Abstract
Objectives
Seroma is a virtually unavoidable early sequela after TEP hernioplasty. This randomised controlled trial evaluated the outcomes of preperitoneal closed-system suction drainage in laparoscopic totally extraperitoneal (TEP) hernioplasty for inguinal hernia.
Methods
Ninety patients aged 18–80 years who presented to our hospital between May 2016 and February 2017 with primary unilateral inguinal hernia were randomised into the preperitoneal drain and no-drain groups. The primary outcome was seroma size on postoperative day 6. Secondary outcomes included clinical seroma formation and seroma size on day 1, day 6, 1 and 7 months postoperatively, length of postoperative stay, pain score, and recurrence.
Results
There was no significant difference in age, sex, co-morbidities, hernia side, mean hernia size, operating time, fixation adjuncts, or postoperative stay. The overall incidence of clinical seroma formation was 25.6% on postoperative day 1, 60.3% on postoperative day 6, 13.2% 1 month and 0% 7 months postoperatively. The mean drain output was 57.9 ml. The drain group had significantly fewer patients with seroma on day 1 (6 vs 14, p = 0.022) and day 6 (17 vs 30, p = 0.000), and a smaller mean seroma size on days 1 and 6 (p = 0.000). Subgroup analysis showed that sac ligation versus reduction, peritoneal perforation, and fixation adjuncts had no significant effects on seroma formation or size. There is a trend of lower early post-operation VAS score and more urinary retention in drain group was observed but not reaching statistical significance. No differences in postoperative pain score or complications were observed at 1 and 7 months’ post operation.
Conclusions
Preperitoneal drainage for 23 h after laparoscopic TEP hernioplasty for inguinal hernia can effectively decrease seroma formation in the early postoperative period, and potentially improving postoperative pain. The benefit is short-term and no significant difference was demonstrated after 1-month post operations. This tradition technique applied to novel operative repair of inguinal hernia is safe and feasible with no significant morbidity demonstrated. Preperitoneal drainage after TEP can be considered as an option to improve patient satisfactions and recovery in selected patient group for maximal benefit, especially for those with prolonged operation which may associate with higher chance of seroma formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic inguinal hernioplasty is the gold standard to repair recurrent and bilateral hernia [1,2,3]. Despite the merits of non-penetrating fixation methods with self-gripping mesh in open hernioplasty—a shorter operating time and comparable long-term outcomes with an easier surgical approach [4]—the indications of laparoscopic repair have been further extended to unilateral disease with the use of advanced techniques like a needlescopic approach [5, 6] or a single-port approach [7, 8] to further minimise the access trauma. The significant drop in the recurrence rate after laparoscopic hernioplasty is attributed by a better optical system, more structural training, a better understanding of preperitoneal anatomy, and a better understanding of the pattern of recurrent disease after laparoscopic repair. However, seroma is still a commonly encountered sequela in the early postoperative period; in the first week postoperatively its incidence is as high as 37.9% [9]. Fluid collection at the inguinal region may mimic early hernia recurrence and cause psychological distress to patients and their relatives. Although numerous techniques have been described to minimise the incidence of this complication, none have been shown to be highly effective [10, 11]. In most situations, seromas will subside spontaneously with conservative management, but detailed explanations are needed to reassure patients, affecting the doctor–patient relationship and patient satisfaction.
