Abstract
Agricultural reclamation activities during the last few decades in the Western Nile Delta have led to great changes in the groundwater levels and quality. In Egypt, changing the desert land into agricultural land has been done using transferred Nile water (through irrigation canal systems) or/and groundwater. This research investigates the hydrogeochemical changes accompanying the reclamation processes in the small basin of Abu Mina, which is part of the Western Nile Delta region. In summer 2008, 23 groundwater samples were collected and groundwater levels were measured in 40 observation wells. Comparing the groundwater data of the pre-reclamation (1974) and the post-reclamation (2008) periods, groundwater seems to have been subjected to many changes: rise in water level, modification of the flow system, improvement of water quality, and addition of new salts through dissolution processes. Generally, Abu Mina basin is subdivided into two areas, recharge and discharge. The dissolution and mixing were recognized in the recharge areas, while the groundwater of the discharge region carries the signature of the diluted pre-reclamation groundwater. The salts of soil and aquifer deposits play an important role in the salt content of the post and pre-reclamation groundwater. NaCl was the predominant water type in the pre-reclamation groundwater, while CaSO4, NaCl and MgSO4 are the common chemical facies in the post-reclamation groundwater. The post-reclamation groundwater mostly indicates mixing between the pre-reclamation groundwater and the infiltrated freshwater with addition of some ions due to interaction with soil and sediments.
Résumé
Les activités d’habilitation agricole au cours des dernières décennies dans la partie occidentale du Delta du Nile ont amené à d’importants changements des niveaux et de la qualité des eaux souterraines. En Egypte, l’habilitation de terres désertiques en terres agricoles a été réalisée en transférant les eaux du Nil (via un système de canaux d’irrigation). La présente étude se propose d’étudier les changements hydrogéochimiques accompagnant les processus d’habilitation de terres désertiques en terres agricoles dans le petit bassin d’Abu Mina situé dans la région ouest du delta du Nil. Durant l’été 2008, 23 échantillons d’eau souterraine ont été collectés et les niveaux piézométriques mesurés dans 40 forages d’observation. En comparant les données sur les eaux souterraines collectées avant (1974) et après (2008) l’habilitation des terres, les eaux souterraines semblent être sensibles à plusieurs changements ; augmentation du niveau d’eau, modification du système d’écoulements, amélioration de la qualité de l’eau et addition de nouveaux sels par processus de dissolution. En règle générale, le bassin d’Abu Mina est divisé en deux parties, avec une zone de recharge et une zone de décharge. Des processus de dissolution et de mélange ont été reconnus dans les aires de recharge alors que les eaux souterraines des secteurs de décharge ont la signature des eaux souterraines diluées avant l’habilitation des terres désertiques en terres agricoles. Les sels des dépôts des sols et des aquifères jouent un rôle important sur la teneur en sels des eaux souterraines post et pré-habilitation des terres. NaCl est le type d’eau prédominant dans les eaux souterraines pré-habilitation des terres, alors que CaSO4, NaCl et MgSO4 sont des faciès communs des eaux souterraines post-habilitation des terres. Les eaux souterraines post-habilitation des terres indiquent principalement un mélange entre les eaux souterraines pré-habilitation des terres et les eaux d’infiltration récentes ayant un supplément d’ions dû aux interactions avec les sols et sédiments.
Resumen
Las actividades de recuperación agrícola durante las últimas décadas en el delta del Nilo Occidental han dado lugar a grandes cambios en los niveles y la calidad del agua subterránea. En Egipto, el cambio del terreno desértico en tierra agrícola que se ha hecho utilizando agua transferida del Nilo (a través de los sistemas de canales de riego) y / o aguas subterráneas. Esta investigación estudia los cambios hidrogeoquímicas que acompañaron los procesos de recuperación en la pequeña cuenca de Abu Mina, que es parte de la región occidental del Delta del Nilo. En el verano de 2008, se recolectaron 23 muestras de agua subterránea y los niveles de agua subterránea se midieron en 40 pozos de observación. Comparando los datos de agua subterránea de los períodos de la pre-recuperación (1974) y del posterior a la recuperación (2008), el agua subterránea parece haber estado sometida a muchos cambios: el ascenso del nivel del agua, la modificación del sistema de flujo, la mejora de la calidad del agua, y la adición de nuevas sales a través de los procesos de disolución. Generalmente, la cuenca de Abu Mina se subdivide en dos zonas, la de recarga y la de descarga. Se reconocieron procesos de disolución y la mezcla en las áreas de recarga, mientras que el agua subterránea en la región de descarga lleva la marca del agua subterránea diluida de la pre-recuperación. Las sales de los depósitos del suelo y de los acuíferos desempeñan un papel importante en el contenido de sal del agua subterránea pre y post-recuperación. El NaCl fue el tipo predominante en el agua subterránea antes de la recuperación, mientras que CaSO4, NaCl y MgSO4 son las facies químicas comunes en el agua subterránea después de la recuperación. El agua subterránea de la post-recuperación indica principalmente una mezcla el agua subterránea pre-recuperación y el agua dulce infiltrada con adición de algunos iones debido a la interacción con el suelo y los sedimentos.
