Abstract
Background
To evaluate the predisposing factors and characteristics of recurrent ventral hernia (RVH) along with the feasibility and outcome of laparoscopy in managing RVH.
Methods
This study is a retrospective analysis of all patients with reducible or irreducible, uncomplicated RVH who underwent surgical management from January 2012 to June 2018.
Results
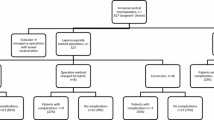
Out of 222 patients, 186 (83.8%) were female, and 36 (16.2%) were male. The mean age was 54.1 ± 10.1 years; an average body mass index was 31 kg/m2 (19–47.9). The most common previous abdominal operations among female patients were cesarean sections (43.5%) and abdominal hysterectomy (36.6%). Most of the patients had a history of open mesh repair (43.7%) and open anatomical repair (36.9%). The median time of recurrence was 4 years (1–33 years). The median defect size was 10 cm2 (range 2–150 cm2), and 73% defects were in the midline. Total 181 of 222 (81.6%) patients underwent laparoscopic intraperitoneal onlay mesh plus (L-IPOM+), 19 (8.5%) laparoscopic-assisted IPOM+, 17(7.7%) laparoscopic anatomical repair, while remaining 5 (2.3%) patients required open mesh reconstruction. The median size of the composite mesh used was 300 cm2 (150–600 cm2). The mean operating time was 145 (30–330) min, and median blood loss was 15 (5–110) ml. The median hospital stay was 3 days, and median follow-up period was 37 months. The post-operative symptomatic seroma rate was 3.1%, and re-recurrence rate was 1.4%.
Conclusion
Obesity, old age, female sex, previous lower abdominal surgeries, and previous open repair of a hernia are factors associated with recurrence. Laparoscopic repair is feasible with excellent outcome in most of the patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is a challenging task for any surgeon to deal with a recurrent ventral hernia (RVH). The difficulty of surgical management is mainly due to altered abdominal wall anatomy, the presence of the previous prosthesis, and diffuse intra-abdominal adhesions. The traditional surgical management of recurrent ventral hernia is open repair preferably with synthetic non-absorbable mesh. Although laparoscopic repair of primary ventral hernia is well established, the use of laparoscopy in managing RVH is yet to become familiar, with a lack of data highlighting the role of laparoscopic management. This study aims to share our 5-year experience of laparoscopic management of RVH.
Materials and methods
It is a retrospective observational study conducted in a tertiary care center for gastrointestinal and minimally invasive surgery, with an annual volume of 500 plus cases of a ventral hernia per year. All patients who were diagnosed and operated for RVH during from January 2012 to June 2018 were included. However, those patients who presented with features of obstruction or strangulation and those with large defects (Length > 10 cm and/or width > 4 cm), who underwent component separation, or staged reconstruction were omitted. The institutional review board approves the study.
The data regarding demographic characteristics, clinical history and examination, perioperative details, the course in the hospital and follow-up after discharge were retrieved from a prospectively maintained computer database. Particular attention was given to co-morbidities, body mass index, details of previous abdominal operations, type, and a number of previous ventral hernia repairs and associated complications, if any.
The details of intra-operative findings especially the defect size, location, type of the previous prosthesis (if any), new mesh type/size/fixation techniques, associated surgeries (if any) and postoperative course were gathered and analyzed.
All patients with RVH underwent routine pre-operative blood investigations, ultrasonography (USG) of the abdomen. Contrast-enhanced computerized tomography (CECT) scan of the abdomen was done selectively for patients with a history of multiple abdominal operations and to rule out associated complex intra-abdominal pathology suspected on USG. At discharge, patients were advised for follow-up after 7 days, 3 months, and 1-year post-surgery and once in a year after that. On each postoperative follow-up visit, a detailed clinical examination was done to rule out any complications like a recurrence of a hernia, symptomatic seroma, surgical site infections. Persistent symptomatic seromas were subjected to repeated aspiration under USG guidance.