Drainage after inguinal hernioplasty was first reported in a randomised controlled trial by Beacon et al. in 1980 [12], and it was shown to be highly effective, particularly in cases of complicated open hernia repair. On the contrary, Rodrigues et al. concluded that drainage was not useful after Stoppa procedures [13], but their sample size was too small to draw a valid conclusion. Peiper et al. also failed to prove the effectiveness of subcutaneous drainage with significant morbidities in their randomised controlled trial [14]; therefore, drain placement is not considered to be a routine practice for open inguinal hernioplasty. Recently, there were two reports of large-scale retrospective cohorts who had preperitoneal drains placed after totally extraperitoneal (TEP) hernioplasty [15, 16]. Both studies showed that drainage can effectively reduce the incidence of seroma formation, but because of heterogeneity in the studied group and retrospective analysis, the scientific value has been challenged. Therefore, our study aimed to determine whether preperitoneal drainage can effectively decrease the incidence and size of seroma formation by a randomised controlled trial with all potential confounding factors controlled. This is the first prospective double-blind randomised trial evaluating preperitoneal closed-system suction drainage after laparoscopic inguinal hernioplasty.
Method
This study was conducted in the Department of Surgery, The University of Hong Kong-Shenzhen Hospital. Shenzhen, China—a tertiary referral centre with case volume more than 200 per year.
Inclusion criteria
Patients between the ages of 18 and 80 with a unilateral inguinal hernia who presented to our surgical outpatient clinic were eligible for inclusion in the study.
Exclusion criteria
Patients were excluded if they had bilateral or recurrent inguinal hernia, incarcerated hernia, irreducible hernia, or significant co-morbidities.
Definition of seroma formation
Seroma was defined as (1) fluid collection with no Doppler signal at the preperitoneal space on ultrasound examination OR (2) clinically palpable, irreducible swelling after TEP hernioplasty with no cough impulse.
Primary outcome
To compare the ultrasonic seroma size over the inguinal region at day 6 after laparoscopic TEP hernioplasty for inguinal hernia.
Secondary outcomes
Clinical and ultrasonic seroma sizes at the inguinal region 1 day, 6 days (clinical seroma formation), 1 month and 7 months postoperatively. Total operative time, total drain output, urinary retention, wound complications, early and late postoperative pain scores, and recurrence at 7 months were also evaluated.
Sample size calculation and statistics
Since there is no literature reporting the reduction of seroma size with preperitoneal drain, the sample size was calculated based on previously published retrospective review articles with end points measuring seroma incidence [15, 16]. Assuming a 65% reduction in the incidence of seroma formation by intervention, a confidence interval of 95%, a power of 80%, and a dropout rate of 15%, the minimum number of subjects required was determined to be 90. Prospective data on patient demographics, the nature of the hernia, and hernia treatment were collected and analysed using the SPSS® 24.0 for Mac® (SPSS Inc. IBM Corp.). All continuous variables were expressed by mean and standard deviations and calculated with the independent sample t test. All categorical variables were calculated with the Chi-square test. A p value < 0.05 was considered statistically significant.
Randomisation and blinding
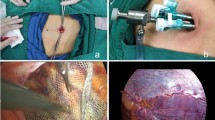
Computer-generated randomisation was performed after adequate extraperitoneal dissection and placement of prosthetic mesh with or without fixation (if applicable). A closed-system silicon suction drain was placed at the extraperitoneal space in the drain group (Fig. 1), OR a dummy drain was attached to the dressing at the skin level for the no-drain group. A dummy drain was placed at the enclosed space, and a knot was tied at its tip to achieve a vacuum-sealed connection to the collection bottle to simulate the drain (Fig. 2). Both the preperitoneal and dummy drains were removed 23 h after the operation. Patients were assessed by independent blinded clinicians (surgeons not involved in the patients’ operations) and radiologists (who were blinded to the study group) for clinical and ultrasonic evidence of seroma formation 24 h after the initial operations. Patients were followed up in the outpatient clinic 1 week and 1 month postoperatively to assess operative outcomes and complications. Both the assessors and patients were blinded to the group they were assigned to, but the operating surgeons were not blinded.
Emergency unblinding
If emergency unblinding was needed, clinicians could retrieve operative details and the drain group assigned in the hospital’s clinical management system.
