (EHighF-gw) and (4) pore water in the aquitards below 20 m with high F concentration and low δ18O value (EPore). Most shallow and middle groundwater samples within Tri 1 present the combined effects of evaporation and fluorite dissolution. The nonlinearity in the relationship attributes to the mixing processes in the shallow and middle groundwater. Most of the extremely high F (>4 mg/L) samples are distributed in Tri 2, where the evaporation effects are neglected for isotopic signals and show no obvious evaporation effect. Pore water in the aquitards seems to affect the groundwater fluoride concentrations, particularly in the Xiayedi Oasis. The mixing processes of three of the end members are corroborated by the difference in the fluoride concentration and δ18O value for the two groups of groundwater. Results of contribution ratios of the defined end members in the Tri 1 and Tri 2 groups are calculated by the linear mixing model (Eqs. 7–9) and shown in Table 5. The generally small (mean value 9%) contribution of evaporation to the F concentrations in Tri 1 clearly indicates that the evaporated recharge, along with the products of fluorite dissolution, infiltrated quickly from the soil into the groundwater system (Datta et al. 1996), particularly the irrigation water from the Manas River with low F concentrations (0.13 mg/L). The contributions of pore water are underestimated because the lowest F concentration (3.92 mg/L) and δ18O value (−13.97‰) along the ZK-01 profile are used. The mean contribution ratio, 21% (1–45%), indicates that the pore water in the aquitards affects the groundwater hydrochemistry in the aquifers and can increase the F concentration. Pore water in the aquitards is believed to preserve large amount of chemicals such as high F contents and depleted δ18O isotopes. Aquitards prolong the migration residence time owing to their high porosity and low permeability, thereby increasing water–rock interactions under natural conditions (Hendry and Wassenaar 2000, 2011; Hendry and Woodbury 2007). However, overexploitation of fresh groundwater can lead to groundwater cones of depression and the consolidation of aquitards. In depression-cone areas such as the Xiayedi Oasis, the observed inverse hydraulic gradient not only drives shallow groundwater leakage into the deep aquifer, facilitating the downward migration of the NO3-rich water from the shallow to the deep aquifers, but may also facilitate the release of pore water into aquifers during the consolidation processes of the aquitards (Ortega-Guerrero 2003; Ortega-Guerrero et al. 1993; Huang et al. 2012). Accordingly, the elevated-F groundwater evolves in three steps: (1) the fluorite in the soils are dissolved during the infiltration process (e.g. rainfall infiltration and irrigation return flow) into the shallow groundwater with an evaporation effect contribution ratio less than 40% in Tri 1 in Table 5; (2) dissolution of the fluorite occurs in the aquifers along the flow path and there is partial mixing with the existing elevated-F groundwater, e.g. water–rock interactions and leakage from the upper aquifers; (3) the groundwater is affected by the pore water in the aquitards (contribution ratios up to 45% in Tri 2 in Table 5). These results suggest that the Manas River and pumping groundwater for irrigation, at least to some degree, affect the F concentration distribution, migration and enrichment in groundwater.
The following Eqs. (7–9) express the mixing processes in Tri 1 and 2 (Fig. 14b) using the defined end members.
where, FOri, FHighF-gw, FEva and FPore are fractions of the original water, elevated-fluoride groundwater, evaporation, and pore water, respectively, and where E is the end member. Smp (F) and Smp (18O) denote the fluoride concentration and δ18O value for the sample, respectively.
Conceptual model of the fluoride migration in MRB
Pumping also disrupts the dynamic balance between the discharge and recharge processes, changes the groundwater flow paths, and redistributes F concentrations spatially. During the pumping process, the groundwater F is brought up to the surface and partially concentrated and accumulated in the vadose zone through soil sorption and evaporation-precipitation processes. Fresh irrigation return flow with low Ca concentrations (such as channel water for irrigation) promotes the dissolution of fluorite and drives the migration of concentrated F easily into the shallow and middle groundwater with the irrigation-elevated water table (Young et al. 2011). A certain amount of fresh groundwater with extremely high F concentrations is distributed in the middle and deep wells in this study area. F concentrations are further increased in the vadose zone and shallow groundwater when fresh groundwater with an elevated F concentration is used as irrigation water (Datta et al. 1996). In summary, it is common that agricultural activities affect the F ion content of groundwater and F ion migration in Northwest China and the North China Plain, where groundwater with high F concentration is exploited to meet water requirements, and elevated F concentrations in pore water in the aquitards are observed (He et al. 2013; Li et al. 2015; Liu et al. 2015b; Kwong et al. 2015; Fig. 15). Thus, in the Xiayedi Oasis where the elevated-F groundwater is pumped for irrigation, the exploitation of elevated-F groundwater should be reduced and channels should be built to transport the Manas River water for irrigation using the popular mulched-drip irrigation method (Li et al. 2016), which could prevent the soil water from evaporation and reduce the amount of irrigation and return flow.
