Abstract
Purpose
Synthetic mesh for herniorrhaphy has been placed under critical observation regarding the potential association of mesh placement and the subsequent development of autoimmune diseases. We sought to evaluate whether there is a link between synthetic polypropylene mesh repairs and the subsequent development of systemic/autoimmune disorders (SAID).
Study design
Adult men undergoing hernia repair with mesh between January 2008 and December 2009 in New York State were identified using International Classification of Diseases, Ninth Revision, Modification procedure codes and Current Procedural Terminology Coding System, Fourth Edition codes. A control cohort of men undergoing colonoscopy was created with whom to compare outcomes.
Results
A total of 29,712 patients underwent hernia repair between January 2008 and December 2009. In the control cohort, 79,265 patients underwent colonoscopy. During the entire follow-up, 475 patients undergoing hernia repair and 1305 patients in the control cohort were diagnosed with autoimmune disease. When patients were matched based on demographics, comorbidities and procedure date, hernia repair was not associated with an increased risk of developing autoimmune disease over the entire follow-up time period. 1.6% of those in the hernia group vs. 1.7% of those in the colonoscopy group developed SAID [risk ratio (95% CI): hernia vs. colonoscopy 0.93(0.79–1.09)]. No association between mesh surgery and increased risks of SAID was found at any of the specified time points (6 months, 1 year, and 2-year follow-up).
Conclusions
Mesh-based hernia repair was not associated with the development of autoimmune diseases compared to those undergoing routine screening colonoscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the United States, over 8,00,000 hernia repairs are performed each year [1]. Because of the high rate of recurrence, the “metallic mesh” for hernia repair garnered widespread use by 1946 [2]. The use of surgical mesh has been used with the intention of reinforcing weak tissues through the development of scar tissue, stimulated by a foreign body reaction [3]. Over time, there has been an increasing use of this mesh-based repair [3]. With the innumerable types of materials used, the type of mesh chosen is highly dependent on the individual patient with synthetic non-absorbable polypropylene and polyester meshes being the most common for hernia repair [4].
After the Food and Drug Administration’s (FDA) analysis of the scientific literature and medical device adverse event reports regarding hernia repair with mesh, many complications have been associated with hernia mesh. Such complications include adhesions, injuries to neighboring organs, nerves and blood vessels [5], pain, hernia recurrence, infection, mesh migration, mesh shrinkage and bowel obstruction [3]. After hundreds of complaints, in 2011 the FDA issued a safety communication regarding the use of surgical mesh in hernia repair and stated that serious complications may be associated with surgical mesh for hernia repair placing patients in inherent risk [5].
On consumer websites, the use of synthetic mesh for hernia repair has been placed under critical observation. A population of individuals believe there to be an association between the placement of mesh and finally the development of systemic autoimmune/inflammatory disorders (SAID) [6]. One proposed theory discussing this association is a subset of individuals experience an immune response to the mesh implant. This ultimately causes the polypropylene, found in synthetic mesh, to degrade under an oxidative process [7]. The degradation of polypropylene could result in a chronic state of inflammation, which could then lead to the developing SAID [8]. At the present time, mesh degradation has not been confirmed in human cases. It is possible that the onset of SAID succeeding mesh placement is coincidental.
To investigate these claims further, we utilized regional administrative claims data to conduct a retrospective cohort study with matched controls to determine if there is a potential link between placement of synthetic polypropylene mesh for hernia repair and the subsequent development of SAID.
Methods
Data source
Data were obtained from the New York State Department of Health Statewide Planning and Research Cooperative System (SPARCS). SPARCS is an all-age group, all-payer dataset that collects patient and treatment information for every hospital discharge, ambulatory surgery, outpatient service and emergency department admission in New York State since 1979 [9]. The data contains patient characteristics, primary and secondary diagnoses and procedures, and length of hospital stay and charges. Furthermore, a unique personal identifier is assigned to every patient, which is encrypted to allow longitudinal analyses without compromising patient confidentiality.
