Abstract
Purpose
Ventral hernia risk score (VHRS) is a risk assessment tool for predicting the development of surgical site infection (SSI) developed in the Veterans Affairs population by Berger et al. The score was externally validated by the same study group in a diverse population in another study. It was also shown to be better than the existing Centre for Diseases (CDC) wound class and Ventral Hernia Working Group (VHWG) models. Our study aims to test the performance of the score in an Asian-Indian population.
Methods
A prospective database of ventral hernia repairs done in a tertiary care centre between February 2019 and December 2020 was utilized for the study. All patients with a minimum follow-up of 1-month period were included in the study. The CDC definition of SSI was used. The VHRS, VHWG, and CDC class of each of the patients was determined. Receiver-Operating curves (ROC) of the scores and area under the curves (AUC) were used to compare the three scores.
Results
A total of 120 patients were included. During the course of our study, a total of 33 patients developed SSI (27.5%). Important factors which seemed to predict SSI were median operating time, CDC incision class, concomitant hernia repair, and creating skin flaps. The AUC of the VHRS score was 0.76 which was higher than those of VHWG (0.61) and CDC (0.58).
Conclusion
Our study externally validates the novel VHRS which outperforms both CDC incision class and VHWG in predicting SSI following open ventral hernia repair, especially in a group with lower BMI compared to the previous reports.
Trial registration No CTRI/2020/07/026289 registered on 01/07/2020.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ventral hernia repair (VHR) is one of the most commonly performed general surgery procedures [1]. Surgical site infection (SSI) following VHR can result in significant morbidity and hospital cost. Currently, there are a few models in practice for stratifying patients at risk of SSI [2, 3]. The commonly used and popular one is the Centre for Disease Control (CDC) incision class [4]. Another risk score that is used specifically for ventral hernia repair surgery is the Ventral Hernia Working Group (VHWG) model [5]. Both these scoring systems aim to predict the development of surgical site infection following ventral hernia repairs [6]. However, there are limitations to both these scoring systems [7, 8]. The drawback of the CDC scoring system is that it is non-specific. The main problem with VHWG grade is that it is based on expert opinion following literature search, rather than based on direct patient data.
So Berger et al. set out with the goal of identifying the factors associated with the development of SSI in patients undergoing hernia repair. Based on data from Veterans Affairs (VA) population, they developed a prognostic risk-assessment score for predicting SSI—the Ventral Hernia Risk Score (VHRS) [9]. They built stepwise regression models to identify the risk factors and internally validated them using bootstrapping. Based on the odds ratio of the risk factors, they devised a scoring system. And depending on the score, the patients were divided into five groups depicting their risk for SSI. This scoring system was externally validated by the same group in another study [10].
However, these studies were done primarily in the Caucasian population and we need external validation from other ethnic groups [11]. So we tested the validity of the scoring system in the Asian-Indian population.
Methodology
Study design
The study was a prospective observational study that was conducted in a tertiary care center. The study was approved by the institutional ethics committee (JIP/IEC/2018/484). This trail was registered in Clinical Trail Registry India (CTRI/2020/07/026289).
Study participants
All patients undergoing open ventral hernia repair between February 2019 and December 2020 were enrolled for the study after obtaining informed consent. Patients who were below 18 years or did not consent were excluded from the study.
Sample size
Taking the area under the receiver operating characteristic (ROC) curve of the tool from the previous study to be 0.71 and keeping the incidence of SSI to be 20%, power 80%, and confidence interval of 95%, the sample size was calculated to be 108 using MedCalc version 18.2.1. Considering a 10% non-response rate, the total sample size was calculated to be 120.
Study procedure
Patients who were admitted for open ventral hernia repair were followed up during the course of their hospital stay and for a minimum period of one month post-operatively. Their demographic details(gender), preoperative details(BMI, co-morbidities, smoking habit, previous surgical history) and intra-operative details (concomitant hernia repair, creating skin flaps, duration of surgery, placement of mesh) were collected. Once the preoperative and intra-operative details were available, the VHRS score was calculated. Concomitant hernia repair was defined as those repaired during a procedure for another surgical indication [9]. The fascial release was defined as the release of fascia through incisions to achieve medial release and closure. Creating skin flaps was defined as the dissection done deeper to the subcutaneous plane to create a plane for placement of mesh. Whenever mesh repair was done, we used onlay meshplasty technique in all our patients.