Conclusions
The F concentrations in the groundwater in the MRB generally increase along the flow path from the southeast piedmont to the northwest alluvial-fluvial plain, and groundwaters with elevated F concentration are mostly distributed in the arable land irrigated by groundwater. Particularly in the Xiayedi Oasis, F concentrations in the groundwater, soils, and pore water in the aquitards are higher than the concentration limit set by the WHO and generally show an increasing trend with increasing depth.
F ions in groundwater are mainly from the fluorite dissolution in sediments and accumulate through prolonged water–rock interactions and as a result of agricultural activities. The relationship between oxygen and hydrogen isotopes in the groundwater reveals a moderate evaporation effect on low-F groundwater. Elevated F concentrations coexist with high Na concentrations, low Ca concentrations, and high F/Cl and Na/Ca ratios under alkaline pH conditions. These indicate that the cation exchange processes between Na and Ca concentrations affect F migration and promote the dissolution of F-bearing minerals in the groundwater over a wide span of residence time. Meanwhile, the presence of high NO3 in some elevated-F groundwater suggests that agricultural activities affect groundwater hydrogeochemistry and partially contribute to the migration and enrichment of F, particularly in shallow and middle aquifers. Wide variation in F and Cl concentrations along with slight differences in depleted δ18O values demonstrate that leaching and mixing with vertical recharge could be the dominant hydrological processes that increase F concentrations.
Groundwater pumping not only disturbs the natural groundwater flow system and reverses hydraulic gradients but also increases the F content of the soils as well as shallow and middle groundwater due to the high F concentrations of the groundwater used for irrigation. Pore water with relatively high F concentrations in the aquitards is released into aquifers, resulting in an increase in F concentrations because of the aquitards consolidation due to the overexploitation of aquifer groundwater. This means that groundwater pumping for irrigation is responsible for F enrichment and migration and therefore must be strictly controlled in areas with high rates of endemic fluorine poisoning. This paper presents findings that have a common pattern and provides evidence to support the fact that both the extensive pumping of groundwater and irrigation control the enrichment and migration mechanisms of toxic ions such as F in Northwest China.
References
Ali S, Thakur S, Sarkar A, Shekhar S (2016) Worldwide contamination of water by fluoride. Environ Chem Lett 14:291–315. https://doi.org/10.1007/s10311-016-0563-5
Amini M, Mueller K, Abbaspour K, Rosenberg T, Afyuni M, Møller K, Sarr M, Johnson C (2008) Statistical modeling of global geogenic fluoride contamination in groundwaters. Environ Sci Technol 42:3662–3668. https://doi.org/10.1021/es071958y
Ayoob S, Gupta AK (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Environ Sci Tecnol 36:433–487. https://doi.org/10.1080/10643380600678112
Bothe O, Fraedrich K, Zhu X (2012) Precipitation climate of Central Asia and the large-scale atmospheric circulation. Theor Appl Climatol 108:345–354. https://doi.org/10.1007/s00704-011-0537-2
Chae G-T, Yun S-T, Mayer B, Kim K-H, Kim S-Y, Kwon J-S, Kim K, Koh Y-K (2007) Fluorine geochemistry in bedrock groundwater of South Korea. Sci Total Environ 385:272–283. https://doi.org/10.1016/j.scitotenv.2007.06.038
