Abstract
Asymbiotic nitrogen (N) fixation (ANF) is an important source of N in pristine forests and is predicted to decrease with N deposition. Previous studies revealing N fixation in response to N deposition have mostly applied understory N addition approaches, neglecting the key processes (for example, N retention and uptake) occurring in forest canopy. This study evaluated the effects of N deposition on N fixation in the soil, forest floor, mosses, and canopy leaves in a temperate forest (in central China) and a tropical forest (in southern China) with different treatments: control, understory N addition, and canopy N addition. Results showed that total ANF rates were higher in the temperate forest (2.57 ± 0.19 mg N m−2 d−1) than in the tropical forest (1.34 ± 0.09 mg N m−2 d−1). N addition inhibited the soil, forest floor, moss, and foliar N fixation in the temperate forest, whereas it inhibited only the soil N fixation in the tropical forest. Compared to canopy N addition, understory N addition overestimated the inhibitory effects of N deposition on total ANF slightly in the tropical forest (by 35%) but severely in the temperate forest (by 375–472%) due to neglecting canopy retention of N. In summary, our findings indicate that ANF has different rates and sensitivities to N addition between tropical and temperate forests and that understory N addition overestimates the N deposition effects on ANF in forests, particularly in the temperate forest. These findings are important for our accurate understanding and estimate of terrestrial N fixation under N deposition scenarios.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Highlights
-
Lower ANF rates in the tropical forest than in the temperate forest
-
Less ANF sensitivity to added N in the tropical forest than in the temperate forest
-
Overestimates of N deposition effects on ANF in forests by understory N addition
Introduction
Nitrogen (N) is a key nutrient to organisms, and its availability regulates ecosystem structure and function (Vitousek and others 2002, 2013; Galloway 1998). Asymbiotic N fixation (ANF), a process by which free-living N-fixing microbes transfer dinitrogen gas (N2) into biologically available N (NH3), is an important pathway of N input into unmanaged terrestrial ecosystems (Cleveland and others 1999; Reed and others 2011). To date, free-living N-fixing microbes have been found in numerous ecosystem substrates, such as the surface soil (Cusack and others 2009), forest floor (Reed and others 2007), epiphytes (for example, mosses and lichens; Cusack and others 2009), and canopy foliage (that is, epiphylls and/or endophytes, Cusack and others 2009; Moyes and others 2016). Previous studies have indicated that ANF constitutes a major source of new N in many high-latitude forests, where rates of N deposition are low (DeLuca and others 2008; Gundale and others 2011; Moyes and others 2016). In several N-rich mature tropical forests, ANF remains active in the soil and forest floor (Reed and others 2007, 2008; Cusack and others 2009), thereby driving and sustaining tropical N richness (Hedin and others 2009). Despite the key roles of N fixation in terrestrial ecosystems, our knowledge of N fixation rates and of their controls is still limited (Cleveland and others 1999; Reed and others 2011; Vitousek and others 2013).
Over the past decades, human activities have led to a dramatic increase in atmospheric N deposition rates (Galloway and others 2004; Chen and others 2015). In most regions of the world, average rates of N deposition have exceeded 10 kg N ha−1 y−1 and are predicted to double by the year 2050 (Galloway and others 2008). Elevated N deposition has adverse impacts on forest ecosystems, resulting in soil acidification, nutrient losses, and decreases in plant and microbial diversity (Galloway and others 2004; Matson and others 1999). Globally, N inputs down-regulate ANF rates (Dynarski and Houlton 2018) because N fixation is more energy expensive than either soil N uptake or the direct utilization of exogenous N (Gutschick 1981). For individual studies, however, the sensitivities of N fixation to N inputs are divergent.
First, previous studies in temperate forests showed that short-term (for example, < 1 year) addition of N suppressed N fixation in the lichens (Hällbom and Bergman 1979), leaf litter (Nohrstedt 1988), and soil (Perakis and others 2017); however, in humid tropical forests, N addition did not affect N fixation (Reed and others 2007) or down-regulated N fixation after chronic (for example, > 3 years) treatment (Barron and others 2008; Cusack and others 2009; Matson and others 2015; Wang and others 2017). Second, a previous study in two tropical rainforests showed that N addition down-regulated N fixation rates in some substrates (for example, soil and forest floor) but not in others (for example, epiphytic mosses; Cusack and others 2009). Third, several field and greenhouse studies revealed that low N addition had no or slightly inhibitory effects on N fixation, whereas high N addition had strong inhibitory effects on N fixation (Gundale and others 2011; Batterman and others 2013; Zheng and others 2016). The above observations indicate our incomplete understanding of the N deposition controls over N fixation.
Importantly, our current knowledge of how N deposition affects N fixation is derived from field experiments with understory N addition (N is directly added to the forest floor), which neglects key processes (that is, retention and uptake of N) occurring in the forest canopy (Zhang and others 2015; Shi and others 2016). There is increasing evidence that forest canopy retains substantial N under atmospheric N deposition (Klopatek and others 2006; Fenn and others 2013; Houle and others 2015) or after canopy N addition (Gaige and others 2007; Dail and others 2009). The N retained by the forest canopy is absorbed by foliage, epiphytes, bark, and branches (Adriaenssens and others 2012), which thereby reduces the inorganic N concentrations (Klopatek and others 2006; Gaige and others 2007; Fenn and others 2013) and alters the ratio of NH4+ to NO3− (Houle and others 2015) in throughfall. Thus, the traditional approach of understory N addition likely overestimates the negative effects of N deposition on understory processes and underestimates the effects on canopy processes. Although ANF has been found in both aboveground and understory compartments in forest ecosystems (Reed and others 2011), no published study has used a canopy N addition approach to determine the effects of N deposition on N fixation.