Study population and follow-up
Adult male patients undergoing hernia surgery with mesh between January 2008 and December 2009 were identified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes and Current Procedural Terminology Coding System, Fourth Edition (CPT-4) codes (Appendix 1). Due to the near-universal adoption of mesh in inguinal hernia repair, patients undergoing inguinal hernia repair in our cohort were assumed to have undergone the repair with mesh [3, 10, 11]. A control cohort was selected during the same time period, which included male patients undergoing colonoscopy. Patients who underwent hernia repair were not included in the control cohort. Control patients who had a colorectal cancer diagnosis within 1 month before and 1 month after colonoscopy were excluded. Participants who relocated or held residence in other states during the study period were excluded.
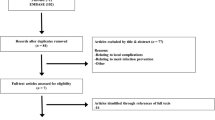
Patients who had less than 1 year’s record in SPARCS system before index admission were excluded. Patient histories were queried from the database from 1995 (first year available to us). Patients who had previous mesh related procedures (inguinal hernia repair, ventral, umbilical or incisional hernia repair with mesh or male urethral sling procedures) were also excluded. Additionally, we excluded patients with a previous diagnosis of SAID. Detailed patient selection process is depicted in the flow chart (Fig. 1).
Patients were followed until the end of the study time period (Dec 2014). The primary outcomes of interest were the development of SAID at 6 months, 1 year, 2 years and during the entire follow-up period. The SAID disorders of interest included Grave’s disease, Hashimoto’s thyroiditis, pernicious anemia, autoimmune hemolytic anemia, autoimmune thrombocytopenic purpura, amyotrophic lateral sclerosis, multiple sclerosis, Guillain–Barré Syndrome, myasthenia gravis, Goodpasture syndrome, vasculitis, celiac disease, pemphigus vulgaris, systemic lupus erythematosus, systemic sclerosis, Sjogren’s syndrome, dermatomyositis, polymyositis, rheumatoid arthritis, ankylosing spondylitis and fibromyalgia (Appendix 2).
Analyzed patient characteristics included age, race, insurance status (Medicare, Medicaid, Commercial and other), place of residence (New York State or out-of-state resident), comorbidities and previous cancer diagnosis. Relevant comorbidities were identified using algorithms validated by Elixhauser et al. [12], including coronary artery disease, hypertension, congestive heart failure, diabetes, chronic pulmonary disease, obesity, anemia, peripheral vascular disease, renal failure, cerebrovascular disease and depression. An unknown category was created for missing race information.
Statistical analysis
Baseline characteristics were compared between mesh and the control cohort. Events and percentages were presented for patient demographics and comorbidities. We performed individual matching (1 case:2 controls) based on patient characteristics and comorbidities to account for difference between mesh and control cohorts. Matching variables included age, race, insurance, place of residence, year and quarter when the procedure was performed, all major comorbidities included and previous cancer diagnosis. Age was matched based on 5-year groups. Flexibility was given when matching major comorbidities to allow for difference in three of the eleven comorbidities. Exact match was performed for other characteristics. Balance achieved by matching was assessed by examining differences in baseline variables between mesh and control cohorts before and after matching.
The presence of SAID was determined after matching for 6-month, 1-year, 2-year intervals, and for the entire follow-up time period. Events and percentages for the matched cohort were presented and risk ratios were calculated. Differences between groups were assessed using stratified Mantel–Haenszel χ 2 tests for paired data in the matched cohort. All analyses were performed using SAS v9.3 (SAS Institute Inc., Cary, NC).
Results
From January 2008 to December 2009, a total of 53,409 male patients were identified who underwent hernia repair. Of these, 29,712 male patients did not have previously diagnosed SAID, and were included in the final analysis. The control cohort without pre-existing SAID included 79,265 male patients having colonoscopy.
Mean ages of patients undergoing mesh-based hernia repair POP and colonoscopy were 58.2 and 58.5, respectively (Table 1). Most patients were white (hernia: 75.5%, colonoscopy: 70.0%) and had commercial insurance (50.0, 60.0%, respectively). When compared to the control group, patients undergoing hernia repair were older, had more medical comorbidities, and were more likely to be white and have commercial insurance. The prevalence of previous cancer among patients undergoing hernia repair and colonoscopy was similar (11.8 vs. 11.3%).