Chen TD, Chen XG, Wang WK et al (2009) Investigation and assessment of groundwater resources and its environmental issues in the Junggar Basin (in Chinese). Geological Publishing House, Peking, pp 25–79
Currell M, Cartwright I, Raveggi M, Han D (2011) Controls on elevated fluoride and arsenic concentrations in groundwater from the Yuncheng Basin, China. Appl Geochem 26:540–552. https://doi.org/10.1016/j.apgeochem.2011.01.012
Datta PS, Deb DL, Tyagi SK (1996) Stable isotope (18O) investigations on the processes controlling fluoride contamination of groundwater. J Contam Hydrol 24(1):85–96. https://doi.org/10.1016/0169-7722(96)00004-6
Gaciri SJ, Davies TC (1993) The occurrence and geochemistry of fluoride in some natural waters of Kenya. J Hydrol 143:395–412. https://doi.org/10.1016/0022-1694(93)90201-J
Guo Q, Wang Y, Ma T, Ma R (2007) Geochemical processes controlling the elevated fluoride concentrations in groundwaters of the Taiyuan Basin, northern China. J Geochem Explor 93:1–12. https://doi.org/10.1016/j.gexplo.2006.07.001
Guo H, Zhang Y, Xing L, Jia Y (2012) Spatial variation in arsenic and fluoride concentrations of shallow groundwater from the town of Shahai in the Hetao basin, Inner Mongolia. Appl Geochem 27:2187–2196. https://doi.org/10.1016/j.apgeochem.2012.01.016
Handa (1975) Geochemistry and genesis of fluoride-containing ground waters in India. Ground Water 13:275–281. https://doi.org/10.1111/j.1745-6584.1975.tb03086.x
Han D, Song X, Currell M, et al. (2011) A survey of groundwater levels and hydrogeochemistry in irrigated fields in the Karamay Agricultural Development Area, northwest China: Implications for soil and groundwater salinity resulting from surface water transfer for irrigation. J Hydrol, 405(3-4):217–234. https://doi.org/10.1016/j.jhydrol.2011.03.052
He X, Ma T, Wang Y, Shan H, Deng Y (2013) Hydrogeochemistry of high fluoride groundwater in shallow aquifers, Hangjinhouqi, Hetao Plain. J Geochem Explor 135:63–70. https://doi.org/10.1016/j.gexplo.2012.11.010
Hendry M, Wassenaar L (2000) Controls on the distribution of major ions in pore waters of a thick surficial aquitard. Water Resour Res 36:503–513. https://doi.org/10.1029/1999WR900310
Hendry M, Wassenaar L (2011) Millennial-scale diffusive migration of solutes in thick clay-rich aquitards: evidence from multiple environmental tracers. Hydrogeol J 19:259–270. https://doi.org/10.1007/s10040-010-0647-4
Hendry MJ, Woodbury AD (2007) Clay aquitards as archives of Holocene paleoclimate: δ18O and thermal profiling. Ground Water 45:683–691. https://doi.org/10.1111/j.1745-6584.2007.00354.x
Hendry MJ, Kelln CJ, Wassenaar LI, Shwa J (2004) Characterizing the hydrogeology of a complex clay-rich aquitard system using detailed vertical profiles of the stable isotopes of water. J Hydrol 293(1–4):47–56. https://doi.org/10.1016/j.jhydrol.2004.01.010
Huang Y, Scanlon BR, Nicot JP, Reedy RC, Dutton AR, Kelley VA, Deeds NE (2012) Sources of groundwater pumpage in a layered aquifer system in the Upper Gulf Coastal Plain, USA. Hydrogeol J 20:783–796. https://doi.org/10.1007/s10040-012-0846-2
IAEA/WMO (2002) Global network of isotopes in precipitation. The GNIP database. IAEA, Vienna
Irigoyen M, Molina N, Luengas I (1995) Prevalence and severity of dental fluorosis in a Mexican community with above-optimal fluoride concentration in drinking water. Community Dent Oral 23:243–245. https://doi.org/10.1111/j.1600-0528.1995.tb00239.x
Jacks G, Bhattacharya P, Chaudhary V, Singh KP (2005) Controls on the genesis of some high-fluoride groundwaters in India. Appl Geochem 20:221–228. https://doi.org/10.1016/j.apgeochem.2004.07.002
