Abstract
Background
Transabdominal preperitoneal (TAPP) inguinal hernia repair requires the surgeon to have good manual skills in laparoscopic surgery, as well as an understanding of the laparoscopic features of the groin anatomy. This is why TAPP is considered a more difficult surgical procedure compared to open techniques. Realistic training model for TAPP inguinal hernia repair would enhance surgeons’ skills before they enter in the operation room. Our aim was to create a realistic, inexpensive, and easily reproducible model for laparoscopic TAPP inguinal hernia repair and to assess its effectiveness.
Methods
The applied TAPP inguinal hernia repair training simulator consists of a laparoscopic box and an inguinal region model placed in it. The model of the groin area is made of the porcine stomach and assembling materials. Uniaxial tensile and T-peel tests were performed to compare the mechanical properties of the porcine stomach and the human cadaver peritoneum. Thirty eight surgeons performed TAPP inguinal hernia repair using this model. Their opinions were scored on a five-point Likert scale.
Results
Close elastic modules of the porcine and human tissues (13.5 ± 4.2 kPa vs. 15.8 ± 6.7 kPa, p = 0.531) gave to trainees a realistic tissue feel and instrument usage. All participants strongly agreed that model was highly useful for TAPP inguinal hernia repair training. They also put the following points: the model as a whole 5 (3–5), simulation of anatomy 5 (3–5), simulation of dissection and mobilization 5 (3–5), and simulation of intracorporeal suture 5 (4–5).
Conclusions
We successfully created a model for TAPP inguinal hernia repair training. The model is made of inexpensive synthetic and biological materials similar to the human tissue. The model is easy to reproduce and can be used in the training programs of surgical residents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inguinal hernia repair is one of the most common operations performed by general surgeons. TAPP inguinal hernia repair is becoming increasingly frequent due to the lower incidence of postoperative pain, the lower chance of mesh infection, and faster patient recovery compared to open techniques. When performed by experienced surgeons, laparoscopic TAPP for inguinal hernia repair is an operation of choice for bilateral and recurrent hernias after open surgery procedures [1].
Despite the proven advantages in current guidelines, the widespread introduction and use of TAPP operation has not yet been noted [2]. Recent studies showed the low rate of utilization of minimally invasive techniques for inguinal hernia repair ranges from 10 to 48% [3], and almost half of the surgeons have never performed laparoscopic operations for inguinal hernias [4].
The main problem for introduction of TAPP is the lack of required manual skills for this procedure [5]. At the same time, the loss of tactile feedback has created a prolonged learning curve for this approach [6]. It should not forget the absence of a similar open procedure, which also makes learning more difficult. Moreover, laparoscopic TAPP inguinal hernia repair is one of the few operations, which must be completed totally laparoscopically, and it is impossible to perform a conversion.
In this aspect, TAPP procedure simulation models should play an invaluable role, being as close as possible to live surgery, allowing trainee to feel the features of dissection in the preperitoneal space, the difficulty in positioning of the mesh, and applying intracorporeal suture on the peritoneum [7].
All hopes for virtual simulators failed, because they do not allow us to feel feedback and have low efficiency for such kind of procedure [8]. Unfortunately, training on cadavers is a complex problem and very expensive [9]. In this regard, only specially created models that maximally reflect the features and feedback of the dissection during an operation can have an importance.
We think that an inexpensive and realistic model could improve the learning outcomes and make laparoscopic TAPP inguinal hernia repair more accessible for surgeons.
In this study, we aimed to create a biological model for the main stages of laparoscopic TAPP inguinal hernia repair training and to assess its efficiency.
Materials and methods
Model creation
We made our model using the biological tissue and synthetic materials: a large flap of the porcine stomach, a polyurethane board, and polyethylene tubes of different diameters and rigidity.
Creation of the biomodel includes four stages: preparation of biomaterial, the creation of an inguinal area simulating board made of polyurethane foam and synthetic tubes, the connection of the gastric flap and the board, and the placement of the model in a laparoscopic box.