摘要
过去几十年尼罗河三角洲西部的农业开垦活动致使地下水位和地下水质发生了很大变化。在埃及,一直通过(灌溉渠道)输送尼罗河水或/及地下水把沙漠变成农田。本研究调查了尼罗河三角洲西部阿布迈那流域的水文地球变化及开垦过程。2008年夏天,采集了23个地下水样,测量了40口观测井的地下水位。比较(1974年)开垦前地下水资料和(2008年)开垦后地下水资料后发现,地下水似乎经历 了许多变化:水位上升、水流系统的改变,水质的改善及溶解过程中新盐分的增加。一般来说,-阿布迈那流域分为两个区域,补给区和排泄区。发现补给区有溶解和混合现象,而排泄区的地下水为稀释的开垦前的地下水。土壤和含水层沉积层的盐分在开垦后和开垦前地下水中的盐分含量上发挥着重要作用。NaCl是开垦的前地下水中的主要水类型,而CaSO4、NaCl和 MgSO4是开垦后地下水中常见的化学相。开垦后地下水主要表明,开垦前地下水和入渗的淡水之间发生混合,并由于入渗的淡水和与土壤和沉积层互相反应增加了一些例离子。
Resumo
Atividades de recuperação de áreas agrícolas durante as últimas décadas no Delta Oeste do Nilo levaram a grandes mudanças nos níveis e na qualidade das águas subterrâneas. No Egito, a mudança de deserto a terra agriculturável foi realizada usando água transferida do Nilo (através de sistemas de canais de irrigação) e/ou águas subterrâneas. Esta pesquisa investiga as mudanças hidrogeoquímicas que acompanham os processos de recuperação na pequena bacia de Abu Mina, que é parte da região do Delta Oeste do Nilo. No verão de 2008, 23 amostras de águas subterrâneas foram coletadas e níveis de águas subterrâneas foram medidos em 40 poços de observação. Comparando os dados de águas subterrâneas do período anterior à recuperação (1974) e após a recuperação (2008), a água subterrânea parece ter sido submetida à várias mudanças: aumento do nível da água, modificação do sistema de escoamento, melhora da qualidade da água e adição de novos sais, através de processos de dissolução. De forma geral, a bacia de Abu Mina é subdividida em duas áreas, recarga e descarga. A dissolução e mistura foram reconhecidas em áreas de recarga, enquanto as águas subterrâneas da região de descarga tem a assinatura de águas subterrâneas anteriores à recuperação, diluída. Os sais no solo e dos depósitos do aquífero têm um papel importante no conteúdo de sal da água anterior e posterior à recuperação. NaCl foi o tipo predominante de água nas águas subterrâneas anteriores à recuperação, enquanto CaSO4, NaCl e MgSO4 são os compostos químicos mais comuns nas água subterrâneas após recuperação. As águas subterrâneas após a recuperação majoritariamente indicam a mistura entre águas subterrâneas anteriores à recuperação e as águas infiltradas com adição de alguns íons, devido à interação com o solo e sedimentos.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing water demands in semiarid and arid regions which have limited water supplies result in a critical status of groundwater recharge. (Semi-) arid regions are expanding and represent 30 % of the global terrestrial surface area (Dregne 1991). Currently, an estimated 1.1 billion of the 6 billion world population lack access to sources of clean drinking water (WHO 2003). Water scarcity will become more critical in the future as population growth in (semi-) arid regions surpasses that in more humid settings.
Impacts of land use/land cover changes on recharge are most obvious in Australia. Recharge rates in native mallee eucalyptus vegetation in Australia range from 0.1 to 1 mm/year, whereas recharge in deforested areas is up to about two orders of magnitude higher (1–50 mm/year). Water tables in remnant eucalyptus vegetation are about 2–7 m deeper than those under adjacent cleared areas (McFarlane and George 1992; Le Maitre et al. 1999). Increased recharge related to deforestation in Australia has resulted in large increases in groundwater salinity.
If irrigation water is derived from surface-water sources, increases in recharge can result in shallower water tables and water logging of soils. In groundwater-fed irrigation systems, increased irrigation pumpage can greatly outweigh increased recharge rates, resulting in great decline in groundwater level, e.g. 20–30 m in China; ≤25 m in southern High Plains (US); 20–30 m in Spain (Bromley et al. 2001; Foster et al. 2004; Liu and Xia 2004; Scanlon et al. 2005). The mobilization of unsaturated-zone salts is critical for groundwater quality in many areas, particularly in Australia, where extensive groundwater and surface-water salinization has resulted from dryland agriculture (Allison et al. 1990; Cook et al. 2001). Nitrate mobilization is critical in the US High Plains, where a large fraction of the wells exceed the maximum contaminant level of 10 ppm NO3–N. Therefore, if conversion of rangeland to dryland agriculture is being considered to increase recharge, the reservoir of stored salts in the rangeland setting should be characterized to assess potential impacts on groundwater quality.
The studied area is called Abu Mina Basin which is a part of the Western Nile Delta region (Fig. 1). Western Nile Delta was the first region in Egypt where land reclamation projects started in the 1950s. The reclamation of the northern ( Nubaria, Bustan, Bustan extension and El Tahrir areas) and northwestern parts (Burg El arab, and Abu Mina Basin) depends mainly on River Nile water derived from a set of main and secondary canals. In the southern parts including Wadi El-Natrun, Sadat City, South Khattaba and Wadi El Farigh, the reclamation depends mainly on groundwater (Fig. 1c). The rise in groundwater level and its quality improvement were noticed in the areas irrigated by surface water. In some locations, water logging and increase in soil salinity were detected (Ahmed 2002 and Fekry 2004). Compared to 1960 groundwater level data, 2010 data show a 30-m rise in the Nubaria area (Osman 2014). On the other hand, the groundwater-irrigated areas suffered from water level decline and salinity increases. 2010 data show up to 25-m decline in the water level in south Khataba area compared to 1960 data (Bayumy 2014). Therefore, it is important to study the changes in groundwater condition at a small closed basin with saline soil due to reclamation effects, such as seen regarding the situation at Abu Mina. As will be discussed in the next sections, although there is saline soil, water level rise is accompanied by reduction in groundwater salinity. Water level rise has occurred due to excessive irrigation and seepage from the irrigation channels. Soils and host rocks have added new salts to the groundwater, but without much effect in salinity modification of the post-reclamation groundwater.