Ethics
The study was conducted in accordance with the CONSORT Statement (www.consort-statement.org), and the CONSORT checklist has been fulfilled. Informed consent was obtained from all individual participants included in the study after detailed explanation of possible complications of laparoscopic TEP hernioplasty. Patients were then given at least 24 h to consider participating in the clinical trial before operations. The trial was approved by the Ethics Committee of the University of Hong Kong-Shenzhen Hospital. Shenzhen, China and registered at www.clinicaltrial.gov (ID# NCT02762747). This study was carried out according to the ethical principles for medical research involving human subjects—the Declaration of Helsinki of World Medical Association. No interim analysis was performed to avoid study bias. No source of funding was received for this trial.
Operative details
After obtaining informed consent, patients were cleared for surgery by the anaesthetists. The operations were performed either by hernia specialists or by advanced surgical trainees under the supervision of specialists. Quality control of operations was monitored by surgeons’ operating records and review of recorded video by primary and secondary investigators. Under general anaesthesia, the patients were placed in the supine position with both arms adducted. Antibiotics were not routinely given except for high-risk patients such as those with diabetes mellitus. The laparoscopic unit was placed at the caudal side of the patient. A sub-umbilical transverse skin incision was made slightly towards the operative side after the skin was prepared with a chlorhexidine solution as a disinfectant. An anterior rectus sheath incision was made at the operative side near the midline, and a 10 mm port was placed. A 30° optical laparoscope was inserted for visualisation and initial preperitoneal dissection. Two 5 mm ports were inserted at the midline below the 10 mm port under direct laparoscopic view. After trocars were inserted, the patient was placed in the Trendelenburg position. Carbon dioxide was insufflated at a pressure of 12 mmHg to establish pneumopreperitoneum.
Extraperitoneal space was then created by blunt and sharp dissection with monopolar diathermy. The direct sac was reduced at this stage. Bogros’ space was entered and opened from the lateral edge of the inferior epigastric vessels. The deep transversalis fascia and arcuate ligament were divided, and the preperitoneal space was created up to the level of the umbilicus over the lateral side. Testicular vessels and vas deferens were skeletonised, and the indirect sac was reduced or transected, with the peritoneal edge retracted at least 4 cm from the deep inguinal ring (peritonealisation). A three-dimensional configured polyester mesh (Parietex Anatomical, Covidien-Medtronic®, US) was placed into the extraperitoneal space, overlapping the defects by at least 2 cm. The mesh was fixed with either (1) titanium tacks (ProTackTM Fixation Device, Covidien-Medtronic®, US) over the superior and medial aspects; (2) N-butyl cyanoacrylate glue (CompontTM Medicinal Adhesive Glue, Compont®, China) over all aspects of the mesh, or (3) no fixation. An 8 Fr silicon suction drain (BDA-YS-0100, Branden®, China) was placed at the extraperitoneal space and exited via the upper operating port wound and connected to a closed-system collection bottle. The extraperitoneal space was then deflated under direct vision to ensure correct positioning of the mesh upon wound closure in the drain group, OR a “dummy drain” was attached to the dressing at the skin level for the no-drain group. A knot was tied at the tip of the dummy drain to achieve a vacuum-sealed connected to the collection bottle to simulate drain placement at the enclosed space (Fig. 2). Vacuum status of the system was maintained by emptying and re-charging the silastic collection bottle at four-hourly interval.
Postoperatively, the patients resumed a normal diet 4 h after surgery. Paracetamol 500 mg was administered every 8 h for pain control in all cases. The extraperitoneal drain/dummy drain was removed 23 h after the operation, followed by clinical and ultrasound assessments. Ultrasonography was performed in the Radiology Department by independent specialist radiologists who were blinded to the study group with a standardised protocol; a high-frequency 2.5–8 MHz linear transducer (Acuson® S2000, Siemens) OR a 2.8–5 MHz (GE Logiq book, C1-5) linear transducer were used for ultrasonic examination of the inguinoscrotal region without excessive compression by the probe. Any collection around the operative region was recorded and measured in three dimensions (the largest two dimensions were used for calculations for simplicity considerations) (Fig. 3). Valsalva manoeuver and Doppler ultrasound were performed to exclude subtle early recurrence. Patients were subsequently discharged according to their clinical recovery status.