Preparation of biomaterial
The fresh porcine stomach was washed with water through the stump of the esophagus. With straight scissors, we cut the stomach along a small curvature and set it on the table with a mucous layer outside. We made incisions of about 5 cm along a large curvature to straighten the folds of the stomach. The flap of the stomach was placed on a board and the edge excess of tissue was cut off. The prepared gastric flaps were laid in a straightened form on top of each other in a plastic box. A preserving solution was added to the box, which consisted of three equal parts of alcohol, glycerin, and distilled water. After 2–3 h, boxes with tissues were placed in a refrigerator with a temperature of +6 ℃. The next day, the tissues were ready for use. After such preparation, the tissues can be stored for up to 2 weeks at a temperature of 6–8° without loss of their properties.
The creation of an inguinal area simulating board
Schematic view of the model is shown in Fig. 1a. We used a board of polyurethane foam with a thickness of about 2 cm and a size of 33 by 24 cm. Tubes of different diameters which simulated the basic anatomical structures of the inguinal region were placed on the board (Fig. 1b). A thick-walled tube, 10 mm in diameter and about 20 cm long, was used to simulate the iliopubic tract. It was located in the oblique longitudinal direction: 5 cm to the left and 8 cm to the right above the lower edge and fastened with 5–6 sutures to the board. A thin-walled elastic tube, 4–5 mm in diameter, was used to simulate the epigastric artery. The tube that simulates the artery was filled with red water-soluble paint and attached transversely to the left side of the board. Soft thin tubes, 3 mm in diameter, that simulate the vas deferens (filled with white water-soluble paint) and the spermatic vessels (filled with red water-soluble paint) were fixed above the tube of the iliopubic tract. The board was ready to be connected to the gastric flap after fixing all the tubes.
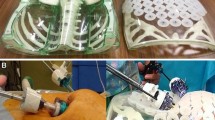
Schematic view (a) and stages of TAPP hernia repair model preparation; b the tubes for simulation of the basic anatomical structures of the inguinal region placed on the board; c, d pulling of the tubes through the tunnels in submucosal layer; e the model placed to the laparoscopic box; f laparoscopic view of the created model with marked anatomical structures
Creation of a biomodel
The porcine stomach flap was placed on the board. The upper part of the flap was fixed with 2–3 seams to the board to prevent its displacement during manipulation. Then, in the lower part of the flap, we created tunnels between the seromuscular and mucosal layers and carried through them the tubes simulating the artery and the vas deferens (Fig. 1c, d). The stomach tissue in the central part was fixed to the board with six seromuscular sutures without a submucosal layer. Then, a flap of the stomach was fixed along the edge of 8–10 sutures through all layers to the polyurethane foam board. Thus, a biomodel of the right inguinal region was created. Prepared biomodels for prevention of drying were covered with a semi-moist cloth and placed in tightly closed boxes, in which they were stored from several hours to 3 days.
Placement of the model
Before training, the prepared biomodel was placed into a box under the control of a laparoscope (Fig. 1e, f). The biomodel was placed at an angle, which is similar to the angle of the anterior abdominal wall of the patient during the laparoscopic TAPP inguinal hernia repair. After placing the model, we fixed it to the bottom and top of the box.
The simulator consists of a plastic box located on a table, which simulates the patient's position on the operating table. The surgeon and the assistant stand in front of the box. They could stand in a position similar to laparoscopic TAPP inguinal hernia repair and use laparoscopic tower and instruments normally used in the operating room (Fig. 2a).
Mechanical testing
Sample collection
The samples of parietal peritoneum for the study were collected from two male and two female non-fixed cadavers. The local ethics committee at Pirogov Russian National Research Medical University approved this study experimental design. Specimens of the peritoneum together with underlying tissues were collected from both sides of lower part of abdominal wall.
The samples of gastric mucosa were taken from four pig stomachs prepared for the biomodel the next day after conservation.
All samples of mucosa layer were excised from the porcine stomach along curvature major and of the human peritoneum along linea alba.
The specimen thickness was measured with a digital thickness gauge.