The VHWG grade was also given for each patient-based on their co-morbidities, active smoking, immunosuppressed status, COPD, earlier wound infections, stomas, and presence of infected mesh [12]. Immunosuppression was defined as the use of medications that can suppress immunity such as chemotherapy, corticosteroids, etc. [13, 14].
Following this, the patients were followed up for 1 month on an outpatient basis, during their routine post-operative visits to the outpatient division (OPD) and surgery casualty. Patients who developed SSI, as defined by CDC, were managed according to the grade of the SSI [4]. The nature of the SSI was recorded into the original database containing the baseline details of the patients along with their VHRS and VHWG grades.
Outcome measures
The primary objective of the study was to externally validate the VHRS in predicting surgical site infections in open ventral hernia repair. The secondary objective was to compare the score to VHWG and CDC incision class.
The study population of the original research by Berger et al., from which the VHRS was developed, was taken as our development cohort [9]. Our patient population in which we conducted the study to validate the VHRS externally is our validation cohort. We compared the SSI rates of these two populations in our study.
Statistical analysis
All of the statistical analyses were done using the STATA software Version 14.0. The patients were split into two groups—those who developed SSI and those who did not develop SSI. The difference between the two groups was compared using statistical tests as follows: Variables were compared using the Pearson Chi-square test, and two-sample Wilcoxon rank-sum test (Mann–Whiney) depending upon the nature of the variables. The predictive accuracy of the VHRS was assessed using the receiver-operating curves (ROC) and precision–recall curve (PRC). The area under the curve (AUC) for VHRS obtained from our study was compared with that of the previous study done for the same scoring system.
Results
A total of 120 patients who underwent open ventral hernia repair were enrolled for the study and their details were collected. They were followed up for a period of 1 month after their surgery during their routine hospital visits to the outpatient division in the post-op period. Overall, the rate of surgical site infection in patients following ventral hernia repair in our study group was 27.5% which was higher than the expected rate of 5.6 to 7.6% [2, 15].
The baseline characteristics of the patients who developed surgical site infection and those who did not develop surgical site infection are given in Table 1. The number of patients with chronic obstructive pulmonary disease (COPD), recurrent hernia repair, and previous history of surgical site infection differed between the two groups significantly (P = 0.03; 0.006; 0.07).
Patients were divided into five risk groups based on their scores. As with the development cohort, the VHRS was strongly associated with the rates of SSI in our validation cohort. The rate of SSI in the initial group 1 is comparable with that of the development cohort. However, in the latter groups, the rates of SSI increase substantially (Table 2).
The odds ratio for each component of the VHRS obtained from our external validation cohort is compared with those from the original study in Table 3. The odds ratios were obtained for concomitant hernia repair, creating skin flaps, and wound class more than 4. The odds ratio arrived for these factors was much higher than those obtained in the original study. The odds ratios of two factors (ASA score more than or equal to 3 and BMI more than or equal to 40) could not be assessed due to a small number of patients in that category. The number of patients with an ASA score of 3 was just one in the entire study group of 120 patients. And there were no patients with a BMI of 40 or more in our study group.