The average time between procedure and end of follow-up was 6 years. A total of 475 (1.6%), and 1305(1.7%) patients undergoing mesh hernia repair and colonoscopy, respectively, were diagnosed with SAID during follow-up until the end of 2014 (Table 2). After matching, mesh-based hernia repair was also not associated with increased risks of developing SAID over the entire follow-up time period (Table 2) [risk ratio (RR) 0.93, 95% CI 0.79–1.09]. Furthermore, no association between hernia mesh repair and the development of SAID was found at 6 months, 1 year and 2-year follow-up (Table 2).
Discussion
Our main findings suggest there is no association of mesh placement with SAID. We found after matching with the control cohort, patients receiving mesh-based hernia repair were not associated with an increased risk of developing SAID during the follow-up period. There was no association between hernia mesh repairs and developing SAID found at 6 months, 1 year and 2-years postoperatively.
Mesh usage provides a new strength layer through the development of scarring, obviating the need to reapproximate a fascial defect. Mesh repairs gained rapid popularity such that they became the dominant hernia repair beginning in year 1998 with the introduction of light-weight mesh [13]. Patients receiving mesh-based hernia repairs tend to have a lower recurrence rate compared to those undergoing repair without mesh. Studies have demonstrated that mesh repairs result in a significant decrease in recurrence compared to non-mesh repairs using sutures alone (2.7 vs. 8.2%) [14]. Soon after its rapid adoption, mesh has been considered a viable treatment option and often preferred over basic suture repairs [11]. These advantages need to be balanced against the known risks associated with mesh placement, such as pain, bleeding, infection, adhesion and bowel obstruction [3]. The FDA has published a number of safety communications which summarize the risks associated with the use of hernia mesh [3, 5]. It is important for clinicians to explicitly discuss these risks with patients to ensure together they are making an informed decision.
In addition to these known risks, consumers’ concerns about the potential association of surgical mesh and the development of autoimmune disease have brought mesh under further scrutiny [6]. Because of these allegations, there has been increasing uncertainty among patients when considering the long-term safety and efficacy of these procedures for their own health outcomes, despite high success rates. The proposed rationale for the development of SAID is that some individuals may experience an immune response to mesh implantation. This may cause the polypropylene to oxidatively degrade [7] causing the individual to undergo a chronic state of inflammation, which could in theory result in SAID [8]. One study assessing various types of mesh in animal models found that all meshes induced varying levels of inflammatory responses [15]. These included foreign body reactions and strong fibrotic responses [15]. Another study using rabbit models found after 4-months implantation the “mean number of inflammatory cells was greater around the polytetrafluoroethylene (ePTFE) when compared with the mid-weight polypropylene, but equal to others (p = 0.02) [16].
The fibrotic response to mesh is what provides strength and support in patients with inherently weak tissue. Nonetheless, certain individuals and patient advocate groups claim that adverse reactions to hernia mesh lead to subsequent diagnoses of autoimmune disorders, such as lupus, fibromyalgia, and rheumatoid arthritis [17]. To date, there remains a lack of evidence that polypropylene leads to SAID, with no available data from controlled trials or prospective cohort studies. To our knowledge, this is the first population-based study evaluating the effect of hernia mesh repair and onset of SAID. After matching based on patient characteristics and procedure time, we found no relationship between the implantation of synthetic mesh for hernia repair and subsequent development of SAID.
There were few limitations associated with using the New York State cohort data. Clinical variables such as the extensiveness of the hernia repair were not available in administrative data. Every attempt was made to adjust for observed confounding factors when running statistical methods by making individual matching, but it is possible that unmeasured differences may exist between the case and control groups. It is possible some of the cases we included in our hernia mesh-based repair cohort may include hernia repair without the use of mesh. This is because ICD-9 code is ambiguous including mesh “or other prosthesis for repair of hernia”. However, based on the existing literature related to hernia repair practice patterns we expect usage of techniques using native tissue repair or with other prosthesis such as biological graft to be very rare. Finally, there may be some patients who developed symptoms of SAID but not yet had an assigned diagnosis of interest represented in the cohort.
Despite these limitations, our findings provide strong evidence that patients receiving mesh-based hernia repair were not at increased risk of developing SAID at up to 6 years follow-up. These findings can be used to reassure patients who are contemplating inguinal hernia repair with mesh. Furthermore, future prospective randomized trials must be utilized to study the different types of mesh and different types of hernias, where exposure to mesh type and breakdown can vary.