Data collection and follow-up
Postoperative assessment was performed by independent parties: both patients and assessors (surgeons not involved in the patients’ operations) were blinded to the group. Patients were asked to fill out pain score forms 7 days postoperatively. Outcomes were assessed in the outpatient clinic using standardised protocol for different aspects of outcomes including patient satisfaction, chronic pain and discomfort, paresthesia, and recurrence at different pre-set time intervals (1 week, 1 month, 7 months), and patients were subject to clinical and/or ultrasound assessments with the same protocol as described above.
Results
From May 2016 to February 2017, 126 consecutive patients were screened for eligibility. Most excluded patients declined study participation or had significant medical co-morbidities. Other causes included previous lower abdominal surgery, declining surgical treatment, or defaulting after the first consultation (Table 1). Ninety patients were included and randomised. One week postoperatively, seven patients in the drain group and five in the no-drain group defaulted and were excluded (n = 12, post-randomisation exclusion = 13.3%) (Diagram 1). Eventually, 41 patients were allocated to the drain group, and 37 patients were allocated to the no-drain group for statistical calculations (n = 78).
Patients in the drain group tended to be older than those in the no-drain group (p = 0.32). There were no differences in other demographic parameters between the two groups including sex, drinking history, significant co-morbidities, history previous hernia operations over the contralateral side, side of hernia, or mean size of hernia defects (Table 2), except there were more smokers in the no-drain group (3 vs 10). Operations were performed as described above. There was no difference in the mean total operative time (p = 0.458), time for the different stages of operation (port insertion, preperitoneal dissection, sac dissection, randomisation), or the distance between the camera and two working ports. However, more patients in the drain group had peritoneal perforation during dissection (p = 0.031) (Table 3). Since this might influence preperitoneal collection, subgroup analysis was carried out. There was no difference between groups in the use of fixation adjuncts after TEP hernioplasty. Total postoperative stay was comparable between groups (p = 0.669) (Table 4). There were no wound complications, infections, or early recurrence observed, but two patients did develop postoperative urinary retention in the drain group (p = 0.495).
The overall incidence of clinical seroma formation was 25.6% one day postoperatively (n = 20) and 60.3% 6 days 6 postoperatively (n = 37). The overall incidence of clinical seroma in the inguinal/scrotal region dropped to 13.2% at the 1-month follow-up. The mean drain output was 57.9 ml (range 5–270 ml). There were significantly fewer patients with seroma on postoperative day 1 and 6 in the drain group compared with the no-drain group (p = 0.022, p = 0.000, respectively). Ultrasound assessment further validated the clinical findings, showing a smaller mean seroma size in the drain group on postoperative day 1 and 6 (p = 0.005, p = 0.000, respectively) (Table 5).
Subgroup analysis was performed to evaluate possible confounding factors, which may have influenced the occurrence and size of preperitoneal seroma after TEP hernioplasty. One proposed cause for seroma formation is hernia sac management after dissection. We compared simple reduction to ligation and transection, there was no significant difference demonstrated. Similarly, we observed a trend of increasing seroma size with time in both groups. On ultrasound, there was no difference in seroma size between groups (Table 6). Since there were significantly more patients with peritoneal perforation during dissection, theoretically, any preperitoneal fluid could be drained intraperitoneally through the small defects at the repaired peritoneum. However, we could not find any statistical difference between the groups with and without peritoneal perforation (Table 7). Comparison was also made between different types of fixation method of the prosthetic mesh; there was no significant difference in clinical seroma formation among different types of fixation adjuncts (Table 8).