SPSS Version 24 (IBM Corp. NY, US) was used to analyze the data. A p value of less than 0.05 was considered as statistically significant.
Surgical techniques
The technique of L-IPOM+ (Laparoscopic Intra-Peritoneal Onlay Mesh plus) is standardized; the details are as follows. We believe, five essential steps for successful completion of IPOM + in the setting of a recurrent ventral hernia, i.e., optimum port positions, meticulous adhesiolysis, near complete sac excision, defect closure, and adequate sized mesh placement and fixation.
Under general anesthesia, patients are placed in supine position. We used Veress needle to create pneumoperitoneum staying away from the previous surgical scar commonly at the sub-xiphoid region or Palmar’s point (Raoul Palmer, French Gynecologist, 1974).
Port placement
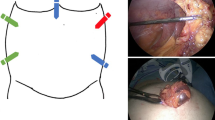
Routine placement of 10 mm camera port at sub-xiphoid region and two 5 mm working port in the right and left subcostal region (Fig. 1) is done. In patients with upper abdominal or lateral wall defects, lateral wall port technique is used (Fig. 2).
Adhesiolysis
Thorough and meticulous adhesiolysis is performed to reduce the contents, clear area for mesh placement, and to rule out occult defects (Figs. 3, 4). Sharp dissection by scissors is preferred with minimal use of energy sources as much as possible. In situations where laparoscopic adhesiolysis is difficult, mini-laparotomy is done to reduce the content of hernial sac. The number, size, and type of defects are identified after adhesiolysis (Figs. 5, 6).
Sac excision
Adequate time is spent near complete hernial sac excision, which authors believe as an essential step for reducing seroma. External compression by an assistant surgeon or scrub nurse dramatically facilitates this step. The excised sac is removed through 10 mm port under vision, after the completion of the procedure. Iatrogenic thermal injury involving the overlying skin (button-hole) can be avoided by assessing the skin thickness, manipulating the external compression and at times letting the sac remain unexcised, where the overlying skin is thinned out.
Defect closure
Most of the defects are closed intracorporeally using number one, non-absorbable synthetic monofilament (Nylon, Ethicon, NJ, US) suture loop, in a continuous fashion (Figs. 7, 8). Cases with assisted repairs (mini-laparotomy) to facilitate content reductions, defect closure is done from outside, before re-establishing pneumoperitoneum. Multiple defects involving different quadrants were closed separately, whereas, Swiss-cheese defects in the same line are approximated together.
Mesh placement
All patients had hernioplasty using a composite mesh [Parietex™ Composite (Covidien, New Haven, CT, USA)]. The choice of size dimensions used was determined by the operating surgeon, after considering the defect size, keeping the mesh coverage 5 cm beyond the original defect size. The contraindications of laparoscopic mesh placement were the presence of intra-abdominal septic focus, iatrogenic bowel/ biliary tract injury (spillage of bile) or small bowel resection-anastomosis. In those situations, mere laparoscopic anatomical (L-Anat) repair was considered. However, all those patients underwent laparoscopic mesh placement after 4–6 weeks. Polypropylene meshes (Ethicon, NJ, US) were used for open mesh repair (O-Mesh). The defects were covered minimum 5 cm all around with mesh and transfixed via transfascial and intra-corporeal suturing. In cases where defects present in multiple quadrants, either bigger meshes or two separate mesh were used, depending upon the distance between the two locations. We seldom used absorbable tackers along with suture fixation to fix bigger meshes.
At the end of the procedure, hemostasis checked and omentum placed over the bowel to prevent adhesions with the intestine. Compression dressings were kept over the defect site for 24 h. Patients were advised to wear an abdominal binder for the next 6 weeks.
Results
From January 2012 to June 2017, a total of 222 patients with recurrent ventral hernia who underwent surgical repair was included in this study. The term recurrent ventral hernia includes recurrence following epigastric hernia, umbilical/ a paraumbilical hernia and incisional hernia repairs. The demographic parameters of these patients are described (Table 1) Majority of patients were females and were obese with median BMI of 31 (range 19–47.9 kg/m2). Cesarean operation and other gynecological procedures history constituted the majority (95.7%) in females, while open appendectomy (10.4%) was most frequent previous surgery, overall. Most of the patients presented had a history of first recurrence (87.8%) after primary ventral hernia repair and median time of recurrence was 4 years (range 1–33 years).
