Abstract
Purpose
Chronic postoperative inguinal pain (CPIP) after pre-peritoneal hernia repair is rare but may be severely invalidating. Mesh may be a contributing factor to the development of CPIP. International guidelines acknowledge mesh removal as a treatment option for CPIP after open repair, but experience in laparoscopic mesh removal is limited. Surgeons are hesitant to remove pre-peritoneal meshes because of fear of operative complications. This observational study describes risks and effectiveness of laparoscopic mesh removal in patients with CPIP after endoscopic inguinal hernia repair.
Methods
Questionnaires and operative findings of consecutive patients undergoing a laparoscopic mesh removal for CPIP between August 2014 and February 2019 in the center for groin pain were prospectively recorded. Long-term efficacy was determined using pre and postoperative questionnaires on pain and quality of life.
Results
Forty-four patients were included (37 males, median age 51 years). Complete or sufficient pain relief was reported in every two out of three patients (68%) and quality of life improved significantly. Intraoperative findings included wrinkled mesh (n = 19), meshoma (n = 14) and infected mesh (n = 1). Surprisingly, over half of the meshes (n = 23) did not fully cover the groin, with three clear recurrent hernias. Intraoperative complications included two bladder injuries. One patient undergoing removal of 3 meshes on one side developed a necrotic testicle. During follow-up, three patients developed a recurrent hernia requiring open surgery.
Conclusion
Laparoscopic mesh removal is safe and effective in selected patients with CPIP after endoscopic hernia repair. We believe that this technique should be adopted by dedicated hernia surgeons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inguinal hernia repair is performed over 20 million times each year worldwide [1]. International guidelines recommend the use of mesh via an open and endoscopic technique [2]. Previously, the open Lichtenstein technique was considered gold standard of inguinal hernia repair [3]. In the previous decade however, endoscopic repair has become the preferred approach because of diminished recovery time and improved cost-effectiveness [2], provided that sufficient experience and resources are available. During endoscopic repair, a mesh is most often placed by a total extra-peritoneal technique (TEP) or using the transabdominal pre-peritoneal approach (TAPP). Chronic postoperative inguinal pain (CPIP) is the most disabling and costly complication of inguinal hernia surgery [4]. Rates of CPIP are lower after endoscopic compared to open repair (6–12% vs 11–17%) [5,6,7,8,9]. Minimal dissection without inguinal nerve manipulation and omitting mesh fixation likely contribute to the benefits of endoscopic techniques regarding CPIP [10].
Management of CPIP is complex. If conservative or minimally invasive treatments are to no avail, surgery may be considered. While mesh and entrapped nerves are relatively easily removed after a Lichtenstein procedure, the surgical treatment of CPIP after endoscopic repair is far more challenging. First, the inguinal nerves are not expected to be involved and (triple) neurectomy will therefore be to no avail. Second, the pre-peritoneal space is not easily accessible after previous surgery whereas the proximity of major vessels, gut and bladder complicate simple mesh dissection.
In 2014, almost half of all groin hernias (46%) were treated with an endoscopic technique in the Netherlands and this rate is still growing [11]. As a consequence, the prevalence of patients with CPIP after pre-peritoneal repair is increasing accordingly. The current literature on laparoscopic removal of pre-peritoneal meshes is retrospective reporting on mesh combining removal after inguinal and incisional hernia at various locations in the abdominal wall [13, 14]. Over 400 CPIP patients annually receive treatment at our tertiary referral center for groin pain. A previously published small retrospective case series of 14 CPIP patients undergoing laparoscopic removal of pre-peritoneal meshes demonstrated that this type of mesh removal was feasible and safe in selected patients [12].
Primary objective of the present study was to determine the effect of laparoscopic removal of pre-peritoneal mesh on CPIP in a large case series. Patient satisfaction and quality of life were evaluated using questionnaires. Other aims were to assess intra- and postoperative complications, hernia recurrence rate and predictors of success.