Tensile test
All mechanical tension tests were carried out with help of a single-column universal material testing machine TA.XTplus texture analyzer (Stable Micro Systems Ltd., UK) equipped with a strain gauge load cell of 0.3 kN with a minimum value of scale division of 0.1 cN. A step of crosshead movement was 0.001 mm. The working width of grips was 40 mm. For analyzing the results, interactive stress–strain curves were used. Samples 40 × 20 mm were fastened with standard grips; the operational distance was 25 mm. The initial tension was set at 10 cH, the samples were stretched at the speed of 0.5 mm/s up to breaking. Exponent version 6.0 software was used to register and process the data. A straight line was drawn from the zero mark through the slope of the linear region of the stress–strain curve. Projection of the point of line’s divergence to the stress axis was the elastic limit and to the strain axis was the elastic strain. The elastic modulus (E) was calculated as the ratio of the elastic limit to the elastic strain, and measured in kPa.
T-peel test
We used T-peel test to compare the force needed to separate the porcine gastric mucosa and human peritoneum from underlying tissues.
The layers of tested samples were peeled apart in a distance of 20 mm for forming the two “legs”, which were placed in the grips of a force-testing stand for continuous measurement of the force and displacement of the separation at a constant speed of 0.5 mm/s. The average peeling force over the width of the sample was then calculated and expressed in N/mm.
Model use and assessment
The mentor showed a training video, and then performed a demonstration operation on the biomodel before training operation. During the demonstration operation, the mentor paid attention to various difficult technical moments. After that, the trainees worked in pairs and each performed three operations. The model allows performing 12–14-cm incision, dissection (Fig. 2b), placing a 10 × 15-cm mesh (Fig. 2c), fixing the closing of peritoneum with sutures as during the real surgery (Fig. 2d). After training, all the participants filled out a questionnaire, specially designed for this study, regarding their opinion about the model as a whole, simulation of anatomy, simulation of dissection and mobilization, and simulation of intracorporeal suture on a five-point Likert scale (1—strongly disagree; 5—strongly agree).
Statistical analysis
All analyses were performed using a commercial package of Statistica 13.3 software for Windows (StatSoft Inc., Tulsa, OK, USA). Results are expressed as mean values and standard deviations (SD) for continuous normally distributed variables, as median (range) for continuous non-normally distributed data, and as counts and percentages for categorical data. Analysis of normality was performed with the Kolmogorov–Smirnov and Shapiro–Wilk tests. The Student’s t test for independent samples was used for comparisons of continuous variables. The Mann–Whitney U test was used for nonparametric quantitative data. A p value less than 0.05 was considered statistically significant.
Results
Mechanical testing
Mechanical tests demonstrated that the elastic moduli of the human peritoneum and pig gastric mucosa are close in mean values, reaching 15.8 ± 6.7 kPa and 13.5 ± 4.2 kPa, respectively (p = 0.531). However, the average peeling force is nearly two times higher for gastric mucosa separation (0.212 ± 0.014 N/mm vs. 0.11 ± 0.086 N/mm, p = 0.038).
According to measurements, the thickness of human peritoneum was, on average, 1.74 ± 0.39 mm vs. 1.73 ± 0.32 mm of pig gastric mucosa (p = 0.168).
Model assessment
Thirty eight surgeons participated in the study. Their characteristics are shown in Table 1. Surgeons had different work experience and level of experience in laparoscopic surgery. Twenty one of them have never performed laparoscopic inguinal TAPP hernia repair. Almost a quarter of surgeons have performed less than ten laparoscopic TAPP inguinal hernia repair.
The results of the subjective assessment of the model on a five-point scale were as follows: the model as a whole—5 (3–5), simulation of anatomy—5 (3–5), simulation of dissection and mobilization—5 (3–5), and simulation of intracorporeal suture—5 (4–5). Structured evaluation of the simulation model is shown in Table 2.
Discussion
Based on the guidelines of the European Hernia Society (EHS) [10] and International Endohernia Society (IEHS) [11], laparoscopic hernia repair is advantageous when compared to open surgery and recommended as the first-choice treatment. At the same time, the introduction of TAPP inguinal hernia repair encounters significant difficulties associated with the need for special laparoscopic manual skills.