The median operating time was 145 min (30–330 min), and median blood loss was 15 ml (5–110 ml). The median defect size was 10 cm2 (range 2–150 cm2). Total 181 (81.6%) patients successfully underwent laparoscopic IPOM plus and also another 19 (8.5%) patients underwent laparoscopic-assisted IPOM plus. The laparoscopic anatomical repair was done in 17(7.7%) patients where mesh was contraindicated. The laparoscopic approach was not feasible in 5(2.3%) patients, where open mesh repair was performed. Some selected patients (27) underwent one additional laparoscopic procedure (Cholecystectomy-7, Inguinal Hernia repair-5, total laparoscopic hysterectomy-4, Small bowel resection-anastomosis-4, and Sleeve gastrectomy-4) along with RVH repair. Three patients underwent abdominoplasty with open mesh repair, having an average mesh size of 300 cm2 (150–600 cm2) and median stay of 3 days (1–23 days). The median follow-up period was 37 months (3–64 months): the details of intra and postoperative variables as mentioned in Table 2.
Total of seven patients (3.1%) developed symptomatic seroma within 3 months after operation who were managed by ultrasound-guided aspiration (1–2 times) under prophylactic oral broad-spectrum antibiotic coverage. Three patients (1.3%) presented with recurrence in follow-up period. No patient had a wound-related complication and none required mesh extraction.
Patients were advised to follow-up on outpatient basis with following schedule. First visit at 2 weeks post-surgery, then at 12 weeks, 24 weeks. After 6 months, it was once in 6 month till 2 years, afterwards, yearly once is advised. This was planned as maximum appearance of recurrence was seen within first 2 years from previous repair and rare after that.
Discussion
Since its first description by LeBlanc, laparoscopic ventral hernia repair (LVHR) has evolved as a superior method over its open counterpart [1, 2]. It is advantageous regarding postoperative pain, the incidence of wound-related complications and postoperative recovery. However, there are controversies in managing patients with recurrent hernias. This study comprises recurrent incisional hernia along with all umbilical/paraumbilical, epigastric and lower abdominal recurrences. There are numerous studies in the literature to identify the probable risk factors for recurrence. Old age, male sex, obesity, smoking, co-morbidities like diabetes and chronic obstructive pulmonary disease(COPD), malnourishment and multiple previous abdominal surgeries are well-known risk factors [3,4,5] In our study, most of the patients were middle-aged obese females with one or more co-morbidities like diabetes, hypertension. More than 60% of our patients were in the ASA II category. A significant proportion of female patients had one or more cesarean section and/or open abdominal hysterectomy which in turn led to abdominal muscle weakness predisposing to lower abdominal RVH. In one cohort study by Abakke et al., it has been shown cesarean section influences lower abdominal hernia mostly within the first 3 years [6]. Agbakwuru et al. in their study mentioned obesity, history of emergency CS or laparotomy, use of absorbable suture material for abdominal wall closure and wound infection as the main factors for ventral hernia formation in women [7]. The incidence of other abdominal surgeries like midline laparotomy, open appendectomy or cholecystectomy was not significant (p > 0.05) in our study.
Number and type of previous ventral hernia repair were two essential factors of RVH. In our study, most of our patients had open mesh repair (43.6%) or open anatomical suture repair (36.7%) as the method of last hernia repair. Zhang et al. in his meta-analysis included 11 studies, and over 1000 patients showed no difference in the incidence of recurrence after LVHR or OVHR [8]. Awaiz et al. in their meta-analysis showed no difference in recurrence rate after laparoscopic and open mesh repair [9]. However, only 14.4% of patients had laparoscopic IPOM, and 5% of patients had L-ANAT as the last method of hernia repair in our study. Thus contrary to literature evidence our research suggests that laparoscopy can give better result to prevent future recurrence compared to open repair.