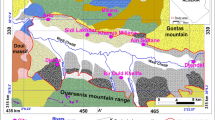
a Map showing the location of the study, b land cover map derived from the enhanced thematic mapper (ETM) image in June 2000 (El-Horiny 2012), and c Western Nile Delta map showing newly reclaimed areas and the constructed irrigation drainages. The yellow quadrangle in b indicates the location of Marmina Monastery
Site description
The present study concerns the Abu Mina basin, which is located in the northwestern Nile Delta, just to the southwest of Alexandria and bounded from the north by Burg Al-Arab area and from the south by latitude 30° 45′ N (Fig. 1). The area of study was considered as a target for reclamation programs by the Egyptian government, which required evaluation of the water-resource sustainability and potentiality in order to cope with prospective development. Abdel Daiem (1976) reported that there was no agriculture activity in Abu Mina basin in the 1970s but now it is fully cultivated (Fig. 1b). The surface water in the study area is supplied by El-Nasr canal and its channel system (Fig. 2). A flood-irrigation system has been employed in the study area since reclamation. A continuous increase of flood-irrigation using different water wells and channels in this area has led to rapid rising of the groundwater levels. This area traverses the Mariut Tableland and slopes gradually towards the NE direction where the ground-surface elevation ranges between 70 m above sea level (asl) to the west and 20 m asl to the east (Fig. 3). Compared to the surroundings, the Marmina Monastery is located in a lowland area, a sink that was formed due to an erosional effect (Mohamed et al. 1979).
The targeted area is characterized by semiarid climatic conditions that are closely related to the Mediterranean climate, where it is moderate to warm, sometimes hot in summer, and cold to moderate in winter. In general, it is characterized by a short rainy season with low and infrequent rainfall, high annual temperature variations, high evaporation rate, high relative humidity and frequent dew formation. The rainiest months are January and February with a mean value of 37.6 mm for each of the 2 months (Egyptian Meteorological Authority, EMA 1994).
Geology
Geologically, Abu Mina basin belongs to the Burg El-Arab area (Fig. 4). This area is generally covered by sedimentary sediments belonging to the Holocene, Pleistocene and late Neogene; however, the depression areas may be occupied largely by alluvial deposits.
a Hydrogeological map of Burg El-Arab and Abu Mina Basin and b hydrogeological cross-section across the two areas (modified after RIGW Research Institute for Groundwater 1991)
The Pleistocene sediments can be tentatively differentiated into two lateral units. The first one is the oolitic limestones which have a wide exposure in the coastal strip of the Mediterranean Sea. These sediments are composed of detrital limestone associated with calcareous clayey soil (Abdel Mogheeth 1968). The second unit (40–60 m thickness) is exposed mainly in the Abu-Mina basin and composed of fluviomarine facies of sands, clays and gypsiferous clays (Mohamed et al. 1979).
The Pliocene sediments are not exposed at the surface. Pliocene sediments underlie the oolitic calcareous bars of the Pleistocene sediments in the coastal zone and are overlain by clastic sediments of the Pleistocene age in Abu Mina basin. The Pliocene sediments overlie Moghra Formation (Miocene) along the western Mediterranean coastal area. A simplified geological section for the study area is presented in Fig. 4.
The Pleistocene clastic aquifer in Abu Mina basin is mostly built up of fluviomarine facies of sands, clays and gypsiferous clays. Groundwater occurs in this aquifer under semi-confined conditions due to covering of the clastic sediments by alluvial deposits of Holocene age. The clastic sediments have a thickness varying between 40 and 60 m, and transmissivity ranges from less than 500 m2/day to about 5,000 m2/day (CEDARE 2009).
The geological sequence from the surface downwards in Abu Mina basin (Water Resources Laboratory 1975) is as follows: 1–15 m Holocene loamy deposits; 5–30 m Pleistocene lagoonal deposits consisting of salt rich sand and sandy clay; 15–40 m Pleistocene deltaic deposits consisting of gravelly sand; 300 m Pliocene clay (water barrier). Recharge takes place through infiltration of rainwater, lateral seepage from El-Nasr Canal and its branches, and downward percolation of excess irrigation water. It is discharged by evaporation, evapotranspiration, and through a number of production wells.
Soil properties
According to Erian and Yacoub (2000), soil salinity of the study area (Fig. 5) falls into the following classes: slightly saline soils (2–4 dS/m), moderately saline soils (4–8 dS/m), strongly saline soils (8–16 dS/m), and the very strong saline soils ( >16 dS/m). The mentioned soils exhibit calcium carbonate content that varies from 28 to 51 %, which permits the formation of calcic horizons. The gypsum content ranges from 0 to 25 %, and is mainly concentrated at the subsurface layers, which permits the formation of a gypsic horizon. With respect to the composition of saturated soil extract (standard soil and water mixture), Ca2+ is the dominant cation followed by Na+ and Mg2+, whereas the anions are dominated by SO4 2−, then Cl− and HCO3 − (Moustafa et al. 2005). The soil texture for the surface horizons of Abu Mina basin is generally of median grain size and varies from sandy loam to sandy clay loam, while the clay content varies from 0 % to more than 40 % (the clay is formed essentially by calcium carbonate). The hydraulic conductivity is low and ranges from 1.3 to 6.2 cm/h. These low values are attributed to the presence of high amounts of both calcium carbonate and gypsum (Mousli 1980; Keren et al. 1980).