This study aimed to determine whether canopy and understory N addition have different effects on ANF. We measured N fixation rates in the substrates of the forest floor, soil, canopy leaves, and mosses in the Jigongshan (JGS) forest (a temperate forest in central China) and the Shimentai (SMT) forest (a tropical forest in southern China) following different N addition treatments: control, understory N addition, and canopy N addition. We selected two forests with different climatic zones because ANF may have different rates (Reed and others 2011) and show different sensitivities to N addition between tropical and temperate forests (Dynarski and Houlton 2018). Moreover, current studies of N fixation and its responses to N addition are lacking in both tropical and temperate forests in Asian regions (Cleveland and others 1999; Dynarski and Houlton 2018). The two selected forests have a similar age (45–50 years), but the SMT forest has a higher mean annual temperature (20.8 vs. 15.2°C), precipitation (2364 vs. 1119 mm), and background N deposition (34.1 vs. 19.6 kg N ha−1 y−1) than the JGS forest. Although high N deposition can inhibit N fixation (Galloway and others 2008), we hypothesized that ANF rates would be higher in the SMT forest than in the JGS forest (H1) because high temperature and precipitation can benefit ANF (Reed and others 2011), and several recent studies have found active ANF in some tropical forests in southern China regardless of high N deposition (Zheng and others 2017, 2018). Given a possible adaption of N fixers to high background N deposition in the SMT forest, we hypothesized that N addition would reduce ANF rates less in the SMT forest than in the JGS forest (H2). Additionally, we hypothesized that understory N addition would overestimate the inhibitory effects on the soil and forest floor N fixation but underestimate those on the moss and foliar N fixation compared to canopy N addition (H3).
Materials and Methods
Study Area
This study was conducted in two forests with different climate zones. The JGS forest is in Jigongshan National Nature Reserve (31°46′–31°52′N, 114°01′–114°06′E) in Henan Province, central China, which lies in a temperate zone. The mean annual temperature is 15.2°C, and the mean annual precipitation is 1119 mm (80% falling from April to October). The rate of ambient N deposition in precipitation is approximately 19.6 kg N ha−1 y−1 (Zhang and others 2015). The main species in the JGS forest are Liquidambar formosana Hance. and Quercus variabilis Bl. in the tree layers and Trachelospermum jasminoide Lem. and Euphorbia humifusa Willd. in the understory layers (Table S6).
The SMT forest is in Shimentai National Nature Reserve (24°22′–24°31′N, 113°05′–113°31′E) in Guangdong Province, southern China, which lies in a tropical zone with a typical monsoon climate. The mean annual temperature is 20.8°C. The mean annual precipitation is 2364 mm, 80% of which occurs from April to October. The rate of ambient N deposition is approximately 34.1 kg N ha−1 y−1 (Zhang and others 2015). The main species in the SMT forest are Castanopsis chinensis Hance., Schefflera octophylla Harms., Elaeocarpus dubius A. DC. and Schima superba Chardn. in the tree layers and Symplocos ramosissima Wall., Macaranga sampsonii Hance. and Lindera metcalfiana Allen. in the understory layers (Table S6).
Experimental Design
At both forest sites, N was added using two approaches (canopy vs. understory N addition). Canopy N addition simulated a realistic pathway of atmospheric N deposition, and understory N addition represented a traditional approach that has been applied in many N deposition studies in the past. Our experiment was a completely randomized block design with four and three blocks in the JGS and SMT forest, respectively. There were five N addition treatments in each block: control (C), understory N addition at 25 kg N ha−1 y−1 (UN25) and at 50 kg N ha−1 y−1 (UN50), canopy N addition at 25 kg N ha−1 y−1 (CN25) and at 50 kg N ha−1 y−1 (CN50). Each treatment was assigned to a circular plot (907 m2 in area and 17 m in semidiameter) with a total of 20 and 15 plots in the JGS and SMT forest, respectively. Each circular plot was surrounded by a more than 20 m wide buffer strip.
From April 2013 to July 2016, the equivalent of 3 mm of precipitation containing NH4NO3 solution was applied to the treated plots monthly during every growing season (from April to October; the 25th treatment event was performed in July 2016) when both forests were at full canopy development. The treated plots received 21 mm of additional water per year, only accounting for less than 2% and less than 1% of the total annual precipitation in the JGS and SMT forests, respectively. Thus, the confounding effect of water addition resulting from the treatment was considered marginal (Zhang and others 2015). No experimental manipulation was performed in the control plots.
Canopy N addition treatment was performed using a 35-m high spraying system in which NH4NO3 solution was pumped to the top (~ 5 m above the canopy) through polyvinyl chloride pipes (which were fixed to a supporting tower) and evenly sprayed on the forest canopy via a pair of crane sprinklers (which could turn 360°automatically). Understory N addition treatment was performed using a 1.5-m high irrigation system that sprayed NH4NO3 solution on the forest floor directly.
Field Sampling
Field sampling was carried out in July 2016 (after 3 years of chronic N addition). Specifically, five samples of forest floor [which included all recognizable leaves (that is, fresh and decomposed leaves) and fine woody tissues; Cusack and others 2009] were randomly collected from each plot with a 30 × 30-cm metal frame and mixed within each plot. Beneath the forest floor, mineral soil (at a depth of 0–10 cm) was sampled with a 2.5-cm soil corer and then mixed by plot. Fresh leaves in the forest canopy were sampled with a 20-m long pole pruner from 2 to 3 individuals of each dominant tree species (L. formosana and Q. variabilis in the JGS forest and C. chinensis, S. octophylla, E. dubius, and S. superba in the SMT forest). The collected leaves were mixed within tree species for a total of 2 and 5 samples per plot in the JGS and SMT forests, respectively. Epiphytic mosses were distributed at the base of the tree trunk, and they received canopy N addition treatments but not understory N addition treatments. The dominant species of mosses are Entodontaceae compressus, Tortula subulata, Barbula tectorum, and Polytrichaceae longisetum in the JGS forest, and Syrrhopodon armatus, Syrrhopodon japonicus, Fissidens javanicus, Paraleucobryum enerve, and Bryum caespiticium in the SMT forest. Epiphytic mosses were sampled by scraping four 10 × 10 cm pieces from each tree whose canopy leaves were sampled. Because different moss species were mixed distributed and each species did not always occur in each plot, the collected mosses were only mixed by plot. Additionally, N-fixing plants were rare at both forest sites (with a coverage of 0.5% and 1.4% in the JGS and SMT forests, respectively; Table S6); thus, their contribution to N input was ignored.