Patients were asked to fill out a standardised Visual Analogue Score (VAS) form assessing their pain. Since patients were blinded to the group assigned, we believe this assessment was valid and objective. VAS score was highest on postoperative day 1 and slowly declined with time. In general, there was no difference between resting and coughing pain scores between the drain and no-drain groups (Table 9); however, the resting pain score on postoperative day 5 was significantly lower in the drain group than the no-drain group (p = 0.017); despite observing a trend of a lower resting pain score in the drain group, the difference was not significant (Charts 1, 2).
At the 1-month follow-up, 48.7% (n = 38) of the studied population came back for clinical and ultrasound assessment. The overall seroma rate dropped to 13.2% (vs 60.3% on postoperative day 6). There was no difference in clinical seroma formation (p = 0.632), mean size of seroma (p = 0.591), or mean postoperative VAS score (p = 0.981) between groups (Table 10). Cross-sectional survey was performed with mean follow-up of 7.3 months (n = 76, 97.4%), there was no statistical difference between drain and no-drain group in terms of recurrence (0), clinical seroma formation (0), pain killer utilization (0), affect daily activities (0), paraesthesia around operative zone (0), infective complication (0), chronic pain (6 vs 5, p = 0.891), chronic discomfort (3 vs 1, p = 0.362), mean VAS score (0.15 vs 0.14, p = 0.892) (Table 11). Among all the potential risk factors analysed, operating time is associate with clinical seroma formation at day 6 post operation in our study (p < 0.003) (Table 12).
Discussion
Among the 78 patients included in the analysis, we demonstrated a significant reduction in the incidence of clinical seroma formation in the drainage group, together with a decrease in the mean seroma size in the preperitoneal space at both postoperative day 1 and day 6. The trial was conducted in a prospective double-blinded manner, i.e., the assessors for clinical outcomes, the radiologists who examined the patients, and patients themselves were blinded to the group assigned. To avoid the possibility that patients and assessors would be aware of the study group assigned, a dummy drain was placed at the skin level to mimic a preperitoneal drain, with the end tied with a knot to maintain the vacuum status over the collection bottle. Moreover, the assessment was performed only after the drain was removed 23 h postoperatively; therefore, during the clinical and ultrasound assessment, there was no drain attached in either group of patients, further eliminating the chance of identifying the patient group from the drain content. We believe these measures helped ensure that independent assessments of clinical seroma formation, ultrasonic seroma size, and pain scores were objective enough to validate our conclusions.
There were a significantly greater proportion of patients in the drain group with peritoneum perforated during dissection, thus suggesting the possibility that any extraperitoneal fluid could be drained intraperitoneally via small holes or defects over the peritoneum and affect the overall outcome. A separate subgroup analysis was performed to exclude this possibility, which showed no significant difference in the perforated and non-perforated groups. However, this conclusion may not be totally valid, as subgroup analysis typically has insignificant statistical power, as the required sample size was not calculated per primary outcome. Nonetheless, no past literature reported the effect of peritoneal perforation during TEP hernioplasty, and we are the first group to include this as a confounding factor for hernia surgery outcomes.
Although generally there was no difference in pain experienced by patients in either group, except for resting pain score on postoperative day 5, we observed a trend towards patients who received preperitoneal drainage having less resting pain compared with the no-drain group with time, which coincided with the seroma reaching its maximal size. There possibly was a relationship between pain and seroma size due to preperitoneal stretching and distention by the seroma with resultant somatic pain. Our findings also in line with the largest retrospective by Gao et al. which preperitoneal drainage can potentially reduce postoperative pain [16]. However, the limited number of patients recruited and the slight difference in pain score between the two groups does not provide statistically sound conclusions, and a larger randomised trial in which the sample size can be calculated based on differences in pain score should be conducted before this is considered as a valid conclusion.