Other important surgical variables of the two groups of patients are summarized in Table 4. The median operative time is significantly higher in those who developed an SSI (P = 0.025). The CDC incision class also seems to be differing significantly between the two groups. Patients with higher wound class were associated with higher rates of SSI (P = 0.005). Performing concomitant hernia repair also seems to increase the rates of surgical site infection (P = 0.002). Ten of the ventral hernia repair were done concomitant with other surgeries. Two of them were done during laparoscopic cholecystectomy. One ventral hernia repair was done with inguinal hernia repair and one was done alongside rectal polyp excision. Four of the remaining concomitant repairs were done during resection and anastomosis of bowel segments. Five of these patients undergoing concomitant repair underwent a meshplasty procedure. Of these five patients, four patients eventually developed SSI. Creating skin flaps during hernia repair was another factor that seemed to be associated with surgical site infection (P = 0.002). Placement of mesh for repair was also shown to have higher rates of surgical site infections (P = 0.007). Of all these factors mentioned above, two factors—concomitant hernia repair and creating skin flaps are components of the Ventral Hernia Risk Score (VHRS) that is being externally validated in our study [9]. In our study, whether the surgery was done in an elective or emergency setting did not affect the rates of SSI in patients. One of the patients who underwent ventral hernia repair had postoperative deep vein thrombosis. The patient was managed with anticoagulants and conservative management. One patient who developed a wound infection had to be re-admitted and managed. No other postoperative complications were recorded in our study.
The ventral hernia working group grade was calculated for each patient in our study population. The development of SSI in each of the groups was calculated as well (Table 5). The VHWG grade seemed to differ significantly between the two groups (P = 0.016), highlighting the fact that the system did predict the development of SSI in those undergoing ventral hernia repairs.
The area under the curve (AUC) for the VHRS obtained from the ROC was 0.75 which was similar to the AUC obtained in the original development cohort (0.71) And AUC for VHWG was 0.609 and for CDC class was 0.58.
The AUC for the VHRS from the Precision-Recall Curve(PRC) was 0.539, slightly higher than for VHWG and CDC, which were 0.404 and 0.470, respectively.
Discussion
As seen from the results discussed previously, our study externally validated the Ventral Hernia risk score for its predictive ability to predict SSI after open ventral hernia repair. The original study was conducted in a large population (n = 888) and it was a retrospective study [9]. The same study group also did an external validation study to check the applicability of the score in other hospital settings with a sample size of 436 [10]. Compared to the original study population, our sample population was much different. For example, our study group had more females than males. The mean BMI was much less compared to the study population (24 vs. 30). Despite these differences, the VHRS was shown to accurately predict SSI in our study population and it did so better than the VHWG score and CDC incision class.
Comparing the baseline characteristics of patients with SSI and without SSI revealed they were comparable in most aspects (Table 1). A significant difference was noted in the number of COPD patients between the groups. This association between COPD and SSI described previously, was established from our analysis as well [16, 17]. A possible explanation was the use of steroids in these patients which could predispose them to infections. Although locally used steroids were unlikely to have systemic immune suppression, nothing else explained the association between higher rates of infection seen in COPD patients [14, 18]. The other baseline factors which differed between the two groups were the previous history of SSI and the previous history of meshplasty. The patients with previous history of SSI would have the same risk factors persisting, to cause the SSI again. As for those who were undergoing repeat surgery, the natural planes of dissection were lost after the first surgery due to scarring and fibrosis. This resulted in the increased dissection of tissues, prolonged surgery, and a higher chance of SSI.
One of the component of VHRS is concomitant hernia repair. In the present study, the surgeries which were done concomitantly with ventral hernia repair were laparoscopic cholecystectomy, resection and anastomosis, inguinal hernia repair, and rectal polyp excision. In patients who underwent resection and anastomosis, rectal polyp excision, there was a potential risk of wound exposure to luminal contents explaining the higher rates of SSI seen in these patients. Duration of surgery and number of operating surgeons are other established factors that would contribute to SSI in these concomitant hernia repairs [19,20,21]. But the latter was not assessed separately in our study.
The second factor, creating skin flap, was also associated with a significant increase in SSI risk. The dissection that is carried out in the process of creating a flap to accommodate the mesh created a potential dead space where seroma or hematoma could accumulate and predispose to SSI. The other problem with flaps was the disruption of vascularity which would have resulted in the development of SSI.