In our study, more than 87% of patients presented with first recurrence and approximately 13% of patients had two or more recurrences. However, irrespective of the number of recurrences they underwent laparoscopic repair (Table 1). Similarly, Picazo-Yeste et al. have mentioned that laparoscopy should be the preferred method of repairing RVH irrespective of the number of recurrences [10].
LeBlanc in his recent article mentioned proper mesh overlap as the critical determinant of recurrence after LVHR [11]. Carter et al. in their study showed the larger defect, some previous hernia repair, improper mesh overlap, and mesh fixation, mesh infection may lead to recurrence or pseudo recurrence after LVHR [12]. Midline defects are more common than non-midline defects(almost 3/4th versus 1/4th) in our study. However, non-midline defects cause more pain and discomfort to the patients [13]. The average defect size in our study was 10 cm2 (2–150 cm2).
As described in surgical techniques, we routinely used three epigastric trocars for umbilical/para-umbilical or lower abdominal recurrent hernia and left lateral abdominal ports for epigastric recurrences. The use of upper abdominal ports where the surgeon stands at the head end of the patient makes it comfortable to suture the most defects in the midline and lower abdomen and is particularly suitable for Indian patients with an average height between 150 and 170 cm, alternatively one can use longer instruments. While, most of the published literature supports the use of left lateral ports for all ventral/ incisional hernias, irrespective of the site [14].
RVH presents with mild to moderate intra-abdominal adhesions which depend on the number of previous abdominal surgeries, previous hernia repair or any associated intra-abdominal pathology (Koch’s abdomen). In all cases, meticulous laparoscopic adhesiolysis was required to identify the hernial defect and repair it accurately. In some cases with severe bowel adhesions with previous mesh or hernial sac, accidental serosal injury or even enterotomy may occur during adhesiolysis. In such situations, our institution practice was to repair the bowel injury, through peritoneal lavage (if there is any spillage of enteric content) and anatomical repair of the hernial defect with synthetic non-absorbable suture. However, laparoscopic composite mesh placement was offered to the most after a gap of 6–8 weeks. In the present study, the iatrogenic bowel injury rate was only 2.7% (6 out of 222 patients) even for RVH repair, and all except one case was managed laparoscopically. Ferrari et al. mentioned 4.3% accidental bowel injury rate during laparoscopic repair of RVH [15]. Other studies reported 0–2% incidence of bowel injury in laparoscopic primary ventral hernia repair [16,17,18]. Perrone et al. and Sharma et al. in their studies mentioned enterotomy during adhesiolysis can lead to severe complications like sepsis and death [19, 20]. Thus all measures to be taken to prevent it.
Adhesiolysis should be done preferably with meticulous sharp dissection using cutting scissors. Additionally, in case of severe adhesions limited open conversions can be done for adhesiolysis and defect closure followed by laparoscopic intra-peritoneal mesh placement [21, 22]. In our institution, this procedure was named as laparoscopic-assisted IPOM (IPOM-A) which has given us the almost equivalent result as IPOM. Yoshikawa et al. and Stoikes et al. described similar approaches for adhesiolysis for difficult RVH repair [23, 24].
Hernial sac excision and defect closure is a routine practice at our institute. Previous studies from our institute already showed that seroma formation, pseudo recurrence, and even recurrence rate could be significantly decreased by doing these two steps [25, 26]. Tandon et al. analyzed 16 RCTs on closure versus non-closure of fascial defects in their meta-analyses(over 3600 patients) and found out a significantly lower rate of seroma formation, pseudo recurrence, hospital stay with no difference in postoperative pain score [27].