After 1 month, we did not observe any differences between groups in terms of seroma rate, seroma size, or VAS pain score. It is likely that the benefits of preperitoneal drainage only occur in the early postoperative period (within 1 week), and the effects diminish or became insignificant at later stages. Alternatively, based on our previous assumption that seroma is one cause for somatic pain, with the decrease in seroma size over time, there would not be any difference in pain score. This phenomenon extended to 7 months’ post operation with no difference demonstrated in both groups and all clinical seroma subsided (0%).
The preperitoneal drain was left in place for 23 h for two reasons: first, to avoid the theoretical risk of bacterial migration and mesh infection caused by drain insertion and yet allow sufficient drainage time to achieve maximal beneficial effect; second, to allow day procedures for laparoscopic hernioplasty without an extended hospital stay; and finally, to avoid affecting the objective assessment by our clinical assessors on postoperative day 1. We initially believed that the maximal seroma size would be reached on postoperative day 1, but ultimately, the seroma size and incidence peaked at 1 week postoperatively (or between 1 week and 1 month postoperatively) and significantly decreasing by 1 month postoperatively. The use of preperitoneal drainage after TEP hernioplasty does not allow same-day discharge, and hence it may not be possible to practice ambulatory surgery for laparoscopic inguinal hernia repair. Further studies comparing the timing of drain removal will help to validate the shortest drainage time providing the maximal benefit in seroma reduction.
We successfully proved that closed-system suction preperitoneal drainage can effectively reduce the incidence and size of seroma after laparoscopic TEP hernioplasty for inguinal hernia. In the latest updated guideline on laparoscopic and endoscopic (TEP) treatment of inguinal hernia by the International Endohernia Society in 2015, there was level 3 evidence concluding that drain use increased the risk of infection or recurrence and only Grade C evidence that a closed-suction drain could be used to reduce the risk of seroma formation without an increased risk of infection [17]. In this study, we are the first group to demonstrate level 1 evidence to support the use of a closed-system preperitoneal drain after laparoscopic TEP hernioplasty to prevent seroma formation without significantly increasing the risk of septic complications or mesh infection. On the other hand, because of the short-term follow-up and relatively small sample size, the recurrence rate and long-term complications need to be explored by a larger scale randomised controlled trial. Moreover, the optimal timing of drain removal is another issue to be studied by comparing outcomes of patients with drains removed at different pre-set time intervals to explore the most appropriate timing of drainage and to further reduce the risk of potential infection. According to our result, operating time is associated with development of clinical seroma at day 6 post-operation, therefore, it is reasonable to advocate the use of preperitoneal suction drain after prolonged dissection or operation.
Conclusion
Extraperitoneal drainage for 23 h after laparoscopic TEP hernioplasty for inguinal hernia can effectively decrease seroma formation in the early postoperative period without compromising surgical outcomes, and potentially improving postoperative pain. The benefit is short-term and no significant difference was demonstrated after 1-month post operations. This tradition technique when applied to novel operative repair of inguinal hernia is safe and feasible with no significant morbidity. This can be considered as option to improve patient satisfactions and recovery in selected patients group for maximal benefit, especially for those with prolonged operation which may associate with higher chance of seroma formation.