The ASA score of the patient had been added as a factor in the VHRS. This can be considered a blanket factor for several other risk factors. For instance, well-known predisposing conditions for SSI such as diabetic status, obesity, immunosuppression were not taken into consideration separately in VHRS. Instead, the ASA score took care of all these factors simultaneously. However, the problem encountered in our study was that there was only one patient with an ASA score of 3 or more. This was because the patients undergoing ventral hernia repair in our hospital were taken up for surgery in the elective setting after adequate optimization. Those who underwent the procedure in the emergency setting were few and even those cases were taken up for surgery before decompensation. So we were unable to comment on the role of this particular factor in our patients.
The fourth factor in VHRS is BMI more than 40. BMI of more than 40 has been classified as morbid obesity in the western population according to the World Health Organization guidelines [22]. However, for the Asian population, the appropriate value is 37.5 [23]. But even then the highest BMI recorded in our study population was 32.5 and only two patients in our study group had a BMI of more than 30. Our study was conducted in a government institute with free medical service, which primarily caters to patients hailing from economically weaker sections of the community. This could explain the lower mean BMI of our patients as compared to the original study group. A detailed nutritional assessment of our study group might have helped better SSI prediction and offered more insights into the nutritional aspects of our patients. However, our study aimed to check if the scoring system applied to our population without any modifications, thereby maintaining its simplicity of usage. So we restricted our assessment to BMI alone, which alone has been shown to have a good ability to assess the nutritional status of patients [24]. A modified score with a BMI cut-off of 37.5 would be a more meaningful scoring system in our Asian-Indian population.
The CDC incision class 4 was the factor that was given the highest points in the scoring system due to its consistent association with the development of SSI. In our study as well, the factor was associated with high rates of SSI. This is portrayed in Tables 3 and 4, which proves beyond doubt the significant association between wound class 4 and SSI. In CDC incision class, wound class 4 translates to dirty wound. It means that the incision site was already teeming with bacteria. These wounds were at the highest risk of developing SSI [8]. So it is no surprise that in our study wound class 4 showed the highest association with SSI.
The odds ratio for concomitant repair in our study was 7.53, which was much greater than the odds ratio from the original study and the previous external validation study. The odds ratio for the factor-creating skin flap was 4.02 which was twice the number from the original study. And for wound class 4 the odds ratio was 15.36, which was more than twice the number in the original study. The confidence intervals calculated for these ratios were also quite wide. This may be explained by the small sample population used in our study group. The sample size was calculated only to test the predictive accuracy of VHRS score for detecting SSI using ROC curve. The sample size was not targeted to obtain the odds ratio for the individual factors of the VHRS score. The factor which occurred most frequently in our study was the creation of skin flaps. The odds ratio and the confidence interval arrived in our study for this particular factor were relatively closer to the numbers from the original study. We can safely presume that in a population of adequate size the odds ratios and confidence intervals tend to become more reliable for the other factors too. Conversely, the odds ratios arrived in our study only highlight how important a role these factors play in our population [9, 10].
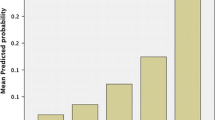
The receiver-operating curve and area under the curve were calculated for VHRS, VHWG, and CDC incision class. As seen from the results, the VHRS score is an accurate predictor of surgical site infection. And the score performed better than the other two scores that it was being compared with. The AUC ROC for the VHRS was 0.755, indicating good performance in discriminating cases with SSI and without SSI (Fig. 1).
As there was an imbalance in the cases, we did precision–recall curve analysis for the three scoring systems. The analysis showed poor performance for the VHWG and CDC scores and a slightly better performance for the VHRS scoring system. This shows that the VHRS performs better even in strict analytic conditions and calls for further studies with a larger sample size.
The CDC incision class system classifies the wounds based on the infection status of the incision site. The system concentrates on the local factor that determines the risk of infection. And as evident from our data, it is the most important factor determining the chances of developing SSI. Hence even as a standalone score, it predicted SSI to a good extent. The area under the curve for this score was 0.58 and the curve was marginally above the reference line of our ROC. Thus the scoring system worked, but not as accurate as of the VHWG or the VHRS.