TAPP inguinal hernia repair is one of the few operations that have no analog in open surgery, so first steps in learning of laparoscopic inguinal hernia repair require a special approach to surgical training. The trainees need manual skills for dissecting, positioning of the mesh, and the intracorporeal peritoneal defect closure alongside proper knowledge of laparoscopic anatomy and features of technique.
A cognitive and technical training curriculum is vital to improve surgeon’s manual skills and patient outcomes [12]. Both surgeons and residents agree that the best educational method would be simulation-based training courses followed by expert proctoring [4], therefore, simulation-based training courses could be part of a standardized curriculum concept for continuing training in hernia surgery [13]. Introduction of various models for TAPP procedure reinforces expectation for improving quality of training and contraction of learning curve.
In recent years, there has been an increasing role of virtual simulators in laparoscopic surgery, and in particular, in the teaching of laparoscopic inguinal hernia repair, but up to date, no studies were encountered using computer-simulated inguinal hernia repair for training [12]. The lack of tactile sensitivity, a significant difference from the real intraoperative situation in the dissection of the peritoneum, and intracorporeal suturing reduce the effectiveness of virtual simulators used for the learning of this intervention.
Different simulators for TAPP and TEP inguinal hernia repair used artificial anatomical structures and tissue placed in a box trainer have been currently developed [14, 15] and their use demonstrates improving of the operative performance and patient outcomes in laparoscopic inguinal hernia repair [16]. Nishihara et al. recently presented a physical simulator for TAPP inguinal hernia repair training using 3D printing technology and a handmade organ replica model [7]. Unfortunately, artificial models also do not give an adequate possibility to simulate dissection of tissue and to get a realistic tissue feeling and instrument usage [17]. An identifying feature of our model is approximate of real haptic feedback of preperitoneal dissection, mesh positioning, and intracorporeal peritoneal defect closure. Nearly all the participants who never performed laparoscopic inguinal TAPP hernia repair rated the likelihood of its performance after training on the model as very high. Placement of the model at an angle similar to the angle of the anterior abdominal wall of the patient during TAPP inguinal hernia repair allows the trainees to experience stress positions of wrist joint during all stages of TAPP procedure with a simulator. Importance of the combination of haptic feedback and stress positions of surgeon’s wrist and arm during laparoscopic TAPP inguinal hernia repair training was noted in study of Nishihara et al. [7]. Therefore, the model also can be used to measure performance of the main procedure-specific surgical steps included in the curriculum for laparoscopic TAPP inguinal hernia repair by many researchers [13, 18].
The use of porcine models for laparoscopic surgery practical skills training has long proved its effectiveness [19, 20]. However, high cost and special requirements for animal housing make using live porcine models very complicated.
The only model that allows simulation of TAPP hernia repair in terms of real anatomy is the cadaver model. However, the implementation of such training requires special facilities and equipment. In addition, both the cadaver and the live pig model can be attributed to single-use models. This also significantly increases the cost of training.
Not having a goal to reconstruct completely an anatomy of human inguinal region in our model, we indicated the main anatomical landmarks for recognizing using synthetic materials filled with colored fluid and placed to the submucosal layer. Liquid leakage in case of damage of tubular structures brings the procedure closer to actual practice. All synthetic parts of our model can be used repeatedly. The porcine stomach is only single-use part of the model, which can be obtained from the slaughterhouse for free.
The similarity of anatomy and mechanical properties of the corresponding organs and tissues opens up opportunities for trainings and medical device testing on isolated pig organs [21]. For the first time, we used dissimilar organ to create TAPP hernia repair model—the porcine stomach. Therefore, for the validation of the model, we performed a comparative assessment of the mechanical properties of the lower part of the human peritoneum, which is dissected for a long distance in TAPP hernia repair, and the mucosa of the porcine stomach, which is dissected in our model. Similar thickness and elasticity made it possible to perform the main steps (dissection, intracorporeal suture) with sensations close to real, and to use real laparoscopic instruments. At the same time, the strength required to separate the gastric mucosa was higher. Many trainees noted this in the comments to questionnaire, but most of them attributed this to the advantages of the model, as a possible element of training for procedure in case of recurrent hernia.