All fresh samples were weighed. Portions of each sample were oven-dried (65°C for the forest floor, leaves, and epiphytic mosses and 105°C for the soil) for 48 h to measure the moisture content. We estimated the standing stocks of the soil and forest floor using dry weights and sampling areas and expressed them as ‘g soil m−2’ and ‘g forest floor m−2, respectively. Because mosses were mainly distributed at the base of the tree surface (that is, ~ 1.6 and ~ 2.4 m above the ground in the SMT and JGS forests, respectively), their densities were estimated by the average percent cover (Zheng and others 2017). Assuming each tree to be a cylinder, we calculated the surface area of the tree by multiplying circumference by height. Thus, the standing stock of mosses was estimated by the moss cover, moss moisture content (for the calculation of dry weight), tree surface area, and tree density (818 and 446 trees ha−1 in the SMT and JGS forests, respectively) and expressed as ‘g moss m−2 ground area.’ The standing stock of leaves (g leaves m−2 ground area) was calculated by the leaf area index [that is, 2.60 (Zhu and others 2017) and 4.43 (personal communication with Zhen Zhang) for the JGS and SMT forests, respectively] and the specific leaf area (leaf area/leaf dry weight).
Measurement of Asymbiotic N Fixation
We measured nitrogenase activity with the acetylene reduction assay (ARA) (Hardy and others 1968), based on the fact that N-fixing microbes can reduce acetylene (C2H2) to ethylene (C2H4). Specifically, about 10 g fresh soil, about 6 g forest floor, about 5 g canopy leaves, or around 4 g mosses was sealed in 120-ml gas-tight glass bottles. In each bottle, 10% (that is, 12 ml) of the headspace gas was removed and replaced with pure C2H2 (99.99%). It is noted that the foliar N fixation that we measured included activities of both epiphylls (Cusack and others 2009) and endophytes (Moyes and others 2016). To approximate the realistic temperature and light of the field, all samples were incubated in situ for 12 h. After incubation, portions (~ 15 ml) of the headspace gas from each bottle were collected and stored in a 12-ml Labco Exetainer™ (Labco, High Wycombe, U.K.).
The C2H4 concentrations of each gas sample were measured using a gas chromatograph (Shimadzu GC14, Japan). Additionally, the background C2H4 concentrations in pure C2H2 gas and the C2H4 concentrations naturally produced by the samples were measured and subtracted. Nitrogenase activity was expressed as the C2H4 production rate (nmol C2H4 g−1 dry weight h−1). N fixation rates (mg N m−2 day−1) were estimated by C2H4 production rates (Figure S1), standing stocks (kg dry weight m−2; Table S3), and conversion ratios (C2H2 reduced/N2 fixed; Table S5). Although a theoretical conversion ratio of 3/1 has been widely used in previous studies (for example, O’Connell and others 1979; Matson and others 2015; Zheng and others 2016), this ratio actually varies across study sites (Barron and others 2008; Keuter and others 2014). In this study, we calibrated the conversion ratios by incubating the subsamples (forest floor, soil, leaves, and mosses) from the control plots (n = 4 for the JGS forest; n = 3 for the SMT forest) with 10% by volume 99 atom% 15N2 for 24 h. The amounts of the subsamples used for incubation were equivalent to those used for the ARA mentioned above. The 15N2 incubation experiment was conducted in parallel with the ARA. After incubation, all samples were dried at 60°C, ground to a fine powder and analyzed for 15N/14N and N% on an isotope ratio mass spectrometer (IsoPrime 100, Elemental Co., Germany). Using the data of ARA (that is, C2H2 reduced) and 15N (that is, N2 fixed), we estimated the conversion ratios for each compartment in the JGS and SMT forests, respectively (Table S5). Total ANF rates within the forests were calculated as the sum of the rates in all compartments.
Measurement of Total N and Inorganic N Concentrations
Concentrations of total N (TN) in each compartment were determined by the indophenol blue method after semimicro-Kjeldahl digestion (Liu 1996). Concentrations of inorganic N (NH4+ and NO3−) in fresh soil and forest floor were analyzed spectrophotometrically after extraction with 50 ml 2 M KCl solution (Bremner and Mulvaney 1982). Concentrations of total dissolved inorganic N (DIN) were the sum of NH4+ and NO3− concentrations.
Data Analyses
To understand how N addition affected N fixation and the N concentrations in each compartment, one-way analysis of variance (ANOVA) was used to determine the differences in each variable among treatments (C, CN25, CN50, UN25, and UN50). Due to the unbalanced experimental design (that is, different plot replicates between the two forests), ANOVA was used to analyze the effects for each forest separately. To understand the initial difference in N fixation between the two forests, an independent two-tailed t test, which does not require equal sample sizes between groups, was used to compare nitrogenase activity and N fixation rates in the control plots of the forests. All data were examined for equality of variances and normality using Levene’s and Kolmogorov–Smirnov tests, respectively. Data that failed to meet the requirements of equality of variance or normality were log-transformed for analysis. Statistical analyses were performed in SPSS 21.0 for Windows (SPSS Inc., Chicago, IL, USA) with a statistically significant level of p < 0.05.
To understand whether understory N addition over- or underestimated the effects on N fixation compared with canopy N addition, we calculated the changes in N fixation by comparing the effects of understory and canopy N addition according to the following formulas:
where F represents N fixation (that is, nitrogenase activity or N fixation rates); C and U represent canopy and understory N addition, respectively; n is the sample size (n = 4 for the JGS forest; n = 3 for the SMT forest); \( \Delta F_{{{\text{C-}}i}} \) and \( \Delta F_{{{\text{U-}}i}} \) are the changes in the effects of canopy and understory N addition compared to the controls, respectively; \( \Delta_{{{\text{estimate-}}i}} \) is the change caused by the effects of understory N addition compared to the effects of canopy N addition.
where \( \bar{\Delta }_{{{\text{estimate-}}i}} \) is the mean change caused by the effects of understory N addition compared to the effects of canopy N addition and \( \Delta_{\text{se}} \) is the standard error of the means. If \( \Delta_{{{\text{estimate-}}i}} \) is positive, understory N addition caused an overestimate compared to canopy N addition, and vice versa. Data shown in figures (Figure 2 and S2) are means (\( \bar{\Delta }_{\text{estimate}} \)) with standard errors (\( \Delta_{\text{se}} \)).