Kim Y, Kim J-Y, Kim K (2011) Geochemical characteristics of fluoride in groundwater of Gimcheon, Korea: lithogenic and agricultural origins. Environ Earth Sci 63:1139–1148. https://doi.org/10.1007/s12665-010-0789-7
Kuang X, Jiao J, Wang Y (2016) Chloride as tracer of solute transport in the aquifer–aquitard system in the Pearl River Delta, China. Hydrogeol J 24:1121–1132. https://doi.org/10.1007/s10040-016-1371-5
Kumar E, Bhatnagar A, Ji M, Jung W, Lee S-H, Kim S-J, Lee G, Song H, Choi J-Y, Yang J-S, Jeon B-H (2009) Defluoridation from aqueous solutions by granular ferric hydroxide (GFH). Water Res 43:490–498. https://doi.org/10.1016/j.watres.2008.10.031
Kwong H, Jiao J (2016) Hydrochemical reactions and origin of offshore relatively fresh pore water from core samples in Hong Kong. J Hydrol 537:283–296. https://doi.org/10.1016/j.jhydrol.2016.03.050
Kwong H, Jiao J, Liu K, Guo H, Yang S (2015) Geochemical signature of pore water from core samples and its implications on the origin of saline pore water in Cangzhou, North China Plain. J Geochem Explor 157:143–152. https://doi.org/10.1016/j.gexplo.2015.06.008
Li J, Liang X, Jin M, Mao X (2013) Geochemical signature of aquitard pore water and its paleo-environment implications in Caofeidian Harbor, China. Geochem J 47:37–50. https://doi.org/10.2343/geochemj.2.0238
Li C, Gao X, Wang Y (2015) Hydrogeochemistry of high-fluoride groundwater at Yuncheng Basin, northern China. Sci Total Environ 508:155–165. https://doi.org/10.1016/j.scitotenv.2014.11.045
Li X, Jin M, Zhou N, Huang J, Jiang S, Telesphore H (2016) Evaluation of evapotranspiration and deep percolation under mulched drip irrigation in an oasis of Tarim basin, China. J Hydrol 538:677–688. https://doi.org/10.1016/j.jhydrol.2016.04.045
Liu Z, Liu S, Chen D, Wang X (2006) Water resources composition and water circulation in plain of Manas River Basin (in Chinese with English Abstract). J Hydraul Eng 37(9):1102–1107
Liu F, Song X, Yang L, Han D, Zhang Y, Ma Y, Bu H (2015a) The role of anthropogenic and natural factors in shaping the geochemical evolution of groundwater in the Subei Lake basin, Ordos energy base, northwestern China. Sci Total Environ 538:327–340. https://doi.org/10.1016/j.scitotenv.2015.08.057
Liu H, Guo H, Yang L, Wu L, Li F, Li S, Ni P, Liang X (2015b) Occurrence and formation of high fluoride groundwater in the Hengshui area of the North China Plain. Environ Earth Sci 74(3):2329–2340. https://doi.org/10.1007/s12665-015-4225-x
Luan F, Zhou Y, Zhou J, Jia R, Zeng Y (2016) Distribution characteristics and enrichment factors of groundwater fluoride in Shihezi area of Xinjiang (in Chinese with English abstract). Yellow River 38(3):64–67
Ma B, Jin M, Liang X, Li J (2017) Groundwater mixing and mineralization processes in a mountain–oasis–desert basin, Northwest China: hydrogeochemistry and environmental tracer indicators. Hydrogeol J 26(D22):1–18. https://doi.org/10.1007/s10040-017-1659-0
Mazor E (2004) Chemical and isotopic groundwater hydrology. Dekker, New York
Meenakshi, Garg VK, Kavita, Renuka, Malik A (2004) Groundwater quality in some villages of Haryana, India: focus on fluoride and fluorosis. J Hazard Mater 106:85–97. https://doi.org/10.1016/j.jhazmat.2003.09.007
Ministry of Health of China (2015) China health statistical yearbook. Peking Union Medical College Press, Beijing
Misra A, Mishra A (2007) Study of quaternary aquifers in Ganga Plain, India: focus on groundwater salinity, fluoride and fluorosis. J Hazard Mater 144:438–448. https://doi.org/10.1016/j.jhazmat.2006.10.057