Methods
Study design
The present study was conducted at SolviMáx, a national reference center for groin pain at Máxima Medical Center (MMC), Eindhoven, The Netherlands. Although the Medical Ethics Committee waived the need for formal approval, the study protocol was reviewed and approved by our local Research Board.
Study population
Each patient who is referred to SolviMáx for potential CPIP standardly completes a set of questionnaires prior to consultation including medical history, quality of life, psychological factors and current pain levels. After evaluation of these questionnaires, patients are invited to undergo a 30 min outpatient assessment including physical examination by a surgeon dedicated to management of groin pain. If patients are suspected to suffer from mesh-related CPIP, they were informed on the specifics and potential complications of laparoscopic mesh removal and informed consent was obtained.
Patients with mesh-related CPIP following endoscopic inguinal hernia repair (TEP or TAPP) who underwent a laparoscopic mesh removal between August 2014 and February 2019 were considered eligible. Mesh-related CPIP after endoscopic hernia repair was determined by previously described criteria [15]. These include sensations of a ‘foreign body feeling’ or tightness in the groin area, pain aggravation during car driving, bending or crossing legs, and pain relief by hip extension. Clues for mesh-related pain during physical examination are a painful deep palpation along the inguinal ligament over more than five centimeters, a diffuse pain and the lack of sensory disturbances. Exclusion criteria were cognitive impairment, inability to complete questionnaires due to language disabilities, bilateral mesh removal, and mesh removal for other reasons than CPIP.
A follow-up survey was conducted in April 2019. Patients were contacted to complete questionnaires on postoperative complications, hernia recurrences, additional treatments, satisfaction and working capabilities. All data were prospectively collected in a database and fully anonymized, according to the European General Data Protection Regulation.
Study intervention
The operation was performed in supine position under general anesthesia with a Foley bladder catheter inserted. Antibiotics were not administered. Two 10 mm trocars were introduced in the upper abdomen and one 5 mm trocar in the ipsilateral flank. Dissection of the mesh started at the cranial or lateral edge. The mesh was gradually released from the underlying structures using scissors and cautery. Epigastric and gonadal vessels were usually tightly adhered to the mesh but were only incidentally sacrificed. Behind the medial umbilical fold, dissection is performed closely along the mesh to avoid damage to the bladder. Once free, the mesh is recovered through a 10 mm trocar, either in one piece, or piecemeal if a meshoma. The technique was described in more detail previously [12]. Intraoperative images are depicted in Fig. 1, 2, 3. An overnight stay was advised as most patients were from other areas of the Netherlands.
Outcome measures
Medical history including pain characteristics, previous treatments was retrieved from the patients’ electronic hospital files. Pain levels (average, maximum, and minimum during the previous week) were assessed using a Numeric Rating Scale (NRS, 0 ‘no pain at all’ to 10 ‘unbearable pain’) at various time points [16]. The Brief Pain Inventory (BPI) was used to assess the impact of pain on functioning. A BPI score of 1–3 was considered to be mild impact of pain, 4–6 is moderate and a score of 7–10 is severe impact of pain [17]. The 12-Item Short Form Health Survey (SF-12) was used to evaluate health status. The SF-12 results in two calculated scores: the Physical Component Score (PCS) and the Mental Component Score (MCS) [18]. In theory, psychological vulnerability can be predictive of the success of mesh removal [19]. Therefore, scores on Pain Catastrophizing Inventory (PCI) and the Hospital Anxiety Depression Scale (HADS) were collected of all patients before mesh removal [20].
Statistical analysis
Data were analyzed between March and July 2019, using the Statistical Package for the Social Sciences (SPSS, SPSS Inc. Chicago, IL, USA), version 25. Data were presented by descriptive statistics. Shapiro–Wilk test was performed to check normality and Wilcoxon sign-rank test was performed to analyze pain scores and quality of life. Mann–Whitney was used to check if responders were similar to non-responders at baseline. Linear regression, Chi-square, and Fisher’s exact test were performed to test univariate relations. Significance levels were set at 0.05.