References
Bittner R et al (2011) Guidelines for laparoscopic (TAPP) and endoscopic (TEP) treatment of inguinal hernia [International Endohernia Society (IEHS)]. Surg Endosc 25(9):2773–2843
Bittner R, Schwarz J (2012) Inguinal hernia repair: current surgical techniques. Langenbecks Arch Surg 397(2):271–282
Wauschkuhn CA et al (2010) Laparoscopic inguinal hernia repair: gold standard in bilateral hernia repair? Results of more than 2800 patients in comparison to literature. Surg Endosc 24(12):3026–3030
Fan JK et al (2017) Randomized trial comparing self gripping semi re-absorbable mesh (PROGRIP) with polypropylene mesh in open inguinal hernioplasty: the 6 years result. Hernia 21(1):9–16
She WH et al (2011) Needlescopic totally extraperitoneal hernioplasty for unilateral inguinal hernia in adult patients. Asian J Surg 34(1):23–27
Wada H et al (2012) Laparoscopic transabdominal preperitoneal inguinal hernia repair using needlescopic instruments: a 15-year, single-center experience in 317 patients. Surg Endosc 26(7):1898–1902
Wijerathne S et al (2014) A prospective randomized controlled trial to compare single-port endo-laparoscopic surgery versus conventional TEP inguinal hernia repair. Surg Endosc 28(11):3053–3058
Tran H (2011) Safety and efficacy of single incision laparoscopic surgery for total extraperitoneal inguinal hernia repair. JSLS 15(1):47–52
Krishna A et al (2012) Laparoscopic inguinal hernia repair: transabdominal preperitoneal (TAPP) versus totally extraperitoneal (TEP) approach: a prospective randomized controlled trial. Surg Endosc 26(3):639–649
Lopez-Monclus J et al (2012) Persistent inguinal seroma managed with sprinkling of talcum powder: a case report. J Med Case Rep 6:391
Berney CR (2012) The Endoloop technique for the primary closure of direct inguinal hernia defect during the endoscopic totally extraperitoneal approach. Hernia 16(3):301–305
Beacon J, Hoile RW, Ellis H (1980) A trial of suction drainage in inguinal hernia repair. Br J Surg 67(8):554–555
Rodrigues AJ Jr et al (2003) The Stoppa procedure in inguinal hernia repair: to drain or not to drain. Rev Hosp Clin Fac Med Sao Paulo 58(2):97–102
Peiper C et al (1997) Value of subcutaneous drainage in repair of primary inguinal hernia. A prospective randomized study of 100 cases. Chirurg 68(1):63–67
Ismail M et al (2009) Impact of closed-suction drain in preperitoneal space on the incidence of seroma formation after laparoscopic total extraperitoneal inguinal hernia repair. Surg Laparosc Endosc Percutan Tech 19(3):263–266
Gao D et al (2015) Clinical research of preperitoneal drainage after endoscopic totally extraperitoneal inguinal hernia repair. Hernia 19(5):789–794
Bittner R et al (2015) Update of guidelines on laparoscopic (TAPP) and endoscopic (TEP) treatment of inguinal hernia (International Endohernia Society). Surg Endosc 29(2):289–321
Acknowledgements
The authors thank ZL; GZ; HZ; JL; JW; JS for assisting with creation of a database and patients care. Expert advice from Prof Anil.Sharma. is greatly acknowledged.
Funding
No funding was received for conducting this trial.
Author information
Authors and Affiliations
Contributions
JKMF—Original idea, data analysis, preparation of manuscript, critical revision of manuscript; JL—data collection and follow-up of patients; KC—data collection and follow-up of patients; XX—data collection and follow-up of patients; HKC—critical revision of manuscript; FSYC—original idea, Critical revision of manuscript; KWHC—data collection and follow up of patients; CML—critical revision of manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
JKMF declare no conflict of interest; JL declare no conflict of interest; KC declare no conflict of interest; XF declare no conflict of interest; XX declare no conflict of interest; HKC declare no conflict of interest; FSYC declare no conflict of interest; KWHC declare no conflict of interest; CML declare no conflict of interest.
Ethical standard
All procedures involving human participants were performed in accordance with the ethical standards of our institutional research committee and the study was conducted according to the ethical standards of the Helsinki Declaration of 1975.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study after detail explanation of possible complications. Patients were then given at least 24 h for consideration of participating in the clinical trial before operation.
Rights and permissions
About this article
Cite this article
Fan, J.K.M., Liu, J., Chen, K. et al. Preperitoneal closed-system suction drainage after totally extraperitoneal hernioplasty in the prevention of early seroma formation: a prospective double-blind randomised controlled trial. Hernia 22, 455–465 (2018). https://doi.org/10.1007/s10029-018-1731-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-018-1731-2