There are studies on an optimal requirement of mesh overlap beyond the defect to prevent future recurrence. Our institution protocol is to cover minimum 5 cm all around the defect, and our recurrence rate for RVH is only 1.4%. Nardi et al. and LeBlanc et al. in their articles have mentioned inadequate mesh fixation as one of the critical factors for recurrence in obese patients with multiple co-morbidities [11, 28]. There are different mesh fixation techniques described in literature starting from tackers, sutures and even fibrin glues. However, none of the single methods is superior over the another [29,30,31]. In our center, we routinely used a combination of transfascial and intra-corporeal sutures for mesh fixation and none of our patients presented with fixation material related complications. This point we authors would like to highlight the given scenario of rising health care related expense, worldwide, especially more relevant to developing economies, where the patient themselves mostly bears the cost. The suture fixation time reduces over the period, significantly brings down the consumables related price and shall help the surgeon to enhance his/her skills in other areas of advanced laparoscopic surgeries.
Praveen Raj et al. in his study has shown the feasibility of doing laparoscopic primary ventral hernia repair with concomitant clean-contaminated procedures with good outcome [32]. Total 13% of patients in our study underwent concomitant clean or clean-contaminated procedures with uneventful post-operative recovery. But those patients have minimal intra-abdominal adhesions with small recurrence. Thus, in RVH concomitant clean or clean-contaminated laparoscopic surgery can be added along with IPOM + in selective patients with good outcome.
Laparoscopic repair offers less postoperative pain, very minimal wound-related complications and early postoperative recovery than its open counterpart [4, 16, 33]. Postoperative seroma formation is one of concerning factor after LVHR [34, 35]. However, sac excision and suture closure of the hernial defect can reduce seroma formation [25, 27]. In the present study, the postoperative incidence of symptomatic seroma formation is only 3.1% in the first 12 weeks and none after that which required some intervention. Ferrari et al. [15] in his study of laparoscopic repair of RVH found 6 (8.7%) who had persisting seroma beyond 8weeks three of them were symptomatic. Nine (4%) patients developed paralytic ileus which stayed beyond 4 days; however, all of them were managed conservatively and responded well after that. No patient had a surgical site infection which is the proven advantage of LVHR [36].
Conclusion
Among patients referred to our center, obesity, old age, female sex, previous lower abdominal surgeries, and previous open repair of a hernia are factors associated with recurrence. Additionally, for surgeons with requisite skill, laparoscopic repair is feasible with excellent outcome in most patients with RVH. Laparoscopic IPOM + has the potential to become a standard approach to managing RVH.
References
LeBlanc KA, Booth WV (1993) Laparoscopic repair of incisional abdominal hernias using expanded polytetrafluoroethylene: preliminary findings. Surg Laparosc Endosc 3:39–41
Bittner R, Bingener-Casey J, Dietz U et al (2014) Guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias (International Endohernia Society (IEHS)-part 1. Surg Endosc 28:2–29. https://doi.org/10.1007/s00464-013-3170-6
Cobb WS, Kercher KW, Heniford BT (2005) Laparoscopic repair of incisional hernias. Surg Clin North Am 85:91–103. https://doi.org/10.1016/j.suc.2004.09.006
Hesselink VJ, Luijendijk RW, de Wilt JH et al (1993) An evaluation of risk factors in incisional hernia recurrence. Surg Gynecol Obstet 176:228–234
Chelala E, Baraké H, Estievenart J et al (2016) Long-term outcomes of 1326 laparoscopic incisional and ventral hernia repair with the routine suturing concept: a single institution experience. Hernia 20:101–110. https://doi.org/10.1007/s10029-015-1397-y