Results
Baseline characteristics
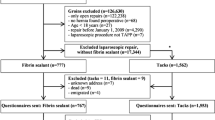
Forty-eight consecutive patients were identified (Fig. 4). As 4 patients were excluded (bilateral mesh removal n = 3; no CPIP but allergy to a titanium requiring mesh removal n = 1), 44 patients met inclusion criteria (male n = 37; median age 51 years, range 26 to 77 years; TEP n = 41; TAPP n = 3). Intake questionnaires were complete in 41 patients (93%). Baseline characteristics are presented in Table 1.
Before mesh removal, most patients had undergone a variety of conservative treatments including injections (n = 26), nerve block/stimulation (n = 18), physical therapy (n = 8) and psychological therapy (n = 1). Moreover, 15 patients had received remedial surgery including neurectomy (n = 15), adductor tenotomy (n = 2), epididymectomy (n = 1), periosteal stripping of pubic bone (n = 4) and correction of femoral hernia (n = 2).
Before mesh removal, median pain score was 7.0 (range 4.0–9.0) and severity of pain and its impact on functioning was scored 7.0 (median BPI, range 1.0–8.7). Quality of life was scored 30 (range 17–57) and 41 (range 25–61) on the physical and mental scale of SF-12, respectively. Pain catastrophizing was scored 26 (range 8–44) out of 52 points and HADS scores on the depression and anxiety scale were 9.0 (range 0–17) and 7.0 (range 0 – 15) respectively.
Intraoperative findings
The time interval between hernia mesh repair and mesh removal was 38 months (median, range 7–332). Operating time was 106 min (median, range 39–210). In 19 patients, the mesh appeared slightly wrinkled. A meshoma, defined as mesh folding into a bulky density [21], was observed in 14 patients. The mesh covered the hernia orifice adequately in just half of the patients (48%). In case of ingrowth of mesh into the pubic bone in locally asymptomatic patients, a small rim of mesh was left in situ (n = 6). Patients that had < 75% of the mesh removed (n = 7) just had complaints attributed to latero-cranial portion of the mesh. Intentional neurectomy of the genitofemoral nerve was performed in two cases. One patient was found to have an infected mesh.
A Lichtenstein procedure was performed in two patients at the end of the endoscopic procedure because of a preoperatively identified hernia recurrence.
Complications associated with mesh removal (n = 5)
Significant bleeding or infection did not occur in any of the 44 patients.
A small bladder laceration was recognized intraoperatively and over sewn using two stitches. Recovery was uneventful. A second patient was readmitted at the referring hospital for persistent abdominal pain due to an unrecognized bladder laceration which resolved after ten days of catheterization. Both patients were free of pain at follow-up. A hematoma at a trocar opening occurred in two additional patients that resolved after temporary mild discomfort. In the fifth patient, 3 pre-peritoneal meshes had formed a tennis ball-sized meshoma that had entrapped the gonadal vessels. He developed necrosis of the testis and a Spigelian hernia. The hernia was treated with open mesh repair and the testicle was removed.
Findings at Follow-up
Follow-up time was 18 months (median, range 2–56) with a 77% response rate. A sensitivity analysis found that baseline characteristics and intraoperative data were similar in responders (n = 33) and non-responders (n = 11) except for a higher BMI in the latter group. Pain scores had significantly dropped from 7.0 to 4.5 (median, range 0–10) (Table 2). Total (n = 7) or partial pain relief (n = 16)) was reported in 23 patients (68%). Nine patients experienced no pain reduction. Two patients reported more pain and were the only two participants regretting surgery.
Satisfaction was excellent in nine patients, good in nine, moderate in eight, poor in three and bad in four patients. The severity of pain and its impact on functioning, as assessed with BPI, was significantly reduced from 7.0 to 3.0 (median, range 0–8.3). A significant improved QoL was demonstrated regarding both physical and mental scale of SF-12, with median scores of 43 and 55 respectively. Both anxiety and depression scales of the HADS reduced at follow-up with a significant reduction in the depression score. Patients reported significantly less days of absenteeism after mesh removal (P = 0.019).