The VHWG grade is broader than the CDC incision class in the sense that it considers more factors. This helps the VHWG grade to predict SSI more reliably than the CDC incision class, as seen from the ROC. The VHRS covers more risk factors for SSI than the VHWG and this translates to better predictability of SSI. Hence the VHRS outperforms the other two scores in our study as expected.
We also studied the effect of few other surgical factors which might affect surgical site infection. Important factors that were affecting the surgical site infection apart from those included in VHRS were the placement of mesh prosthesis and duration of surgery. As seen from Table 4, a longer duration of surgery resulted in higher rates of SSI in our study, just as noted from the literature [19,20,21]. The longer the wound remains exposed to the environment, the more likely it is to get infected. Placement of prosthetic mesh was the other important factor that was associated with higher SSI. The SSI rate in those who underwent meshplasty in the current study was significantly higher than those who underwent only anatomical repair, similar to previous studies [25]. Placement of a mesh requires the creation of skin flaps for accomodating the mesh. Meshplasty itself takes a longer operating time than an anatomical repair. All these factors resulted in an increased rate of SSI in meshplasty patients.
Our study had its limitations. As we have not calculated the sample size for the individual components of the VHRS, we could not check the validity of the odds ratios for it. Since there was a lack of diversity in our study population, the results should be interpreted with caution. We did not have adequate patients with BMI more than 40 or ASA grade 3 and above, so their odds ratios could not be commented on from our study. Despite these limitations, the VHRS was able to predict SSI reliably in our study.
Conclusion
The Ventral Hernia Risk Score (VHRS) was able to predict surgical site infections reliably in our study populations with good accuracy. VHRS is more useful than CDC incision class or VHWG, as a result of examining in the group with lower BMI compared to previous reports. Further studies are required before we can comment on the generalizability of the ventral hernia risk score. One area that needs to be investigated in our population is the modification of BMI cut-off in the score. With that modification, VHRS has the potential to be widely adopted into clinical practice in the form of a conventional checklist for screening patients planned for open ventral hernia repair. Mobile applications incorporating the score can provide avenues for the surgeon and the patient to interact and work in unison on pre-set goals for pre-op optimization.
References
Poulose BK, Shelton J, Phillips S, Moore D, Nealon W, Penson D et al (2012) Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia 16(2):179–183
World Health Organization (2016) Global guidelines for the prevention of surgical site infection. World Health Organization, Geneva
Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK et al (2013) Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 173(22):2039
9 Surgical Site Infection (SSI) Event. 2020;36.
Hodgkinson JD, Worley G, Warusavitarne J, Hanna GB, Vaizey CJ, Faiz OD (2021) Evaluation of the Ventral Hernia Working Group classification for long-term outcome using English Hospital Episode Statistics: a population study. Hernia J Hernias Abdom Wall Surg. 25:977–984
Kanters AE, Krpata DM, Blatnik JA, Novitsky YM, Rosen MJ (2012) Modified hernia grading scale to stratify surgical site occurrence after open ventral hernia repairs. J Am Coll Surg 215(6):787–793
Brahmbhatt R, Carter SA, Hicks SC, Berger DH, Liang MK (2014) Identifying risk factors for surgical site complications after laparoscopic ventral hernia repair: evaluation of the Ventral Hernia Working Group grading system. Surg Infect 15(3):187–193
Ortega G, Rhee DS, Papandria DJ, Yang J, Ibrahim AM, Shore AD et al (2012) An evaluation of surgical site infections by wound classification system using the ACS-NSQIP. J Surg Res 174(1):33–38
Berger RL, Li LT, Hicks SC, Davila JA, Kao LS, Liang MK (2013) Development and validation of a risk-stratification score for surgical site occurrence and surgical site infection after open ventral hernia repair. J Am Coll Surg 217(6):974–982