Soil salinity map of Abu Mina area (after Erian and Yacoub 2000)
Materials and methods
To investigate the groundwater levels and flow regime in 2008, data from 40 piezometers were used (Fig. 2). These piezometers range in depth from 30 to 50 m and are mostly screened in the last 6 m. Twenty-three groundwater samples from production wells and another one from El Nasr Canal were collected for the present study during the summer of 2008 (Fig. 2). All the production wells tap the Quaternary aquifer with the same average screen depth as the piezometers. To interpret the spatiotemporal change in groundwater levels, flow regime and chemistry, data from seven piezometers (average depth 45–60 m) were taken from Abdel Daiem (1976) to represent the groundwater condition of 1974 in the study area. Hydrogen ion activity (pH), electrical conductance, temperature, total dissolved solids (TDS), alkalinity, Ca and Mg concentrations had been measured in the field. The last three parameters were measured by titration. Chloride concentrations were determined in the laboratory by titration; Na and K ions were measured by flame photometer, whereas SO4 and NO3 were measured by spectrophotometer. The results of the hydrochemical analysis are shown in Table 1. The ion-charge-balance error for 2008 data is less than 5 %. All the contour maps used were made by a kriging method using surfer software.
Results and discussion
Flow regime
Depth to groundwater in 1974 (Fig.6a) varied from >51 m in the southwest to 33 m in the northeast. In contrast, the 2008 depth to groundwater (Fig. 6c) ranged between <1 m at Marmina Monastery to 11 m in the northeast at the periphery of the Mariut Tableland, with a general decrease towards the central part of the area. Groundwater in 1974 was recharged from Mariut Tableland in the northwest direction; it flowed to the south and southeast and discharged locally at Marmina Monastery (Fig. 6b). The general distribution of the water-table contour lines in 2008 (Fig. 6d) indicates different conditions compared to 1974 (Fig. 6b). The recharge area has become related to the El Nasr Canal and its branches, and the main direction of flow is now towards the central part of Abu Mina basin (Marmina Monastery). Other minor flow directions are detected in 2008 to the eastern and western parts of this aquifer.
Maps showing 1974 pre-reclamation a depth to water and b water-table contours. Post-reclamation (2008) c depth to water and d water-table contours. Arrows mark the groundwater flow directions. The red circles indicate the location of Marmina Monastery. The units are meters relative to ground surface (in a and c) and sea level (in b and d)
Hydrochemical characteristics and ion spatial distributions
The pre-reclamation groundwater (1974) showed higher values of TDS and total hardness (Fig. 7a,b; Table 1). The enrichment trend of both parameters followed the water level depth and flow regime (Fig. 6a,b) where they increased from northeast (shallower groundwater) to southwest (deeper groundwater) from 8,350 to 31,550 mg/L (for TDS) and from 1,182 to 5,554 mg/l (for TH) respectively. The local discharge area in Marmina Monastery showed abnormal total hardness distribution (Fig. 7b), where Ca and Mg are derived from the northward carbonate rock (Fig. 3).
Most of the 2008 water samples range from slightly saline to moderately saline. The salinity values of the studied groundwater samples vary from 1,260 to 7,070 mg/l at wells 4 and 11, respectively, with an average of 2,947.4 mg/l (Table 1). The areal distribution of salinity (Fig. 7c) shows that the TDS increases from all directions towards the central part of the aquifer (>5,000 mg/l), where Marmina Monastery is located in the lowland areas. Noteworthy, the areal distribution of TDS in the study area follows, more or less, the general pattern of the water level depth map (Fig. 6c), i.e. the higher the water salinity the shallower the water level depth. The total hardness of water samples ranges from 750 to 3,462.3 mg/l at wells 17 and 1, respectively (Table 1; Fig. 7d). The total hardness distribution map follows, more or less, the total salinity distribution.
As shown in Fig.8, the spatial distribution of ion concentrations (except alkalinity) of the pre-reclamation groundwater (1974) showed a similar enrichment trend to TDS. Total alkalinity had a reverse trend with zero value at the deeper groundwater in the southwest. Mg and Na had abnormal distribution trends in the Marmina Monastery area where the groundwater locally discharged. Post-reclamation groundwater data (2008) are spatially presented in Fig. 9. Calcium content varies from 140 to 672 mg/l at wells 5 and 3, respectively, with a mean value of 385 mg/l (Table 1). The distribution of calcium ion concentration increases away from the central part (natural discharge area) towards the east and west directions (Fig. 9a). The magnesium ion concentration ranges from 9.8 to 448.1 mg/l at wells 3 and 1, respectively, with an average of 124.6 mg/l (Table 1). Magnesium areal distribution does not follow that of calcium where it shows alternative parallel high and low concentration zones, which may be related to soil composition or discharge of the deeper old water (Fig. 9b). The sodium ion concentration ranges in 2008 from 138.4 to 1,750 mg/l at wells 6 and 11, respectively, with an average of 474 mg/l (Table 1). The iso-sodium contour map (Fig. 9c) reveals that the dominating trend is an increase from all directions towards the central part. The highest values of sodium are encountered in the areas of Marmina Monastery, where the groundwater discharges. The distribution pattern of the Na ion is relatively comparable with that of the Mg ion, except in the southeastern part. It is thought that the marine salt and the carbonate rocks in the area are the main source of both Na and Mg ions respectively. Potassium concentrations vary from 15.8 to 51.9 mg/l and have no definite areal distribution trend but in general it increases towards the east and the south (Fig. 9d).