Hernia recurrence and prediction of success
Four patients reported a recurrent hernia within six months as diagnosed by their own physician, and three required an operation. One of them is the previously described case with 3 meshes at one side. Seven patients experienced symptoms possibly reflecting a recurrent hernia but none of them reported a bulging.
Not one baseline characteristic or intraoperative finding was predictive of success after mesh removal surgery. Although not statistically significant (P = 0.064), time between endoscopic hernia repair and mesh removal was positively correlated with pain reduction. This finding indicates that mesh removal should not be denied in patients who have been suffering from pain for an extended period of time.
Discussion
This study is the first prospective study reporting on the effect of laparoscopic mesh removal in a series of 44 patients with unacceptable CPIP following endoscopic hernia repair. At a median 18 months’ follow-up, pain was absent in 21%, whereas another 47% reported pain reduction (68% success rate). Average pain scores using NRS dropped significantly from 7.0 to 4.5 with a significant improved quality of life. Overall level of pain also decreased significantly from severe to mild, whereas impact on functioning was attenuated. Short-term and long-term complication rates were acceptable.
Mesh removal for CPIP is controversial in a variety of ways. Not all hernia surgeons engaged in pre-peritoneal mesh placement are convinced that CPIP can be due to the ongoing presence of a mesh. Several of our patients stated that their surgeons had discouraged them to undergo remedial surgery as ‘it is impossible or very dangerous to remove the mesh’. Indeed, one study in 25 patients undergoing endoscopic mesh removal for recurrence or CPIP reported iliac vein damage (n = 1), spermatic duct damage (n = 1) and unintended nerve damage (n = 4) although long-term sequelae were not studied [22]. The present case series found an 11% short-term complication rate including a necrotic testicle in one patient who was very relieved as removal of the three meshes had cured his debilitating pain. We routinely catheterize the bladder during surgery since a bladder laceration had occurred in two. Based on an acceptable short-term complication rate, it may be argued that an endoscopical mesh removal is safe in expert hands and may possibly be preferred over an open removal technique.
We initially thought that success after mesh removal was related to an initial incorrect mesh position leading to CPIP. If mesh would wrinkle, or even turn into a meshoma, an incorrect position may preferentially lead to CPIP. In this series, position and location of the mesh, and percentage of removed mesh could not predict success rate or recurrence. Perhaps this can be explained by the small sample size. Interestingly, just a quarter of the removed meshes (11/44) were in an adequate position whereas 19 meshes were wrinkled and 14 had a meshoma. An inadequate covering of hernia orifices due to wrinkling was found in more than half of the patients (23/44), surprisingly only 3 demonstrated a recurrence during surgery. This might suggest that the presence of mesh, even with inadequate coverage, was still able to help prevent a recurrent hernia in most patients. Another option could be that reduction of the hernia alone appears to be sufficient in some cases or that there never was a hernia at primary surgery. A German study describing open and laparoscopic mesh removal found similar results regarding position and location of pre-peritoneal meshes [23]. In their series, all meshes were folded and in 4 of the 24 patients demonstrated recurrence intraoperatively. It is unknown whether an incorrect mesh position predisposes to wrinkling, or whether wrinkling in itself leads to CPIP, but it is tempting to believe so. Imaging in control patients who do not suffer from CPIP may shed light on these issues.
During follow-up, four recurrent hernias were objectified by a physician and seven additional patients reported complaints potentially caused by a recurrence Although there is no mesh remaining, it is surprising that this did not lead to a hernia recurrence for most patients. When we performed our initial cases, we considered reinforcing the abdominal wall with an open mesh technique simultaneously. It would, however, not be possible to judge the effect of removal on pain if a second procedure was added. Considering the current data, recurrence rates are rather low. A longer follow-up may potentially result in a higher number of recurrences following mesh removal. Up till now, we have no argument for a simultaneous reinforcement.
We found a significant reduction in sick days after removal. In the period of six months prior to surgery, work absence was reported to be 53 days (mean). In six months of follow-up, absence was reported 38 days (mean). So besides a personal benefit for patients in reduction and of pain and a better quality of life, there also is a relevant socioeconomic benefit.