Aabakke AJM, Krebs L, Ladelund S, Secher NJ (2014) Incidence of incisional hernia after cesarean delivery: a register-based cohort study. PLoS One 9:e108829. https://doi.org/10.1371/journal.pone.0108829
Agbakwuru E, Olabanji J, Alatise O et al (2009) Incisional hernia in women: predisposing factors and management where mesh is not readily available. Libyan J Med 4:66–69. https://doi.org/10.4176/081105
Zhang Y, Zhou H, Chai Y et al (2014) Laparoscopic versus open incisional and ventral hernia repair: a systematic review and meta-analysis. World J Surg 38:2233–2240. https://doi.org/10.1007/s00268-014-2578-z
Awaiz A, Rahman F, Hossain MB et al (2015) Meta-analysis and systematic review of laparoscopic versus open mesh repair for elective incisional hernia. Hernia 19:449–463. https://doi.org/10.1007/s10029-015-1351-z
Picazo-Yeste J, Moreno-Sanz C, Sedano-Vizcaíno C et al (2017) Outcomes after laparoscopic ventral hernia repair: does the number of previous recurrences matter? A prospective study. Surg Endosc. https://doi.org/10.1007/s00464-017-5510-4
LeBlanc K (2016) Proper mesh overlap is a key determinant in hernia recurrence following laparoscopic ventral and incisional hernia repair. Hernia 20:85–99. https://doi.org/10.1007/s10029-015-1399-9
Carter SA, Hicks SC, Brahmbhatt R, Liang MK (2014) Recurrence and pseudorecurrence after laparoscopic ventral hernia repair: predictors and patient-focused outcomes. Am Surgeon 80(2):138–48
Moreno-Egea A, Carrillo A, Aguayo JL (2008) Midline versus nonmidline laparoscopic incisional hernioplasty: a comparative study. Surg Endosc 22:744–749. https://doi.org/10.1007/s00464-007-9480-9
Misiakos EP, Patapis P, Zavras N et al (2015) Current trends in laparoscopic ventral hernia repair. JSLS. https://doi.org/10.4293/JSLS.2015.00048
Ferrari G, Bertoglio C, Magistro C et al (2013) Laparoscopic repair for recurrent incisional hernias: a single institute experience of 10 years. Hernia 17:573–580. https://doi.org/10.1007/s10029-013-1098-3
Davies SW, Turza KC, Sawyer RG et al (2012) A comparative analysis between laparoscopic and open ventral hernia repair at a tertiary care center. Am Surg 78:888–892
Stirler VMA, Schoenmaeckers EJP, de Haas RJ et al (2014) Laparoscopic repair of primary and incisional ventral hernias: the differences must be acknowledged. Surg Endosc 28:891–895. https://doi.org/10.1007/s00464-013-3243-6
Mercoli H, Tzedakis S, D’Urso A et al (2017) Postoperative complications as an independent risk factor for recurrence after laparoscopic ventral hernia repair: a prospective study of 417 patients with long-term follow-up. Surg Endosc 31:1469–1477. https://doi.org/10.1007/s00464-016-5140-2
Perrone JM, Soper NJ, Eagon JC et al (2005) Perioperative outcomes and complications of laparoscopic ventral hernia repair. Surgery 138:708–716. https://doi.org/10.1016/j.surg.2005.06.054
Sharma A, Khullar R, Soni V et al (2013) Iatrogenic enterotomy in laparoscopic ventral/incisional hernia repair: a single center experience of 2,346 patients over 17 years. Hernia 17:581–587. https://doi.org/10.1007/s10029-013-1122-7
Bittner R, Bingener-Casey J, Dietz U et al (2014) Guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias (International Endohernia Society [IEHS])—part 2. Surg Endosc 28:353–379. https://doi.org/10.1007/s00464-013-3171-5
Sharma A, Mehrotra M, Khullar R et al (2008) Limited-conversion technique: a safe and viable alternative to conversion in laparoscopic ventral/incisional hernia repair. Hernia 12:367–371. https://doi.org/10.1007/s10029-008-0363-3
Stoikes N, Quasebarth M, Brunt LM (2013) Hybrid ventral hernia repair: technique and results. Hernia 17:627–632. https://doi.org/10.1007/s10029-013-1092-9
Yoshikawa K, Shimada M, Kurita N et al (2014) Hybrid technique for laparoscopic incisional ventral hernia repair combining laparoscopic primary closure and mesh repair. Asian J Endosc Surg 7:282–285. https://doi.org/10.1111/ases.12113