Result
Asymbiotic N Fixation in the Control and N Addition Plots
Epiphytic mosses showed the highest nitrogenase activity (2.33–4.27 nmol g−1 h−1) followed by the forest floor (0.86–1.89 nmol g−1 h−1), leaves (0.10–0.19 nmol g−1 h−1), and soil (0.04–0.07 nmol g−1 h−1) (Figure S1). In the control plots, moss nitrogenase activity was significantly higher in the JGS forest than in the SMT forest (t5 = 2.9, p = 0.035; t test), whereas nitrogenase activity of the soil, forest floor, and leaves were comparable between the forests. In the JGS forest, UN25 and UN50 significantly decreased nitrogenase activity of the forest floor (by 42.3% and 54.4%, respectively; F4,15 = 24.2, p < 0.001; ANOVA) and soil (by 35.9% and 37.7%, respectively; F4,15 = 10.8, p < 0.001), whereas CN25 and CN50 significantly decreased nitrogenase activity of the mosses (by 29.6% and 41.1%, respectively; F4,15 = 9.4, p = 0.001) and CN25 significantly decreased foliar (L. formosana) nitrogenase activity (by 34.2%; F4,15 = 3.7, p = 0.027). N addition had no effect on nitrogenase activity in any of the compartments (except for the soil) in the SMT forest.
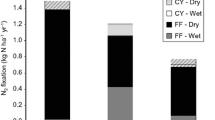
N fixation rates (mg N m−2 d−1) were calculated by standing stocks, nitrogenase activity, and the calibrated conversion ratios (see the Materials and Method section). Soil showed the highest N fixation rates (1.06–1.86 mg N m−2 d−1), followed by forest floor (0.22–0.66 mg N m−2 d−1), epiphytic mosses (0.01–0.03 mg N m−2 d−1), and leaves (0.01–0.02 mg N m−2 d−1) (Figure 1). In the control plots, the forest floor, soil, and moss N fixation rates were significantly higher in the JGS forest than in the SMT forest (t5 = 6.3, 3.7, and 6.7, respectively, p = 0.001, 0.013, and 0.001, respectively; t test), but foliar N fixation rates were comparable between the forests. Total ANF rates were significantly higher in the JGS forest than in the SMT forest (t5 = 5.1, p = 0.004). In the JGS forest, UN25 and UN50 significantly decreased N fixation rates of the forest floor (by 41.5% and 56.2%, respectively; F4,15 = 8.0, p = 0.001; ANOVA) and soil (by 35.3% and 38.1%, respectively; F4,15 = 8.0, p = 0.001), and CN25 and CN50 significantly decreased N fixation rates of the mosses (by 37.8% and 54.7%, respectively; F4,15 = 11.3, p < 0.001), whereas CN25 significantly decreased the foliar (L. formosana) N fixation rates (by 31.1%; F4,15 = 3.7, p = 0.027). In the SMT forest, there was no effect of N addition on N fixation rates in any of the compartments (except for the soil). UN25 and UN50 significantly decreased total ANF rates in the JGS forest (by 36.1% and 41.8%, respectively; F4,15 = 14.8, p < 0.001), whereas UN50 significantly decreased total ANF rates in the SMT forest (by 19.2%; F4,10 = 3.6, p = 0.045).
Effects of N addition on asymbiotic N fixation (ANF) rates in different compartments and total ANF rates in the JGS (Jigongshan) and SMT (Shimentai) forests. C: control; CN25: canopy N addition at 25 kg N ha−1 y−1; CN50: canopy N addition at 50 kg N ha−1 y−1; UN25: understory N addition at 25 kg N ha−1 y−1; UN50: understory N addition at 50 kg N ha−1 y−1; Leaf-1 and Leaf-2 in the JGS forest represent L. formosana and Q. variabilis, respectively; Leaf-1, Leaf-2, Leaf-3, and Leaf-4 in the SMT forest represent C. chinensis, S. octophylla, E. dubius, and S. superba, respectively. Error bar represents standard error of means (n = 4 for the JGS forest; n = 3 for the SMT forest). *Represents a significant difference (p < 0.05) between the treatment and control. Different letters represent significant differences (p < 0.05) between the control plots.
Changes in Asymbiotic N Fixation Comparing Understory Versus Canopy N Addition
The changes in nitrogenase activity comparing the two N treatments (CN vs. UN) were large in the soil and forest floor but small and approximately zero in the mosses and leaves (Figure S2). In the JGS forest, compared with CN25 and CN50, UN25 and UN50 overestimated the inhibitory effects of N deposition on nitrogenase activity in the soil (by 352.1% and 671.6%, respectively) and forest floor (by 278.2% and 879.6%, respectively) but underestimated that in the mosses (by 82.6% and 120.0%, respectively) and the canopy leaves of Q. variabilis (by 139.3% and 170.8%, respectively). Compared with CN25, UN25 underestimated the inhibitory effects on nitrogenase activity in the canopy leaves of L. formosana (by 133.4%) in the JGS forest. In the SMT forest, compared with CN50, UN50 overestimated the inhibitory effects on nitrogenase activity in the soil (by 75.8%) but underestimated that in the canopy leaves of C. chinensis (by 55.5%) and S. superba (by 37.6%). No significant change in nitrogenase activity was observed when comparing canopy and understory N treatments in other compartments in the SMT forest.
Similarly, the changes in N fixation rates comparing the two N treatments were large in the soil and forest floor in the JGS forest but small in the SMT forest (Figure 2). In the JGS forest, compared with CN25 and CN50, UN25 and UN50 overestimated the inhibitory effects on N fixation rates in the soil (by 646.3% and 634.0%, respectively) and forest floor (by 200.7% and 406.3%, respectively) but underestimated those in the mosses (by 81.1% and 118.3%, respectively). In the JGS forest, UN25 underestimated the inhibitory effects on N fixation rates in the canopy leaves of L. formosana (by 125.7%) compared with CN25, and both UN25 and UN50 underestimated the inhibitory effects in the canopy leaves of Q. variabilis (by 144.8% and 77.1%, respectively) compared with CN25 and CN50. In the SMT forest, UN50 overestimated the inhibitory effects on the soil N fixation rates (by 41.3%) compared with CN50. In the JGS forest, UN25 and UN50 overestimated the inhibitory effects on total ANF rates (by 375.1% and 471.8%, respectively) compared with CN25 and CN50, while UN50 overestimated the inhibitory effects on total ANF rates (by 34.9%) in the SMT forest compared with CN50.