Negrel P, Pauwels H, Dewandel B, Gandolfi JM, Mascré C, Ahmed S (2011) Understanding groundwater systems and their functioning through the study of stable water isotopes in a hard-rock aquifer (Maheshwaram watershed, India). J Hydrol 397:55–70. https://doi.org/10.1016/j.jhydrol.2010.11.033
Nordstrom D, Jenne E (1977) Fluorite solubility equilibria in selected geothermal waters. Geochim Cosmochim Acta 41:175–188. https://doi.org/10.1016/0016-7037(77)90224-1
Ortega-Guerrero A (2003) Origin and geochemical evolution of groundwater in a closed-basin clayey aquitard, northern Mexico. J Hydrol 284:26–44. https://doi.org/10.1016/S0022-1694(03)00239-7
Ortega-Guerrero A, Cherry J, Rudolph D (1993) Large-scale aquitard consolidation near Mexico City. Ground Water 31:708–718. https://doi.org/10.1111/j.1745-6584.1993.tb00841.x
Pang Z, Kong Y, Li J, Tian J (2017) An isotopic geoindicator in the hydrological cycle. Procedia Earth Planet Sci 17:534–537. https://doi.org/10.1016/j.proeps.2016.12.135
Perrin J, Mascré C, Pauwels H, Ahmed S (2011) Solute recycling: an emerging threat to groundwater quality in southern India? J Hydrol 398:144–154. https://doi.org/10.1016/j.jhydrol.2010.12.024
Rafique T, Naseem S, Usmani T, Bashir E, Khan F, Bhanger M (2009) Geochemical factors controlling the occurrence of high fluoride groundwater in the Nagar Parkar area, Sindh, Pakistan. J Hazard Mater 171:424–430. https://doi.org/10.1016/j.jhazmat.2009.06.018
Rango T, Vengosh A, Jeuland M, Tekle-Haimanot R, Weinthal E, Kravchenko J, Paul C, McCornick P (2014) Fluoride exposure from groundwater as reflected by urinary fluoride and children’s dental fluorosis in the Main Ethiopian Rift Valley. Sci Total Environ 496(496C):188–197. https://doi.org/10.1016/j.scitotenv.2014.07.048
Rao N (2003) Groundwater quality: focus on fluoride concentration in rural parts of Guntur district, Andhra Pradesh, India. Hydrol Sci J 48:835–847. https://doi.org/10.1623/hysj.48.5.835.51449
Reddy DV, Nagabhushanam P, Sukhija BS, Reddy AGS, Smedley PL (2010) Fluoride dynamics in the granitic aquifer of the Wailapally watershed, Nalgonda District, India. Chem Geol 269:278–289. https://doi.org/10.1016/j.chemgeo.2009.10.003
Rockström J, Falkenmark M, Karlberg L, Hoff H, Rost S, Gerten D (2009) Future water availability for global food production: the potential of green water for increasing resilience to global change. Water Resour Res 45. https://doi.org/10.1029/2007WR006767
Saether OM, Andreassen BT, Semb A (1995) Amounts and sources of fluoride in precipitation over southern Norway. Atmos Environ 29:1785–1793. https://doi.org/10.1016/1352-2310(95)00100-D
Salle CLGL, Marlin C, Leduc C, Taupin JD, Massault M, Favreau G (2001) Renewal rate estimation of groundwater based on radioactive tracers (3 H, 14C) in an unconfined aquifer in a semi-arid area, Iullemeden basin, Niger. J Hydrol 254(1):145–156
Saxena V, Ahmed S (2003) Inferring the chemical parameters for the dissolution of fluoride in groundwater. Environ Geol 43(6):731–736. https://doi.org/10.1007/s00254-002-0672-2
Scanlon BR, Nicot JP, Reedy RC, Kurtzman D, Mukherjee A, Nordstrom DK (2009) Elevated naturally occurring arsenic in a semiarid oxidizing system, Southern High Plains aquifer, Texas, USA. Appl Geochem 24:2061–2071. https://doi.org/10.1016/j.apgeochem.2009.08.004
Schoeller H (1965) Qualitative evaluation of groundwater resources: methods and techniques of groundwater investigations and development. UNESCO, Paris, pp 54–83
Su C, Wang Y, Xie X, Zhu Y (2015) An isotope hydrochemical approach to understand fluoride release into groundwaters of the Datong Basin, northern China. Environ Sci Processes Impacts 17(4):791–801. https://doi.org/10.1039/C4EM00584H