Limitations and recommendations
A few limitations of this study have to be discussed. Response rate was 77% at follow-up which may reduce the power of our findings. However, non-responders did not differ significantly from the responders considering baseline characteristics and intraoperative data. In spite of this, selection bias may have occurred, which may have influenced the results of the mesh removal in either a positive or a negative way. It is possible that patients with severe pain refused to respond because they were dissatisfied. On the other hand, non-responders could also be relieved of pain and therefore, were no longer interested to participate in this study. Due to the cross-sectional design of the study, there was a large spread in the follow-up period, potentially leading to recall bias. However, we did not find a relationship between the follow-up interval and the results of mesh removal. In patients with longer follow-up, there is a greater chance that other treatments have had an effect on pain. It is a legitimate question whether the eventual result in these patients can be explained by a late effect of mesh removal or by to other treatments. Nevertheless, we found a significant difference in success rate between patients with and without additional pain therapies. Though only tree patients were operated for recurrent hernia after removal a true incidence of recurrence cannot be determined without physical or radiological examination.
From personal communications, we know that there are others that perform this technique. Still we recommend that more hernia clinics should focus on the removal of pre-peritoneal inguinal meshes. From our experience, respecting the learning curve, laparoscopic mesh removal should be in the hands of hernia experts. We encourage colleagues to contact us for more detailed information on this technique, to exchange knowledge and establish a network dedicated to this complicated pain problem following inguinal hernia repair.
Conclusion
Laparoscopic mesh removal for CPIP after pre-peritoneal inguinal hernia repair is not a new procedure. The present study is the first to report on success rates of this procedure, and results are satisfying. Pain scores reduced and quality of life improved significantly. We demonstrated that laparoscopic mesh removal is an effective procedure in selected patients with limited complications.
References
Kingsnorth A, LeBlanc K (2003) Hernias: inguinal and incisional. Lancet 362(9395):1561–1571. https://doi.org/10.1016/S0140-6736(03)14746-0
HerniaSurge G (2018) International guidelines for groin hernia management. Hernia 22(1):1–165. https://doi.org/10.1007/s10029-017-1668-x
Kulacoglu H (2011) Current options in inguinal hernia repair in adult patients. Hippokratia 15(3):223–231
Bozuk M, Schuster R, Stewart D, Hicks K, Greaney G, Waxman K (2003) Disability and chronic pain after open mesh and laparoscopic inguinal hernia repair. Am Surg 69(10):839–841
Koning GG, Wetterslev J, van Laarhoven CJ, Keus F (2013) The totally extraperitoneal method versus Lichtenstein’s technique for inguinal hernia repair: a systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. PLoS ONE 8(1):e52599. https://doi.org/10.1371/journal.pone.0052599
Nienhuijs S, Staal E, Strobbe L, Rosman C, Groenewonld H, Bleichrod R (2007) Chronic pain after mesh repair of inguinal hernia: a systematic review. Am J Surg 194(3):394–400. https://doi.org/10.1016/j.amjsurg.2007.02.012
Simons MP, Aufenacker T, Bay-Nielsen M et al (2009) European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia 13(4):343–403. https://doi.org/10.1007/s10029-009-0529-7
Poobalan AS, Bruce J, King PM, Chambers WA, Krukowski ZH, Smith WC (2001) Chronic pain and quality of life following open inguinal hernia repair. Br J Surg 88(8):1122–1126. https://doi.org/10.1046/j.0007-1323.2001.01828.x
Aasvang E, Kehlet H (2005) Chronic postoperative pain: the case of inguinal herniorrhaphy. Br J Anaesth 95(1):69–76. https://doi.org/10.1093/bja/aei019