Changes in N fixation rates with understory N addition compared to those with canopy N addition. JGS: Jigongshan forest; SMT: Shimentai forest; UN25 versus CN25: the effects of understory N addition compared to those of canopy N addition at 25 kg N ha−1 y−1 (n = 4 for the JGS forest; n = 3 for the SMT forest); UN50 versus CN50: the effects of understory N addition compared to those of canopy N addition at 50 kg N ha−1 y−1 (n = 4 for the JGS forest; n = 3 for the SMT forest); Leaf-1 and Leaf-2 in the JGS forest represent L. formosana and Q. variabilis, respectively; Leaf-1, Leaf-2, Leaf-3, and Leaf-4 in the SMT forest represent C. chinensis, S. octophylla, E. dubius, and S. superba, respectively. Error bar represents standard error of means (n = 4 for the JGS forest; n = 3 for the SMT forest). Error bars overlapping with zero indicate insignificant effects.
N Concentrations
Generally, the TN concentrations in each compartment were higher in the SMT forest than in the JGS forest, although the differences were insignificant (Table 1). In the JGS forest, UN25 and UN50 significantly increased the TN concentration in the forest floor (by 49.9% and 42.7%, respectively; F4,15 = 5.1, p = 0.009; ANOVA), while CN25 and CN50 significantly increased the TN concentrations in the mosses (by 49.5% and 69.6%, respectively; F4,15 = 4.2, p = 0.018) and canopy leaves of L. formosana (by 45.0% and 51.3%, respectively; F4,15 = 3.3, p = 0.040; Table S1). In the SMT forest, neither understory nor canopy N addition changed the TN concentrations in any of the compartments, indicating that this forest may have been N saturated.
The total DIN concentrations in the soil and forest floor were significantly higher in the SMT forest than in the JGS forest (t5 = 2.8 and 2.7, respectively, p = 0.037 and 0.041, respectively; t test; Table 2). In the JGS forest, UN25 and UN50 significantly increased soil NH4+ (by 106.6% and 159.6%, respectively; F4,15 = 9.8, p < 0.001; ANOVA) and total DIN (by 64.2% and 105.8%, respectively; F4,15 = 6.9, p = 0.002) concentrations, as well as the forest floor NO3− (by 120.8% and 79.4%, respectively; F4,15 = 4.5, p = 0.014), NH4+ (by 71.5% and 97.9%, respectively; F4,15 = 3.8, p = 0.025), and total DIN (by 97.3% and 88.2%, respectively; F4,15 = 5.6, p = 0.006; Table S2) concentrations. In the SMT forest, CN50, UN25, and UN50 significantly increased the soil NH4+ (by 84.3%, 73.0%, and 71.1%, respectively; F4,10 = 3.5, p = 0.048) and total DIN (by 58.8%, 62.9%, and 58.4%, respectively; F4,10 = 3.6, p = 0.047). concentrations CN50, UN25 and UN50 significantly increased the forest floor NO3− (by 64.8%, 62.5%, and 64.3%, respectively; F4,10 = 3.5, p = 0.048), while CN25, UN25, and UN50 significantly increased the forest floor NH4+ (by 102.6%, 136.8%, and 154.9%, respectively; F4,10 = 5.1, p = 0.017) and total DIN (by 73.7%, 96.4%, and 105.6%, respectively; F4,10 = 4.2, p = 0.029) concentrations in the SMT forest.
Discussion
Asymbiotic N Fixation in Control Plots
Inconsistent with our hypothesis (H1), we found that total ANF rates were higher in the temperate forest (2.57 ± 0.19 mg N m−2 d−1) than in the tropical forest (1.34 ± 0.09 mg N m−2 d−1) (Figure 1). Assuming that N fixation occurs and remains constant during the growing season (April to October), we estimated the total ANF rates to be 5.4 and 2.8 kg N ha−1 y−1 in the temperate and tropical forests, respectively. This result is inconsistent with a prior biome-scale estimate showing that average ANF rates were higher in tropical forests (3.3–7.8 kg N ha−1 y−1) than in temperate forests (1.7 kg N ha−1 y−1) (Reed and others 2011). Compared with neotropical forests where N-fixing trees are abundant (Gei and others 2018), many tropical/subtropical natural forests in southern China may lack N-fixing trees (for example, Zheng and others 2017, 2018). Therefore, it is likely that the patterns of total N fixation (symbiotic + asymbiotic) in China may be inconsistent with an early estimate showing that tropical forests had higher rates of total N fixation (21.7–36.5 kg N ha−1 y−1) than temperate forests (16.0 kg N ha−1 y−1) (Cleveland and others 1999).
Generally, high temperature and precipitation in most tropical regions are thought to drive N fixation (Houlton and others 2008; Reed and others 2011), but the low ANF rates observed in our tropical forest (Figure 1) indicate that other factors may affect N fixation more than temperature and precipitation. One factor could be background N deposition. Previous studies have revealed that increases in N deposition (or fertilization) rates inhibit N fixation (DeLuca and others 2008; Cusack and others 2009; Leppänen and others 2013; Zheng and others 2016). Spatially robust estimates have shown that N fixation rates in tropical forest biomes decreased by 80% over the past 15 years due to N deposition (Sullivan and others 2014). China has experienced rapid economic growth in recent decades, and agricultural and industrial activities have dramatically increased the emission of reactive N nationally, particularly in the southern regions (Liu and others 2013). The SMT forest (located in southern China) has experienced high N pollution (~ 34 kg N ha−1 y−1), nearly twice that of the JGS forest (~ 19 kg N ha−1 y−1); thus, N fixation may have been greatly inhibited. Another factor could be related to the N demand. Compared to the JGS forest, the SMT forest has become N saturated possibly due to high N deposition, as evidenced by a lack of response of soil and plant N concentrations to N addition (Table 1). This finding suggests that the availability of N has satisfied plant and microbial demand in the SMT forest and thus, plants and microbes may reduce dependence on the energy-intensive pathway of N fixation.