Palanivelu C, Jani KV, Senthilnathan P et al (2007) Laparoscopic sutured closure with mesh reinforcement of incisional hernias. Hernia 11:223–228. https://doi.org/10.1007/s10029-007-0200-0
Palanivelu C, Rangarajan M, Parthasarathi R et al (2008) Laparoscopic repair of suprapubic incisional hernias: suturing and intraperitoneal composite mesh onlay. A retrospective study. Hernia 12:251–256. https://doi.org/10.1007/s10029-008-0337-5
Tandon A, Pathak S, Lyons NJR et al (2016) Meta-analysis of closure of the fascial defect during laparoscopic incisional and ventral hernia repair. Br J Surg 103:1598–1607. https://doi.org/10.1002/bjs.10268
Nardi M, Millo P, Brachet Contul R et al (2017) Laparoscopic ventral hernia repair with composite mesh: analysis of risk factors for recurrence in 185 patients with 5 years follow-up. Int J Surg 40:38–44. https://doi.org/10.1016/j.ijsu.2017.02.016
Chatzimavroudis G, Kalaitzis S, Voloudakis N et al (2017) Evaluation of four mesh fixation methods in an experimental model of ventral hernia repair. https://doi.org/10.1016/j.jss.2017.01.013
Reynvoet E, Deschepper E, Rogiers X et al (2014) Laparoscopic ventral hernia repair: is there an optimal mesh fixation technique? A systematic review. Langenbeck’s Arch Surg 399:55–63. https://doi.org/10.1007/s00423-013-1126-x
LeBlanc KA (2007) Laparoscopic incisional hernia repair: are transfascial sutures necessary? A review of the literature. Surg Endosc 21:508–513. https://doi.org/10.1007/s00464-006-9032-8
Praveen Raj P, Ganesh MK, Senthilnathan P et al (2015) Concomitant laparoscopic intraperitoneal onlay mesh repair with other clean contaminated procedures—study of feasibility and safety. J Laparoendosc Adv Surg Tech 25:33–36. https://doi.org/10.1089/lap.2014.0001
Ross SW, Wormer BA, Kim M et al (2015) Defining surgical outcomes and quality of life in massive ventral hernia repair: an international multicenter prospective study. Am J Surg. https://doi.org/10.1016/j.amjsurg.2015.06.020
Tsimoyiannis EC, Tsimogiannis KE, Pappas-Gogos G, Nikas K, Karfis E, Sioziou H (2008) Seroma and recurrence in laparoscopic ventral hernioplasty. JSLS J Soc Laparoendosc Surg 12(1):51
Yang PG, Tung LK (2016) Preperitoneal onlay mesh repair for ventral abdominal wall and incisional hernia: a novel technique. Asian J Endosc Surg 9:344–347. https://doi.org/10.1111/ases.12295
Arita NA, Nguyen MT, Nguyen DH et al (2015) Laparoscopic repair reduces incidence of surgical site infections for all ventral hernias. Surg Endosc 29:1769–1780. https://doi.org/10.1007/s00464-014-3859-1
Funding
The authors have not received any financial help for this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We authors Drs, Sumanta Dey, Ramakrishnan Parthasarathy, Sandeep C. Sabnis, Rohan Jain, Palanivelu Praveen Raj, Palanisamy Senthilnathan, Subbaiah Rajapandian, Chinnusamy Palanivelu, states that they have no conflict of interest to disclose.
Ethical approval
This study was approved by institutional ethical committee for human studies, and waiver of consent of was obtained, in view of retrospective study design, where atmost care has been taken to avoid disclosure of identity of any individual participant.
Human and animal rights
All procedures were carried out following the ethical standards of the responsible (institutional) committee on human experimentation and following the Helsinki Declaration of 1964 and later versions.
Informed consent
Informed and written consent was obtained from all the patients for the mentioned surgical procedure.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dey, S., Parthasarathi, R., Sabnis, S.C. et al. Laparoscopic management of recurrent ventral hernia: an experience of 222 patients. Hernia 23, 927–934 (2019). https://doi.org/10.1007/s10029-019-01912-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-019-01912-0