Liang MK, Goodenough CJ, Martindale RG, Roth JS, Kao LS (2015) External validation of the ventral hernia risk score for prediction of surgical site infections. Surg Infect 16(1):36–40
Matthews BD, Poulose BK, Rosen MJ (2014) Ventral hernia risk score: the importance of patient selection and technique. J Am Coll Surg 218(5):1075–1076
Ventral Hernia Working Group, Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS et al (2010) Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery 148(3):544–558
Meidani M, Naeini AE, Rostami M, Sherkat R, Tayeri K (2014) Immunocompromised patients: review of the most common infections happened in 446 hospitalized patients. J Res Med Sci Off J Isfahan Univ Med Sci 19(Suppl 1):S71–S73
Sabroe I, Postma D, Heijink I, Dockrell DH (2013) The yin and the yang of immunosuppression with inhaled corticosteroids. Thorax 68(12):1085–1087
Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L et al (2011) Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet Lond Engl 377(9761):228–241
Kaye KS, Sloane R, Sexton DJ, Schmader KA (2006) Risk factors for surgical site infections in older people. J Am Geriatr Soc 54(3):391–396
Lee Y-P, Feng M-C, Wu L-C, Chen S-H, Chen Y-H, Chiu C-C et al (2010) Outcome and risk factors associated with surgical site infections after cardiac surgery in a Taiwan medical center. J Microbiol Immunol Infect Wei Mian Yu Gan Ran Za Zhi 43(5):378–385
Arbid SA, El-Khoury H, Jamali F, Tamim H, Chami H (2019) Association of preoperative systemic corticosteroid therapy with surgical outcomes in chronic obstructive pulmonary disease patients. Ann Thorac Med 14(2):141–147
Cheng H, Chen BP-H, Soleas IM, Ferko NC, Cameron CG, Hinoul P (2017) Prolonged operative duration increases risk of surgical site infections: a systematic review. Surg Infect 18(6):722–735
Poruk KE, Hicks CW, Trent Magruder J, Rodriguez-Unda N, Burce KK, Azoury SC et al (2017) Creation of a novel risk score for surgical site infection and occurrence after ventral hernia repair. Hernia J Hernias Abdom Wall Surg 21(2):261–269
Mukagendaneza MJ, Munyaneza E, Muhawenayo E, Nyirasebura D, Abahuje E, Nyirigira J et al (2019) Incidence, root causes, and outcomes of surgical site infections in a tertiary care hospital in Rwanda: a prospective observational cohort study. Patient Saf Surg 13:10
Purnell JQ. Definitions, Classification, and Epidemiology of Obesity. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000. http://www.ncbi.nlm.nih.gov/books/NBK279167/. Accessed 20 May 2021
Shiwaku K, Anuurad E, Enkhmaa B, Kitajima K, Yamane Y (2004) Appropriate BMI for Asian populations. Lancet Lond Engl 363(9414):1077
Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S et al (2015) Diagnostic criteria for malnutrition—an ESPEN Consensus Statement. Clin Nutr Edinb Scotl 34(3):335–340
Liang MK, Holihan JL, Itani K, Alawadi ZM, Gonzalez JRF, Askenasy EP et al (2017) Ventral hernia management: expert consensus guided by systematic review. Ann Surg 265(1):80–89
Acknowledgements
The authors would like to thank Dr. Stuti Pramod, Junior Resident, Department of Preventive and Social Medicine (PSM), Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry, India, for her help in the statistical analysis
Funding
Nil.
Author information
Authors and Affiliations
Contributions
SA did the acquisition and analysis of the data; AA contributed to the conception and design of the research, interpretation of the data; SS contributrd in analysis and interpretation of data, drafting the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Subramaniyan Ashuvanth, Amaranathan Anandhi and Sathasivam Sureshkumar don’t have any conflict of interest.
Ethical approval
The study was approved by the institutional ethics committee (JIP/IEC/2018/484). This trail was registered in Clinical Trail Registry India (CTRI/2020/07/026289).
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
All patients undergoing open ventral hernia repair between February 2019 and December 2020 were enrolled for the study after obtaining informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ashuvanth, S., Anandhi, A. & Sureshkumar, S. Validation of ventral hernia risk score in predicting surgical site infections. Hernia 26, 911–917 (2022). https://doi.org/10.1007/s10029-021-02537-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-021-02537-y