Chloride ion concentration for 2008 varies from 140 to 2,100 mg/l at wells 4 and 11, respectively, with an average of 537 mg/l (Table 1). The highest values of chloride (>2,500 mg/l) are also encountered near Marmina Monastery (Fig. 9e). The distribution of Cl ion concentration follows, more or less, that of the Na, Mg and sulfate ions reflecting the same source. Sulfate concentrations increase from 700 mg/l at well 15–3,200 mg/l at well 11, with an average of 1,647 mg/l (Table 1). The sulfate ion represents between 24 and 83 % of the total dissolved anions in the groundwater and this is attributed to the occurrence of lagoonal deposits and saline soils in the area (Fig. 9f). Sulfate ion concentration increases from all directions toward the central part. The highest values of sulfate (>2,500 mg/l) are encountered in the Marmina Monastery low relief discharge area (Fig. 6d).
The bicarbonate ion concentration for 2008 ranges between 18 and 286 mg/l at wells 2 and 4, respectively, with a mean value of 104 mg/l (Table 1). The majority of the samples have bicarbonate content of less than 100 mg/l. The bicarbonate iso-concentration map (Fig. 9g) shows a decreasing trend from north and northwest to south and southeast. Such a trend is lithological controlled where the carbonate rocks of the Mariut Tableland is closer to the high concentration zones. Nitrate content ranges widely from 4.9 mg/l at well 15 to 66.9 mg/l at well 6, with a mean value of 33.4 mg/l (Table 1). In contrast to bicarbonate, the high values of nitrate are encountered in the southern part of the area towards the extension of El-Nasr Canal and away from both the tableland and the central area, where the large-scale irrigation and intensive use of fertilizers are known to occur leading to such high values of nitrate (Fig. 9h).
From the spatial distribution of the chemical parameters shown in Figs. 7, 8 and 9, it is concluded that the excessive irrigation and water seepage from the intensive channel system led to a great change in the groundwater chemistry. Generally, the TDS, TH and ion concentrations (except bicarbonate) become lower in 2008 compared with the pre-reclamation time (1974). It could be said that the Abu Mina basin groundwater has been subjected to freshening and dissolution processes. Groundwater flow direction and water-level depths were the main factors controlling the groundwater chemistry in 1974 where the TDS, TH and ion concentrations (except alkalinity) increased along the flow path. Marmina Monastery area is considered the main discharge area in 2008 where the highest salinity and ion concentrations are represented. Channel locations, use of fertilizers, presence of carbonate rocks, soil salinity and flow systems are the factors controlling the hydrochemistry of the groundwater in 2008.
Hydrochemical coefficients (ionic ratios)
The relationships between TDS and different major ion concentrations are shown in Fig. 10. Generally, TDS data of the 2008 groundwater set show a strong direct relation with Na + K, SO4 and Cl where the correlation coefficients (R 2) are higher than 0.85 (Fig 10a,b,f,g). Calcium and bicarbonate have a weak inverse relation with TDS (Fig. 10c,e, respectively). TDS shows an intermediate direct relation with Mg (Fig. 10d) where R 2 equals 0.59. Moreover, samples points in the NO3-TDS plot (Fig. 10h) are scattered with no definite trend (R 2 = 0.1). The distribution of the plotted samples reflects that the high concentrations of Na, Mg, Cl and SO4 in the 1974 groundwater are responsible for the salinity of the 2008 groundwater. On the other hand, recently infiltrated fresh water is responsible for salinity modifications through dilution of the 1974 groundwater and dissolution of the carbonate and sulfate minerals.
The Na/Cl parameter expresses the sodium–chloride imbalance in the water. It differs greatly according to the total concentration of the dissolved solids. The values of Na/Cl ratio for the collected samples range between 0.46 and 3.83 (samples 1 and 21 respectively, Table 1). Most water samples are above the unit value. In Fig. 11a, the Na/Cl relationship is strong (R 2 = 0.78) and the majority of the samples are clustered around the 2008-channel-water/1974-groundwater mixing line. Five samples deviate above the mixing lines. These results suggest two processes: (1) simple mixing between the 1974 groundwater and the 2008 channel water, as the general case, and (2) addition of sodium of terrestrial origin to the water, which is probably due to ion exchange where Na replaces Ca of the dissolved soil salts.
The Ca/Mg ratio helps in tracing seawater intrusion or rainwater recharge where it equals 0.25 and 5.63 (Table 1), respectively. Thus, higher values of this ratio may indicate surface water mixing (Ca/Mg = 10.62). However, the Ca/Mg ratio is highly influenced by the activity of the base-exchange process. The value of the Ca/Mg ratio is higher than unity in most of the samples (Table 1; Fig. 11b) where calcium exceeds magnesium (R 2 = 0.25) with ratios ranging from 0.58 to 41.7. Higher values of this ratio and distribution above and away from the mixing lines indicate a surface water (El-Nasr Canal) effect, which dissolves the calcium-rich minerals. Samples with Ca/Mg ratio of less than unity are located in the discharge area and are characterized by high TDS due to little mixing and higher percentage of pre-reclamation (1974) groundwater.