Grant AM, Scott NW, O’Dwyer PJ (2004) Group obotMLGHT Five-year follow-up of a randomized trial to assess pain and numbness after laparoscopic or open repair of groin hernia. BJS 91(12):1570–1574. https://doi.org/10.1002/bjs.4799
Wegdam JA, de VriesReilingh TS, Nienhuijs SW, Simons MP (2020) Abdominal wall hernia surgery in The Netherlands: a national survey. Hernia 24(3):601–611. https://doi.org/10.1007/s10029-019-02048 (-x)
Slooter GD, Zwaans WAR, Perquin CW, Roumen RM, Scheltinga MR (2018) Laparoscopic mesh removal for otherwise intractable inguinal pain following endoscopic hernia repair is feasible, safe and may be effective in selected patients. Surg Endosc 32(3):1613–1619. https://doi.org/10.1007/s00464-017-5824-2
Ramshaw B, Vetrano V, Jagadish M, Forman B, Heidel E, Mancini M (2017) Laparoscopic approach for the treatment of chronic groin pain after inguinal hernia repair : Laparoscopic approach for inguinodynia. Surg Endosc 31(12):5267–5274. https://doi.org/10.1007/s00464-017-5600-3
Sharma R, Fadaee N, Zarrinkhoo E, Towfigh S (2018) Why we remove mesh. Hernia 22(6):953–959. https://doi.org/10.1007/s10029-018-1839-4
Zwaans WA, Perquin CW, Loos MJ, Roumen RM, Scheltinga MR (2017) Mesh removal and selective neurectomy for persistent groin pain following lichtenstein repair. World J Surg 41(3):701–712. https://doi.org/10.1007/s00268-016-3780-y
Hawker GA, Mian S, Kendzerska T, Frensh M (2011) Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 63(Suppl 11):S240–S252. https://doi.org/10.1002/acr.20543
Kumar SP (2011) Utilization of brief pain inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J Palliat Care 17(2):108–115. https://doi.org/10.4103/0973-1075.84531
Gandek B, Ware JE, Aaronson NK et al (1998) Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project International Quality of Life Assessment. J Clin Epidemiol 51(11):1171–1178. https://doi.org/10.1016/s0895-4356(98)00109-7
Darnall BD, Sturgeon JA, Cook KF, Taub CJ, Roy A, Burns JW, Sullivan M, MackeySC (2017) Development and validation of a daily pain catastrophizing scale. J Pain 18(9):1139–1149. https://doi.org/10.1016/j.jpain.2017.05.003
Bjelland I, Dahl AA, Haug TT, Neckelmann D (2002) The validity of the hospital anxiety and depression scale an updated literature review. J Psychosom Res 52(2):69–77. https://doi.org/10.1016/s0022-3999(01)00296-3
Amid PK (2004) Radiologic images of meshoma: a new phenomenon causing chronic pain after prosthetic repair of abdominal wall hernias. Arch Surg 139(12):1297–1298. https://doi.org/10.1001/archsurg.139.12.1297
Truong A, Al-Aufey BS, Towfigh S (2019) Step-by-step guide to safe removal of pre-peritoneal inguinal mesh. Surg Endosc 33(8):2680–2685. https://doi.org/10.1007/s00464-018-6558-5
Arlt GD, Lamm T, Klosterhalfen B (2003) Mesh removal in inguinal hernia repair. Eur Surg 35(1):42–44
Funding
No funding was used to support this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study was conducted according to the Declaration of Helsinki and the guidelines of Good Clinical Practice (World Medical Association, 2013). Written informed consent was obtained from all patients. The study was approved by the local ethics committee of Máxima MC, protocol number N19.008.
Conflict of interest
Charlotte Slooter declares no conflict of interest in the manuscript. Christel Perquin declares no conflict of interest in the manuscript. Willem Zwaans declares no conflict of interest in the manuscript. Rudi Roumen declares no conflict of interest in the manuscript. Marc Scheltinga declares no conflict of interest in the manuscript. Gerrit Slooter declares no conflict of interest in the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Slooter, C.D., Perquin, C.W., Zwaans, W.A. et al. Laparoscopic mesh removal for chronic postoperative inguinal pain following endoscopic hernia repair: a cohort study on the effect on pain. Hernia 27, 77–84 (2023). https://doi.org/10.1007/s10029-022-02712-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-022-02712-9