Our study has several limitations. Relatively small homogeneous group of general surgeons tested the model, and further studies are needed to confirm whether skills obtained with our model can be transferred directly to operative setting. Our model does not include the step of hernia sac identification and reduction, but we are currently working with improving the model.
The recommendation for a goal-directed curriculum including anatomy, procedure steps, intraoperative decision-making and proficiency-based, simulation-enhanced technical skills training has been strongly upgraded in the International guidelines for groin hernia management [22]. Therefore, further models must be developed for intensive preclinical training courses [23]. At the moment, no simulation model can combine the real anatomy of the human inguinal area, an intraoperative variety of hernias and all procedural steps. We consider our model not as an alternative, but as part of a comprehensive multi-modal program with focus on key procedure-specific practical skills training. Considering the difficulties of performing TAPP procedure, we suppose that our model, which reflects the main anatomical landmarks, allows building a mental model of the inguinal area for TAPP inguinal hernia repair even at the training level.
Conclusions
We successfully created a model for TAPP inguinal hernia repair training. The model is made of inexpensive synthetic and biological materials similar to human tissue. This model is easy to reproduce and can be used in the training programs of surgeons and residents.
References
Andresen K, Friis-Andersen H, Rosenberg J (2016) Laparoscopic repair of primary inguinal hernia performed in public hospitals or low-volume centers have increased risk of reoperation for recurrence. Surg Innov 23:142–147
Trevisonno M, Kaneva P, Watanabe Y, Fried GM, Feldman LS, Andalib A, Vassiliou MC (2015) Current practices of laparoscopic inguinal hernia repair: a population-based analysis. Hernia 19:725–733
Vu JV, Gunaseelan V, Krapohl GL, Englesbe MJ, Campbell DA, Dimick JB, Telem DA (2018) Surgeon utilization of minimally invasive techniques for inguinal hernia repair: a population-based study. Surg Endosc. https://doi.org/10.1007/s00464-018-6322-x
Trevisonno M, Kaneva P, Watanabe Y, Fried GM, Feldman LS, Lebedeva E, Vassiliou MC (2015) A survey of general surgeons regarding laparoscopic inguinal hernia repair: practice patterns, barriers, and educational needs. Hernia 19:719–724
Suguita Fabio Yuji, Essu FF, Oliveira LT, Iuamoto LR, Kato JM, Torsani MB, Franco AS, Meyer A, Andraus W (2017) Learning curve takes 65 repetitions of totally extraperitoneal laparoscopy on inguinal hernias for reduction of operating time and complications. Surg Endosc 31:3939–3945
Leblanc F, Champagne BJ, Augestad KM, Neary PC, Senagore AJ, Ellis CN, Delaney CP (2010) A comparison of human cadaver and augmented reality simulator models for straight laparoscopic colorectal skills acquisition training. J Am Coll Surg 211:250–255
Nishihara Y, Isobe Y, Kitagawa Y (2017) Validation of newly developed physical laparoscopy simulator in transabdominal preperitoneal (TAPP) inguinal hernia repair. Surg Endosc 31:5429–5435
Sharma M, Horgan A (2012) Comparison of fresh-frozen cadaver and high-fidelity virtual reality simulator as methods of laparoscopic training. World J Surg 36:1732–1737
Yiasemidou M, Roberts D, Glassman D, Tomlinson J, Biyani S, Miskovic D (2017) A multispecialty evaluation of thiel cadavers for surgical training. World J Surg 41:1201–1207
The Herniasurge Group (2018) International guidelines for groin hernia management. Hernia 22:1–165