The Na/Cl ratio and relationship indicate cation exchange reaction (Edet and Okereke 2001). In Fig. 11c, the relationship shows a reverse trend (R 2 = −0.36). Such relation could be related to an ion exchange process where sodium replaces calcium. The Ca/SO4 ratio (Table 1) has mean values of less than unity for all 2008 samples (except sample 19) indicating Ca removal possibly by calcite precipitation or ion exchange with Na. Figure 11d shows a weak relationship between Ca and SO4 (R 2 = 0.1). Most of the samples are clustered around the rain–seawater mixing line, indicating that most of dissolved CaSO4 are related to dissolution of soil and aquifer marine salts by rainwater before reclamation.
The Ca/Cl ratio is useful in studying the modification of groundwater composition due to leaching and dissolution of dolomite, limestone, gypsum, and anhydrite. The Ca/Cl ratio ranges from 0.34 to 7.92 (Table 1) and most of the samples have a ratio over the unit value. There is no definite trend (R 2 = −0.004) in the relation shown in Fig. 11e and the samples are located over the mixing lines, reflecting the addition of calcium by freshwater dissolution of the calcium rich minerals. On the other hand, the recorded lower values of this ratio represent the discharge area where salinities have their highest values and calcium addition is minimal.
The Cl/Mg ratio ranges from 0.33 to 14.04 and their relation (Fig. 11f) shows a direct trend (R 2 = 0.7), indicating the same source. The relation of the two elements is not affected, neither by rain–seawater mixing nor by 2008-channel-water/1974 groundwater mixing. The SO4/Cl ratio could be taken as a guide for detecting any increase of sulfate in groundwater due to CaSO4 dissolution or CaCO3 precipitation. The values of this ratio are high and range from 1.12 to 9.35, which are higher than that of seawater (Table 1). There is a direct relation (R 2 = 0.72) between the two parameters (Fig. 11g) and the samples have no relation with the mixing lines. Such a high ratio and direct relation reflect the same terrestrial source and high concentration of sulfate soluble salts which are easily dissolved by freshwater.
The mean values of Cl/HCO3 (Table 1) in the studied area vary from 0.84 to 45.18 in 2008. This ratio may indicate a higher control of freshwater in the formation of the present groundwater. The reverse trend (R 2 = −0.1) shown in Fig. 11h and the clustering of the samples around the 2008-channel-water/1974-groundwater mixing line indicate that bicarbonate-rich freshwater diluted a chloride-rich groundwater.
Gibbs (1970) diagrams (Fig. 12) illustrate the distribution of sample points, as shown as a cluster, suggesting that, groundwater chemistry is controlled by rock weathering and evaporation processes, leading to the poor quality of groundwater (Rao 2002). Pre-reclamation (1974) groundwater salinity, Na, Ca, Cl, and HCO3 concentrations depend mainly on the evaporation process. For 2008 groundwater, freshwater mixing with 1974 groundwater could be the main source of Na and Cl, whereas soil and aquifer materials are the most reasonable source of Ca.
According to the afore-mentioned relations and ratios, the following points could be predicted: (1) the salinity of the 2008 groundwater is affected by Na, SO4, Cl concentrations that may relate to old groundwater; (2) dilution or mixing between the recently infiltrated and the pre-reclamation (1974) groundwater seemed to have occurred; (3) freshwater interaction with soil and aquifer materials adds new salts of Ca, HCO3, Na and SO4 to the groundwater; (4) 1974-groundwater salts content is mainly controlled by an evaporation process, while the post-reclamation (2008) groundwater hydrochemical composition is related to freshwater mixing with the pre-reclamation groundwater and water–rock interaction processes including dissolution and ion exchange.
Hydrochemical classification
The distribution of the studied samples in Sulin’s (1946) diagram (Fig. 13) shows that most of 2008 samples are of the sulfate type (Na2SO4). In the current study, such a water type could not be explained as it is of deep meteoric origin but it might be related to dissolution of gypsum contained in the soils followed by ion exchange with Na contained in the aquifer sediments. A few of the 2008 samples (1, 6, 15, 19 and 23) are similar to 1974 groundwater where they are of the chloride type (MgCl2) of marine origin. This indicates the larger influence of the recent source of groundwater which dissolves the marine salts of the soil and aquifer sediments.
A Piper diagram of 2008 and 1974 groundwater samples of Abu Mina basin shows a change of the water’s ion content over time (Fig. 14). Cl and SO4 are the predominant anions in 1974 and 2008 groundwaters, respectively. Na is the most predominant cation in the 1974 groundwater; the 2008 groundwater has variable content of Na, Ca and Mg; therefore, 1974 groundwater is characterized by NaCl chemical type, while the 2008 samples are characterized by CaSO4, NaCl and MgSO4 types. Derivation of the latter three types from NaCl old groundwater reflects the modification of the groundwater chemistry through mixing, soil minerals dissolution and water/aquifer-sediment interaction.