Conclusions
Mesh-based hernia repair was not associated with the development of autoimmune diseases, supporting the safety of these devices and refuting the claims that mesh leads to systemic illness.
References
Rutkow IM (2003) Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin North Am 83(5):1045–1051 (v-vi)
Gandhi D, Marcin S, Xin Z, Asha B, Kaswala D, Zamir B (2011) Chronic abdominal pain secondary to mesh erosion into cecum following incisional hernia repair: a case report and literature review. Ann Gastroenterol 24(4):321–324
FDA (2016) Hernia surgical mesh implants. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/HerniaSurgicalMesh/default.htm. Accessed 22 Nov 2016
Bilsel Y, Abci I (2012) The search for ideal hernia repair; mesh materials and types. Int J Surg 10(6):317–321
FDA (2014) Safety communications—surgical mesh: FDA safety communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm142636.htm. Accessed 22 Nov 2016
Akre J (2014) Autoimmune diseases and surgical mesh—causation or correlation? Available from: http://meshmedicaldevicenewsdesk.com/autoimmune-diseases-and-surgical-mesh-causation-or-correlation
Ostergard DR (2010) Polypropylene vaginal mesh grafts in gynecology. Obstet Gynecol 116(4):962–966
Clave A et al (2010) Polypropylene as a reinforcement in pelvic surgery is not inert: comparative analysis of 100 explants. Int Urogynecol J 21(3):261–270
Health N.Y.S.D.O (2014) Statewide planning and research cooperative system (SPARCS). 2014 06/2014 19 August, 2014. Available from: http://www.health.ny.gov/statistics/sparcs/
Treadwell J, Tipton K, Oyesanmi O, Sun F, Schoelles K (2012) AHRQ Comparative Effectiveness Reviews. In: Surgical Options for Inguinal Hernia: Comparative Effectiveness Review. Agency for Healthcare Research and Quality (US), Rockville (MD)
Brown CN, Finch JG (2010) Which mesh for hernia repair? Ann R Coll Surg Engl 92(4):272–278
Elixhauser A et al (1998) Comorbidity measures for use with administrative data. Med Care 36(1):8–27
Klinge U (2008) Mesh for hernia repair. Br J Surg 95(5):539–540
Nguyen MT et al (2014) Comparison of outcomes of synthetic mesh vs suture repair of elective primary ventral herniorrhaphy: a systematic review and meta-analysis. JAMA Surg 149(5):415–421
Orenstein SB et al (2012) Comparative analysis of histopathologic effects of synthetic meshes based on material, weight, and pore size in mice. J Surg Res 176(2):423–429
Harrell AG et al (2007) Prospective histologic evaluation of intra-abdominal prosthetics 4 months after implantation in a rabbit model. Surg Endosc 21(7):1170–1174
Widerman N (2013) The links between surgical mesh complications and the development of autoimmune diseases. Available from: http://meshproblems.weebly.com/linking-the-research-from-fbr-to-implant-materials-to-autoimmune-disease-initiation-or-exacerbation.html
Acknowledgements
None to report at this time.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
BC declares no conflict of interest. DT declares no conflict of interest. JM declares no conflict of interest. KE declares conflict of interest related to being an investigator for Boston Scientific, Astellas and Allergan not directly related to the submitted work. JA declares conflict of interest related to being an investigator/expert witness for Boston Scientific Corporation not directly related to the submitted work. JQC declares no conflict of interest. AS declares a conflict of interest receiving grants from the US Food and Drug Administration not directly related to the submitted work.
Ethical statement
For this type of study formal consent was not required.
Statement of human and animal rights
This article does not contain any studies with animals performed by any of the authors. This study used deidentified administrative data from humans.
Informed consent
This study did not require informed consent because this was a retrospective study using deidentified administrative data.
Funding
This work was supported by the American Urological Association Data Grant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chughtai, B., Thomas, D., Mao, J. et al. Hernia repair with polypropylene mesh is not associated with an increased risk of autoimmune disease in adult men. Hernia 21, 637–642 (2017). https://doi.org/10.1007/s10029-017-1591-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-017-1591-1