Additionally, we cannot rule out other environmental (for example, organic matter, phosphorus, molybdenum, and pH) and biotic (for example, abundance of N-fixing microbes) factors that may also contribute to the differences observed in N fixation between the forests (Figure 1). Therefore, more studies of forest N fixation and its controlling factors are needed in the future, as this information is important for improving estimates of terrestrial N fixation (Cleveland and others 1999; Reed and others 2011).
Responses of Asymbiotic N Fixation to N Addition in Tropical Versus Temperate Forests
Consistent with our hypothesis (H2), we found that N addition reduced total ANF rates by 36–42% in the JGS forest but reduced the rates by only 19% in the SMT forest (Figure 1). This finding indicates that ANF was less sensitive to N addition in the tropical forest than in the temperate forest. Similarly, previous studies in temperate forests showed that N addition significantly reduced ANF rates by 56–97% (Hällbom and Bergman 1979; Nohrstedt 1988; Perakis and others 2017). In contrast, N addition reduced ANF rates by 19–79% in tropical forests (Vitousek and Hobbie 2000; Barron and others 2008; Cusack and others 2009; Matson and others 2015) and did not affect ANF in some old-growth tropical forests in Hawaii (Crews and others 2000) and Costa Rica (Reed and others 2007).
The mechanisms underlying the less ANF sensitivities to N addition in tropical forests than in temperate forests are currently unclear. We speculated that N fixers may have adapted to N richness in tropical forests. Compared to temperate forests, many tropical forests are relatively rich in N (for example, Fang and others 2008; Hedin and others 2009; and Tables 1, 2). The N richness results in a low rate of background N fixation and thus limits N fixation in response to additional N, as evidenced by a negative relationship between soil N availability and the absolute declines in N fixation following N addition revealed by a previous study (p < 0.01; Perakis and others 2017). Additional evidence is derived from a recent study showing that long-term N deposition and fertilization had minor effects on total ANF rates in a N-saturated tropical forest (Zheng and others 2018). Similarly, ANF had almost no response to N addition in the SMT forest (Figure 1) with high N availability (Table 2). Given the possible adaption of N fixers to high N environments, N fixers remain active and show less response to further N inputs (Zheng and others 2018). This mechanism exerts positive feedback on ecosystem N richness and may help us understand a long-term N paradoxical phenomenon—many tropical forests sustain N accumulation regardless of soil N richness (Hedin and others 2009).
Changes in Asymbiotic N Fixation Comparing Understory Versus Canopy N Addition
Compared with canopy N addition, understory N addition overestimated the inhibitory effects on N fixation in the soil and forest floor but underestimated those in mosses and canopy leaves, which supports our hypothesis (H3). This phenomenon was severe in the JGS forest (overestimated by 200–880% for the soil and forest floor and underestimated by 77–170% for the mosses and canopy leaves) but slight in the SMT forest (overestimated by 41–76% for the soil).
Several mechanisms account for the above observation. First, the capacities of canopy N retention (absorption) were different between the forests. Compared to temperate forests, many tropical rainforests are rich in N and show large losses of DIN (Hedin and others 2009), indicating a lack of N limitation in these tropical forests (Brookshire and others 2012). In our study, the JGS forest was limited by N and thus retained more added N than the SMT forest because canopy N (NH4NO3) addition increased the soil and forest floor DIN in the SMT forest but not in the JGS forest (Table 2 and S2). The retained DIN was absorbed by leaves and epiphytic mosses in the JGS forest, as evidenced by a significant increase in foliar and moss TN concentrations following canopy N addition (Table 1 and S1). Similarly, previous studies have also found a significant retention of deposited DIN by plants in temperate forests (Gilliam and others 1996, 2016; Gaige and others 2007; Dail and others 2009) but no or slight N retention in tropical forests (Fang and others 2008; Umana and Wanek 2010). Second, high precipitation may lead to canopy N leaching. The SMT forest has higher annual rainfall (2364 mm) than the JGS forest (1119 mm); rainfall may remove the added N from the canopy and drop it into the understory layers in the SMT forest. Third, we currently cannot judge whether the differences in tree species and leaf physiological structure (for example, drip tips, waxes, and roughness) between the SMT and JGS forests may contribute to the observed differences in ANF responses. However, the higher tree density, leaf area index (see the Method section), standing stock of canopy leaves (Table S3), and coverage of tree layers (Table S6) in the SMT forest seem not to help retain the added N (Table 1); instead, the lower representation of the shrub and herb layers (Table S6) in the SMT forest than in the JGS forest may explain the lack of N retention in the former (Table 1), which deserves further research.
Conclusions
To our knowledge, this study was the first to evaluate the effects of N deposition on asymbiotic N fixation in a tropical and a temperate forest using both understory and canopy N addition approaches. The ANF rates were lower in the tropical forest than in the temperate forest mainly because of the high background N deposition rates and the low N demand in the tropical forest. ANF was less sensitive to N addition in the tropical forest than in the temperate forest; a potential mechanism for this phenomenon is that N fixers in N-rich tropical forests may have adapted to N richness, which limits their responses to additional N. Compared with canopy N addition, understory N addition overestimated the inhibitory effects of N deposition on total ANF rates slightly in the tropical forest (by 35%) but severely in the temperate forest (by 375–472%). Mechanisms for this phenomenon are that the tropical forest has a weak canopy retention (absorption) of added N and that high precipitation may remove the added N from the canopy and drop it into the understory layers. Overall, our findings reveal the differences in ANF and in its sensitivities to N inputs between tropical and temperate forests, which are important for our understanding of N fixation in different climatic regions. More importantly, our findings indicate that understory N addition may have overestimated the N deposition effects on ANF in forests, which may lead to an inaccurate estimate of terrestrial N fixation and N cycles under N deposition scenarios.
References
Adriaenssens S, Staelens J, Wuyts K, Samson R, Verheyen K, Boeckx P. 2012. Retention of dissolved inorganic nitrogen by foliage and twigs of four temperate tree species. Ecosystems 15:1093–107.