As an alternative to graphical methods, multivariate statistical techniques such as hierarchical cluster analysis (HCA) are now routinely used in groundwater studies as an effective tool to analyse large data sets (e.g. Gǜler et al. 2002; Daughney and Reeves 2005; O’Shea and Jankowski 2006; Menció and Mas-Pla 2008; Woocay and Walton 2008). However, in order to understand the physical and chemical processes that control groundwater evolution, the results of the multivariate statistical analysis have to be placed in the spatial context (Thyne et al. 2004). The dendrogram (Fig. 15) classified the 2008 water samples into two main groups: group 1 includes all the samples, except samples 1, 5, 11 and 16, which belong to group 2. The spatial distribution of the two groups is strongly correlated with the flow system (Fig. 6d) and the spatial distributions of TDS and ions concentrations (Figs. 7c and 9). Group 2 samples are located in the discharge areas and have higher concentrations of TDS, EC, Mg, Na, SO4 and Cl compared to group 1. Group 1 has higher Ca and HCO3 than group 2 and characterizes the recharge areas. Both groups have the same anionic concentration order similar to that of the soil, but for cations, group 1 has the order Ca > Na > Mg > K, whereas group 2 has the order Na > Ca > Mg > K.
According to the hydrochemical classification depending on the main anions and cations, the pre-reclamation (1974) groundwater is only of the NaCl type but the post-reclamation (2008) water is of sulfate type with different proportions of Na, Mg and Ca. According to the origin, some samples of 2008 groundwater still carry the signature of 1974 groundwater, i.e. of marine origin (MgCl2 type in Sulin’s diagram). Most of 2008 groundwater samples have Na2SO4 due to gypsum dissolution followed by ion exchange with Na. Statistically, the 2008 groundwater is classified into two groups depending on the cations concentration order. Group 1 is of discharge type but group 2 is of recharge type.
Conclusions
The Abu Mina basin is a good example for monitoring the effect of reclamation processes on the groundwater in a semi-arid area environment. The excessive use of irrigation systems and intensive channel networks in such areas (with saline soil and a small basin topographic shape) led to great changes in the groundwater environment. This is reflected by the groundwater level rising especially in the lowland areas, together with great changes in the flow regime and hydrochemistry. The water table has become shallower; it changed from >50–30 m depth to 11–<1 m deep. Groundwater flow direction has been changed from northwest–south and northwest–southeast to flow mainly towards the central part of the basin (Marmina Monastery). Other minor flow directions could be detected in 2008 to the eastern and western parts of this aquifer.
The hydrochemical characteristics of the basin groundwater have changed too—TDS, TH, Na, Ca, Mg, Cl and SO4 in 1974 groundwater were enriched along the flow direction, while in contrast, alkalinity reached zero concentration in the southwest and increased to the northwest. Recently, in 2008, the distribution of all the chemical data became quite different where most of the parameters increased toward the discharge area in the central part reflecting different proportions of 1974 groundwater along the flow path, but bicarbonate increase close to Mariut Tableland which consists of carbonate rock. On the other hand, NO3 for the 2008 groundwater distribution increases to the south and carries the signature of irrigation processes.
Ionic ratios and relations reveal that 2008 groundwater is a mixture of pre-reclamation (1974) and irrigation freshwater where Na, SO4, Cl are still controlling its salinity content. Simple mixing and dissolution are the main chemical processes affecting the chemistry of 2008 groundwater, with a minor effect of ion exchange. According to the Gibbs diagram, evaporation is the predominant chemical process influencing 1974 groundwater but 2008 groundwater is affected by both mixing and water–rock interaction.
Hydrochemically, the 1974 groundwater was only of the NaCl type but the 2008 groundwater is classified as the SO4 dominant chemical type with different proportions of Na, Ca and Mg. Most of 2008 groundwater samples are of the Na2SO4 type but some are similar to the 1974 groundwater having marine origin (MgCl2 type). Cluster analysis reflects also the variation of salinity and ion content. The recognized two main groups are simply comparable with the flow direction where the samples with high TDS and ion content are located in the discharge area.
Generally, reclamation processes in Abu Mina basin cause freshening of the old (1974) saline groundwater producing a brackish groundwater. Mixing between 1974 groundwater and the infiltrated freshwater is the main chemical process which led to salinity reduction. The groundwater level in Abu Mina basin rose by more than 30 m between 1974 and 2008; such a continuous rising of the water level in a small basinal area may cause severe water logging. As the groundwater level approaches the ground surface, soil salinity could be increased due to evaporation processes; therefore, an effective management policy should be implemented and specific crops might be cultivated to reduce the water consumption in order to control the problem.