Bittner R, Arregui ME, Bisgaard T, Dudai M, Ferzli GS, Fitzgibbons RJ, Fortelny RH, Klinge U, Kockerling F, Kuhry E, Kukleta J, Lomanto D, Misra MC, Montgomery A, Reinpold W, Rosenberg J, Sauerland S, Singh K, Timoney M, Weyhe D, Chowbey P (2011) Guidelines for laparoscopic (TAPP) and endoscopic (TEP) treatment of inguinal hernia [International Endohernia Society (IEHS)]. Surg Endosc 25:2773–2843
Bittner R, Montgomery MA, Arregui E, Bansal V, Bingener J, Bisgaard T, Buhck H, Dudai M, Ferzli GS, Fitzgibbons RJ, Fortelny RH, Grimes KL, Klinge U, Koeckerling F, Kumar S, Kukleta J, Lomanto D, Misra MC, Morales-Conde S, Reinpold W, Rosenberg J, Singh K, Timoney M, Weyhe D, Chowbey P (2015) Update of guidelines on laparoscopic (TAPP) and endoscopic (TEP) treatment of inguinal hernia (International Endohernia Society). Surg Endosc 29:289–321
Lorenz R, Stechemesser B, Reinpold W, Fortelny R, Mayer F, Schröder W, Köckerling F (2017) Development of a standardized curriculum concept for continuing training in hernia surgery: German Hernia School. Hernia 21(2):153–162
Kurashima Y, Feldman L, Al-Sabah S, Kaneva P, Fried G, Vassiliou M (2011) A novel low-cost simulator for laparoscopic inguinal hernia repair. Surg Innov 18:171–175
Hoops HE, Maynard E, Brasel KJ (2017) Training surgeons in the current US Healthcare System : a review of recent changes in resident education. Curr Surg Reports. https://doi.org/10.1007/s40137-017-0195-0
Kurashima Y, Feldman LS, Kaneva PA, Fried GM, Bergman S, Demyttenaere SV, Li C, Vassiliou MC (2014) Simulation-based training improves the operative performance of totally extraperitoneal (TEP) laparoscopic inguinal hernia repair: a prospective randomized controlled trial. Surg Endosc 28:783–788
Schlottmann F, Murty NS, Patti MG (2017) Simulation model for laparoscopic foregut surgery: the University of North Carolina foregut model. J Laparoendosc Adv Surg Tech 27:1–5
Kurashima Y, Feldman LS, Al-Sabah S, Kaneva PA, Fried GM, Vassiliou MC (2011) A tool for training and evaluation of laparoscopic inguinal hernia repair: the Global Operative Assessment of Laparoscopic Skills-Groin Hernia (GOALS-GH). Am J Surg 201:54–61
Lee JS, Hong TH (2015) In vivo porcine training model for laparoscopic Roux-en-Y choledochojejunostomy. Ann Surg Treat Res 88:306–310
Kim EY, Hong TH (2018) In vivo porcine training model of laparoscopic common bile duct repair with T-tube insertion under the situation of iatrogenic common bile duct injury. Ann Surg Treat Res 94:142–146
White EJ, Cunnane EM, Mcmahon M, Walsh MT, Coffey JC, Sullivan LO (2018) Mechanical characterisation of porcine non-intestinal colorectal tissues for innovation in surgical instrument design. J Eng Med 232:796–806
The HerniaSurg Group (2018) International guidelines for groin hernia management. Hernia 22:1–165. https://doi.org/10.1007/s10029-017-1668-x
Köckerling F (2018) What is the influence of simulation-based training courses, the learning curve, supervision, and surgeon volume on the outcome in hernia repair? A Systematic Review. Front Surg. 5:57. https://doi.org/10.3389/fsurg.2018.00057(Published online 25 Sep 2018)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Georgy Ivakhov, Alexey Kolygin, Svetlana Titkova, Michail Anurov, and Alexander Sazhin have no conflicts of interest or financial ties to disclose.
Ethical approval
The study was approved by the institutional Ethics Committee of Pirogov Russian National Research Medical University.
Human and animal rights
This article does not contain any studies with human participants. No procedures were performed on animals in this study.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (MOV 124223 kb)
Rights and permissions
About this article
Cite this article
Ivakhov, G., Kolygin, A., Titkova, S. et al. Development and evaluation of a novel simulation model for transabdominal preperitoneal (TAPP) inguinal hernia repair. Hernia 24, 159–166 (2020). https://doi.org/10.1007/s10029-019-02032-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-019-02032-5