Barron AR, Wurzburger N, Bellenger JP, Wright SJ, Kraepiel AM, Hedin LO. 2008. Molybdenum limitation of asymbiotic nitrogen fixation in tropical forest soils. Nat Geosci 2:42–5.
Batterman SA, Wurzburger N, Hedin LO. 2013. Nitrogen and phosphorus interact to control tropical symbiotic N2 fixation: a test in Inga punctata. J Ecol 101:1400–8.
Bremner J, Mulvaney C. 1982. Nitrogen-total. In: Page AL, Ed. Methods of soil analysis. Part 2. Chemical and microbiological properties. Madison, WI: American Society of Agronomy. p 595–624.
Brookshire E, Gerber S, Menge DN, Hedin LO. 2012. Large losses of inorganic nitrogen from tropical rainforests suggest a lack of nitrogen limitation. Ecol Lett 15:9–16.
Chen H, Li D, Gurmesa GA, Yu G, Li L, Zhang W, Fang H, Mo J. 2015. Effects of nitrogen deposition on carbon cycle in terrestrial ecosystems of China: A meta-analysis. Environ Pollut 206:352–60.
Cleveland CC, Townsend AR, Schimel DS, Fisher H, Howarth RW, Hedin LO, Perakis SS, Latty EF, Von Fischer JC, Elseroad A. 1999. Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Global Biogeochem Cy 13:623–45.
Crews TE, Farrington H, Vitousek PM. 2000. Changes in asymbiotic, heterotrophic nitrogen fixation on leaf litter of Metrosideros polymorpha with long-term ecosystem development in Hawaii. Ecosystems 3:386–95.
Cusack DF, Silver W, McDowell WH. 2009. Biological Nitrogen Fixation in Two Tropical Forests: Ecosystem-Level Patterns and Effects of Nitrogen Fertilization. Ecosystems 12:1299–315.
Dail DB, Hollinger DY, Davidson EA, Fernandez I, Sievering HC, Scott NA, Gaige E. 2009. Distribution of nitrogen15 tracers applied to the canopy of a mature spruce-hemlock stand, Howland, Maine, USA. Oecologia 160:589–99.
DeLuca TH, Zackrisson O, Gundale MJ, Nilsson MC. 2008. Ecosystem feedbacks and nitrogen fixation in boreal forests. Science 320:1181.
Dynarski KA, Houlton BZ. 2018. Nutrient limitation of terrestrial free-living nitrogen fixation. New Phytol 217:1050–61.
Fang YT, Gundersen P, Mo JM, Zhu WX. 2008. Input and output of dissolved organic and inorganic nitrogen in subtropical forests of South China under high air pollution. Biogeosciences 5:339–52.
Fenn ME, Ross CS, Schilling SL, Baccus WD, Larrabee MA, Lofgren RA. 2013. Atmospheric deposition of nitrogen and sulfur and preferential canopy consumption of nitrate in forests of the Pacific Northwest, USA. Forest Ecol Manag 302:240–53.
Gaige E, Dail DB, Hollinger DY, Davidson EA, Fernandez IJ, Sievering H, White A, Halteman W. 2007. Changes in Canopy Processes Following Whole-Forest Canopy Nitrogen Fertilization of a Mature Spruce-Hemlock Forest. Ecosystems 10:1133–47.
Galloway JN. 1998. The global nitrogen cycle: changes and consequences. Environ Pollut 102:15–24.
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland C, Green P, Holland E. 2004. Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226.
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA. 2008. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–92.
Gei M, Rozendaal DM, Poorter L, Bongers F, Sprent JI, Garner MD, Aide TM, Andrade JL, Balvanera P, Becknell JM. 2018. Legume abundance along successional and rainfall gradients in Neotropical forests. Nat Ecol Evol 2:1104–11.
Gilliam FS, Yurish BM, Adams MB. 1996. Ecosystem nutrient responses to chronic nitrogen inputs at Fernow Experimental Forest, West Virginia. Can J Forest Res 26:196–205.
Gilliam FS, Billmyer JH, Walter CA, Peterjohn WT. 2016. Effects of excess nitrogen on biogeochemistry of a temperate hardwood forest: Evidence of nutrient redistribution by a forest understory species. Atmos Environ 146:261–70.
Gutschick VP. 1981. Evolved strategies in nitrogen acquisition by plants. Am Nat 118:607–37.
Gundale MJ, Deluca TH, Nordin A. 2011. Bryophytes attenuate anthropogenic nitrogen inputs in boreal forests. Global Change Biol 17:2743–53.
Hällbom L, Bergman B. 1979. Influence of certain herbicides and a forest fertilizer on the nitrogen fixation by the lichen Peltigera praetextata. Oecologia 40:19–27.
Hardy RW, Holsten R, Jackson E, Burns R. 1968. The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant physiol 43:1185–207.
Hedin LO, Brookshire EJ, Menge DN, Barron AR. 2009. The nitrogen paradox in tropical forest ecosystems. Annu Rev Ecol Evol Syst 40:613–35.
Houle D, Marty C, Duchesne L. 2015. Response of canopy nitrogen uptake to a rapid decrease in bulk nitrate deposition in two eastern Canadian boreal forests. Oecologia 177:29–37.
Houlton BZ, Wang YP, Vitousek PM, Field CB. 2008. A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454:327–30.
Keuter A, Veldkamp E, Corre MD. 2014. Asymbiotic biological nitrogen fixation in a temperate grassland as affected by management practices. Soil Biol Biochem 70:38–46.
Klopatek JM, Barry MJ, Johnson DW. 2006. Potential canopy interception of nitrogen in the Pacific Northwest, USA. Forest Ecol Manag 234:344–54.
Leppänen SM, Salemaa M, Smolander A, Mäkipää R, Tiirola M. 2013. Nitrogen fixation and methanotrophy in forest mosses along a N deposition gradient. Environ Exp Bot 90:62–9.
Liu G. 1996. Standard methods for the observation and analysis of Chinese ecosystem research network: soil analysis and profile description. Beijing: Standards Press of China.
Liu XJ, Zhang Y, Han WX, Tang AH, Shen JL, Cui ZL, Vitousek P, Erisman JW, Goulding K, Christie P. 2013. Enhanced nitrogen deposition over China. Nature 494:459–62.