References
Abdel Daiem AA (1976) Melioration hydrogeology in west El-Nubaria area. PhD Thesis, Mansoura University, Egypt
Abdel Mogheeth SM (1968) Hydrogeochemical studies of Bur El-Arab and vicinities. MSc Thesis, Ain Shams University, Egypt
Ahmed ASA (2002) Environmental impacts of development on the hydrogeology and hydrochemistry, West Nile Delta, Egypt. PhD Thesis, Cairo University, Egypt
Allison GB, Cook PG, Barnett SR (1990) Land clearance and river salinization in the western Murray Basin, Australia. J Hydrol 119:1–20
Bayumy DA (2014) Sedimentological and hydrological studies on the area west of the Nile Delta, Egypt. MSc Thesis, Tanta University, Egypt
Bromley J, Cruces J, Acreman M, Martinez L, Llamas MR (2001) Problems of sustainable groundwater management in an area of over exploitation: the upper Guadiana catchment, central Spain. Int J Water Resour Dev 17:379–396
CEDARE (2009) Assessment of groundwater potential in Alexandria Governorate. CEDARE, Cairo
Cook PG, Leaney FW, Jolly ID (2001) Groundwater recharge in the Mallee Region, and salinity implications for the Murray River: a review. CSIRO Land and Water technical report 45, CSIRO, Clayton, Australia, 133 pp
Daughney CJ, Reeves RR (2005) Definition of hydrochemical facies in the New Zealand National Groundwater Monitoring Programme. J Hydrol (New Zealand) 44:105–130
Dregne HE (1991) Global status of desertification. Ann Arid Zone 30:179–185
Edet AE, Okereke CS (2001) A regional study of saltwater intrusion in southeastern Nigeria based on the analysis of geoelectrical and hydrochemical data. Environ Geol 40:1278–1289
Egyptian Meteorological Authority (EMA) (1994) Climatic records of Egypt. Annual reports (1959/1994), EMA, Cairo
El-Horiny M (2012) Geoelectrical and hydrogeological studies on Burg El-Arab area and its adjacent areas North Western Coast of Egypt. MSc Dissertation, Tanta University, Egypt
Erian WF, Yacoub RK (2000) The use of the ordinary kriging techniques in measuring the sustainability in sugar beet area, Nubariya, Egypt. Int Archives Photogramm Remote Sensing XXXIII (B7):405–419
Fekry AM (2004) Assessment of impacts of groundwater developments in the west Nile Delta region, Proceedings of the 3rd ISG, Tanta, Egypt, December 2003, pp 262–270
Foster S, Garduno H, Evans R, Olson D, Tian Y, Zhang W, Han Z (2004) Quaternary aquifer of the North China plain: assessing and achieving groundwater resource sustainability. Hydrogeology J 12:81–93
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 17:1088–1090
Gǜler C, Thyne GD, McCray JE, Turner AK (2002) Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeology J 10:455–474
Keren R, Kreit JF, Shainberg I (1980) Influence of size of gypsum particles on the hydraulic conductivity of soils. Soil Sci 130:113–117
Le Maitre DC, Scott DF, Colvin C (1999) A review of information on interactions between vegetation and groundwater. Water 25:137–152
Liu CM, Xia J (2004) Water problems and hydrological research in the Yellow River and the Huai and Hai River basins of China. Hydrol Processes 18:2197–2210
McFarlane DJ, George RJ (1992) Factors affecting dryland salinity on two wheat belt catchments in Western Australia. Aust J Soil Res 30:85–100
Menció A, Mas-Pla J (2008) Assessment by multivariate analysis of groundwater–surface water interactions in urbanized Mediterranean streams. J Hydrol 352:355–366
Mohamed SS, Abd Allatif T, Khalil S (1979) Electrical resistivity investigations in clarifying the subsurface geological sequences and groundwater conditions of Abu Mina basin (northwestern coastal zone, Egypt). In: The hydrology of areas of low precipitation, Proceedings of the Canberra Symposium, December 1979. IAHS publ. 128, IAHS, Wallingford, UK, pp 349–354
Mousli OF (1980) Methods of evaluation and classification of gypsiferous soils and suitability for irrigated agriculture. In: Beinroth FH, Osman A (eds) Proceedings of the 3rd International Soil Classification Workshop. The Arab Center for Studies of the Arid Zones and Dry Lands (ACSAD), Damascus, Syria, pp 278–307
Moustafa AM, Ismail HA, El-Menshawy AB, Soliman WA (2005) Current and predicted land evaluation using integrated GIS and modeling tools at El-Bangar area, Egypt. Minufiy J Agric Res 30:365–385
O’Shea B, Jankowski J (2006) Detecting subtle hydrochemical anomalies with multivariate statistics: an example from ‘homogeneous’ groundwaters in the Great Artesian Basin, Australia. Hydrol Process 20:4317–4333
Osman OM (2014) Hydrogeological and geoenvironmental studies of El Behira Governorate, Egypt. PhD Thesis, Tanta University, Egypt
Rao NS (2002) Geochemistry of groundwater in parts of Guntur district, Andhra Pradesh, India. Environ Geol 41:552–562
RIGW (Research Institute for Groundwater) (1991) Hydrogeological map of Egypt, Burg El-Arab area, scale 1:100,000. RIGW, Cairo, Egypt
Scanlon BR, Reedy RC, Stonestrom DA, Prudic DE, Dennehy KF (2005) Impact of land use and land cover change on groundwater recharge and quality in the southwestern USA. Glob Chang Biol 11:1577–1593
Sulin VA (1946) Waters of petroleum formations in the system of natural water. Gostoptekhizdat, Moscow, pp 35–96
Thyne G, Güler C, Poeter E (2004) Sequential analysis of hydrochemical data for watershed characterization. Ground Water 42:711–723
Water Resources Laboratory (1975) Geological and hydrogeological investigations of the 300 000 feddan reclamation project (west of Nubariya). Desert Research Centre (DRC), Cairo
WHO (2003) The right to water. Health and Human Rights Publication Series no. 3, World Health Organization, Geneva, Switzerland, 44 pp
Woocay A, Walton J (2008) Multivariate analyses of water chemistry: surface and ground water interactions. Ground Water 46:437–449
Acknowledgements
The 2008 groundwater data were taken from the master’s thesis of the third author. The authors are thankful to Tanta University, Egypt, for their financial support during the field and laboratory work. The authors are grateful to the associate editor of Hydrogeology Journal and two anonymous reviewers for their constructive remarks, which lead to improvement in the quality of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salem, Z.E., Atwia, M.G. & El-Horiny, M.M. Hydrogeochemical analysis and evaluation of groundwater in the reclaimed small basin of Abu Mina, Egypt. Hydrogeol J 23, 1781–1797 (2015). https://doi.org/10.1007/s10040-015-1303-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10040-015-1303-9