Sujana MG, Pradhan HK, Anand S (2009) Studies on sorption of some geomaterials for fluoride removal from aqueous solutions. J Hazard Mater 161:120–125. https://doi.org/10.1016/j.jhazmat.2008.03.062
Tekle-Haimanot R, Melaku Z, Kloos H, Reimann C, Fantaye W, Zerihun L, Bjorvatn K (2006) The geographic distribution of fluoride in surface and groundwater in Ethiopia with an emphasis on the Rift Valley. Sci Total Environ 367:182–190. https://doi.org/10.1016/j.scitotenv.2005.11.003
Tian H, Wang WK, Jing XY, Deng L (2010) A study on tritium of groundwater in Manas River basin (in Chinese with English abstract). J Arid Land Res Environ 24(3):98–102
Vikas C, Kushwaha R, Ahmad W, Prasannakumar V, Reghunath R (2013) Genesis and geochemistry of high fluoride bearing groundwater from a semi-arid terrain of NW India. Environ Earth Sci 68:289–305. https://doi.org/10.1007/s12665-012-1739-3
Vithanage M, Bhattacharya P (2015) Fluoride in the environment: sources, distribution and defluoridation. Environ Chem Lett 13:131–147. https://doi.org/10.1007/s10311-015-0496-4
Vithanage M, Jayarathna L, Rajapaksha A, Dissanayake CB, Bootharaju MS, Pradeep T (2012) Modeling sorption of fluoride on to iron rich laterite. Colloids Surface A 398:69–75. https://doi.org/10.1016/j.colsurfa.2012.02.011
Wang S, Zhang M, Che Y, Chen F, Qiang F (2016) Contribution of recycled moisture to precipitation in oases of arid Central Asia: a stable isotope approach. Water Resour Res 52:3246–3257. https://doi.org/10.1002/2015WR018135
Wenzel WW, Blum WE (1992) Fluorine speciation and mobility in F-contaminated soils. Soil Sci 153:357–364. https://doi.org/10.1097/00010694-199205000-00003
WHO (2004) Fluoride in drinking water-background document for development of WHO guidelines for drinking water quality. WHO, Geneva
Williams WD (1999) Salinisation: a major threat to water resources in the arid and semi-arid regions of the world. Lakes Reserv Res Manag 4:85–91. https://doi.org/10.1046/j.1440-1770.1999.00089.x
Xie X, Wang Y, Su C, Li J, Li M (2012) Influence of irrigation practices on arsenic mobilization: evidence from isotope composition and Cl/Br ratios in groundwater from Datong Basin, northern China. J Hydrol 424-425:3747. https://doi.org/10.1016/j.jhydrol.2011.12.017
Young SM, Pitawala A, Ishiga H (2011) Factors controlling fluoride contents of groundwater in north-central and northwestern Sri Lanka. Environ Earth Sci 63(6):1333–1342. https://doi.org/10.1007/s12665-010-0804-z
Zhou Z, Guo Q, Dou Z (2013) Delayed drainage of aquitard in response to sudden change in groundwater level in adjacent confined aquifer: analytical and experimental studies. Chin Sci Bull 58:3060–3069. https://doi.org/10.1007/s11434-013-5730-5
Acknowledgements
The authors thank Professor Jinlong Zhou and Dr. Ruiliang Jia (Xinjiang Agricultural University) for providing field work assistance. We thank Dr. Jing Li for her constructive suggestions on this study.
Funding
This study was funded by the National Natural Science Foundation of China (Nos. U1403282 and 41672246) and the Fundamental Research Funds for the Central Universities, China University of Geosciences, Wuhan (CUGQYZX1712).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special issue “Groundwater sustainability in fast-developing China”
Rights and permissions
About this article
Cite this article
Liu, Y., Jin, M., Ma, B. et al. Distribution and migration mechanism of fluoride in groundwater in the Manas River Basin, Northwest China. Hydrogeol J 26, 1527–1546 (2018). https://doi.org/10.1007/s10040-018-1780-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10040-018-1780-8