Matson AL, Corre MD, Burneo JI, Veldkamp E. 2015. Free-living nitrogen fixation responds to elevated nutrient inputs in tropical montane forest floor and canopy soils of southern Ecuador. Biogeochemistry 122:281–94.
Matson PA, McDOWELL WH, Townsend AR, Vitousek PM. 1999. The globalization of N deposition: ecosystem consequences in tropical environments. Biogeochemistry 46:67–83.
Moyes AB, Kueppers LM, Pett-Ridge J, Carper DL, Vandehey N, O’Neil J, Frank AC. 2016. Evidence for foliar endophytic nitrogen fixation in a widely distributed subalpine conifer. New Phytol 2:657–68.
Nohrstedt HÖ. 1988. Effect of liming and N-fertilization on denitrification and N2-fixation in an acid coniferous forest floor. Forest Ecol Manag 24:1–13.
O’connell A, Grove T, Malajczuk N. 1979. Nitrogen fixation in the litter layer of eucalypt forests. Soil Biol Biochem 11:681–2.
Perakis S, Pett-Ridge JC, Catricala CE. 2017. Nutrient feedbacks to soil heterotrophic nitrogen fixation in forests. Biogeochemistry 134:41–55.
Reed SC, Cleveland CC, Townsend AR. 2007. Controls over leaf litter and soil nitrogen fixation in two lowland tropical rain forests. Biotropica 39:585–92.
Reed SC, Cleveland CC, Townsend AR. 2008. Tree species control rates of free-living nitrogen fixation in a tropical rain forest. Ecology 89:2924–34.
Reed SC, Cleveland CC, Townsend AR. 2011. Functional ecology of free-living nitrogen fixation: a contemporary perspective. Annu Rev Ecol Evol Syst 42:489–512.
Shi L, Zhang H, Liu T, Zhang W, Shao Y, Ha D, Li Y, Zhang C, Cai XA, Rao X. 2016. Consistent effects of canopy vs. understory nitrogen addition on the soil exchangeable cations and microbial community in two contrasting forests. Sci Total Environ 553:349–57.
Sullivan BW, Smith WK, Townsend AR, Nasto MK, Reed SC, Chazdon RL, Cleveland CC. 2014. Spatially robust estimates of biological nitrogen (N) fixation imply substantial human alteration of the tropical N cycle. P Natl Acad Sci USA 111:8101–6.
Umana NHN, Wanek W. 2010. Large canopy exchange fluxes of inorganic and organic nitrogen and preferential retention of nitrogen by epiphytes in a tropical lowland rainforest. Ecosystems 13:367–81.
Vitousek PM, Cassman K, Cleveland C, Crews T, Field CB, Grimm NB, Howarth RW, Marino R, Martinelli L, Rastetter EB. 2002. Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 57:1–45.
Vitousek PM, Hobbie S. 2000. Heterotrophic nitrogen fixation in decomposing litter: patterns and regulation. Ecology 81:2366–76.
Vitousek PM, Menge DN, Reed SC, Cleveland CC. 2013. Biological nitrogen fixation: rates, patterns and ecological controls in terrestrial ecosystems. Philos T R Soc B 368:20130119.
Wang Q, Wang J, Li Y, Chen D, Ao J, Zhou W, Shen D, Li Q, Huang Z, Jiang Y. 2017. Influence of nitrogen and phosphorus additions on N2-fixation activity, abundance, and composition of diazotrophic communities in a Chinese fir plantation. Sci Total Environ . https://doi.org/10.1016/j.scitotenv.2017.10.064.
Zhang W, Shen W, Zhu S, Wan S, Luo Y, Yan J, Wang K, Liu L, Dai H, Li P. 2015. CAN Canopy Addition of Nitrogen Better Illustrate the Effect of Atmospheric Nitrogen Deposition on Forest Ecosystem. Sci Rep 5:11245.
Zheng M, Chen H, Li D, Zhu X, Zhang W, Fu S, Mo J. 2016. Biological nitrogen fixation and its response to nitrogen input in two mature tropical plantations with and without legume trees. Biol Fert Soils 52:1–10.
Zheng M, Zhang W, Luo Y, Mori T, Mao Q, Wang S, Huang J, Lu X, Mo J. 2017. Different responses of asymbiotic nitrogen fixation to nitrogen addition between disturbed and rehabilitated subtropical forests. Sci Total Environ 601:1505–12.
Zheng M, Zhang W, Luo Y, Li D, Wang S, Huang J, Lu X, Mo J. 2018. Stoichiometry controls asymbiotic nitrogen fixation and its response to nitrogen inputs in a nitrogen-saturated forest. Ecology . https://doi.org/10.1002/ecy.2416.
Zhu L, Hu Y, Zhao X, Zeng X, Zhao P, Zhang Z, Ju Y. 2017. The impact of drought on sap flow of co-occurring Liquidambar formosana Hance and Quercus variabilis Blume in a temperate forest, central China. Ecohydrology . https://doi.org/10.1002/eco.1828.
Acknowledgements
We appreciate three anonymous reviewers for their constructive comments on the manuscript. We acknowledge financial supports from the National Natural Science Foundation of China (Nos. 31670488, 31370011, 31770523), the Natural Science Foundation of Guangdong Province (No. 2017A030313168), the National Postdoctoral Program for Innovative Talents (BX20180312), China Postdoctoral Science Foundation (2018M640836), and the UCAS Joint PhD Training Program. We thank Ms. Xiaoping Pan and Mr. Peng Mao for their assistance in laboratory and field experiments. The authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Author Contributions
MHZ, WZ, SLF, NL, QY, JHY, and JMM designed the study. MHZ, WZ, SHW, BZ, CLF, YXJ, DLH, and LWZ performed the research. MHZ analyzed the data. MHZ, WZ, YQL, SQW, SLF, and JMM wrote the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, M., Zhang, W., Luo, Y. et al. The Inhibitory Effects of Nitrogen Deposition on Asymbiotic Nitrogen Fixation are Divergent Between a Tropical and a Temperate Forest. Ecosystems 22, 955–967 (2019). https://doi.org/10.1007/s10021-018-0313-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-018-0313-6