Abstract
Litter decomposition is a major driver of carbon (C) and nitrogen (N) cycles in forest ecosystems and has major implications for C sequestration and nutrient availability. However, empirical information regarding long-term decomposition rates of foliage and wood remains rare. In this study, we assessed long-term C and N dynamics (12–13 years) during decomposition of foliage and wood for three boreal tree species, under a range of harvesting intensities and slash treatments. We used model selection based on the second-order Akaike’s Information Criterion to determine which decomposition model had the most support. The double-exponential model provided a good fit to C mass loss for foliage of trembling aspen, white spruce, and balsam fir, as well as aspen wood. These litters underwent a rapid initial phase of leaching and mineralisation, followed by a slow decomposition. In contrast, for spruce and fir wood, the single-exponential model had the most support. The long-term average decay rate of wood was faster than that of foliage for aspen, but not of conifers. However, we found no evidence that fir and spruce wood decomposed at slower rates than the recalcitrant fraction of their foliage. The critical C:N ratios, at which net N mineralisation began, were higher for wood than for foliage. Long-term decay rates following clear-cutting were either similar or faster than those observed in control stands, depending on litter material, tree species, and slash treatment. The critical C:N ratios were reached later and decreased for all conifer litters following stem-only clear-cutting, indicating increased N retention in harvested sites with high slash loads. Partial harvesting had weak effects on C and N dynamics of decaying litters. A comprehensive understanding of the long-term patterns and controls of C and N dynamics following forest disturbance would improve our ability to forecast the implications of forest harvesting for C sequestration and nutrient availability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant litter decomposition is a major driver of carbon (C) and nitrogen (N) in forest ecosystems and contributes to atmospheric CO2 emissions, soil C sequestration, and plant nutrition (Prescott 2010). For more than 50 years, the use of the litterbag technique has contributed to our understanding of factors controlling litter decomposition (Wieder and Lang 1982; Prescott 2010). Experiments have mainly been conducted on foliar litter, over short periods (≤6 years) and within natural stands (Fioretto and others 2005; Moore and others 2006; Strukelj and others 2012). Wood decomposition studies, on the other hand, are often based on log chronosequences over longer periods (Harmon and others 1986; Brais and others 2006; Fukasawa and others 2009; Angers and others 2012). Given the different approaches and time periods underlying the study of foliage and wood decomposition, few experiments have systematically compared these two types of decomposition under similar conditions (Trofymow and others 1995).
Because of its physical structure, its low nutrient content, and its high lignin content (Laiho and Prescott 2004; Cornwell and others 2009; Cornelissen and others 2012), wood is assumed to decompose at rates slower than those of foliar litters (Prescott 2010). However, recent evidence suggests that lignin is less resistant to decomposition than previously hypothesised (Thevenot and others 2010). Additionally, alkyl C compounds, from litter constituents or from microbial products, could be the main precursors of stable organic matter (Von Lützow and others 2006; Lorenz and others 2007; Cotrufo and others 2013). Fresh foliage is richer in alkyl C compounds than fresh wood (Strukelj and others 2012, 2013), and its decomposition yields more microbial products due to its higher initial labile constituents (Trofymow and others 1995; Cotrufo and others 2013). Hence, foliage could generate more stable compounds with decomposition than wood, resulting in a slower long-term decay rate.
Decomposition models generally employ a single negative exponential equation (Olson 1963) to assess the initial decay rates. However, this model does not perform well once organic matter stabilises in more advanced decomposition states (Brais and others 2006; Harmon and others 2009). Asymptotic and double-exponential models are recommended alternatives (Wieder and Lang 1982; Harmon and others 2009). They assume an initial decomposition phase, that is, dominated by the decay of labile components, and a stable phase, that is, characterised by a null (asymptotic model) or slow (double-exponential model) decay rate. Compared to the single-exponential equation, these models could better reflect differing long-term patterns of decay for both foliar litter and wood (Berg 2000; Manzoni and others 2012; Rajala and others 2012).
In response to the unbalanced supply of C and N provided by fresh litter, saprotrophic fungi maintain their biomass stoichiometry by regulating their element use efficiency or by mycelial translocation of external N (Mooshammer and others 2014). These mechanisms increase litter N concentrations and decrease C:N ratios as decomposition progresses. Eventually, available C becomes more limiting than N to the growth of saprotrophic organisms (Boddy and Watkinson 1995; Hart 1999) and a critical C:N ratio is reached as net N mineralisation begins and litter N content starts declining (Manzoni and others 2008). The C:N ratio of litter seems to stabilise at some point during decomposition, depending upon the initial litter quality and N availability in the forest floor and mineral soil (Hart and Firestone 1991; Moore and others 2011; Strukelj and others 2013). Critical and stabilised C:N ratios have been shown to increase with increasing initial litter C:N ratios and with increasing forest floor C:N ratios (Parton and others 2007; Moore and others 2011; Ågren and others 2013). Again, these results stem from undisturbed stands where litter inputs and forest floor environmental conditions were relatively constant.
Forest harvesting may affect microclimate (Barg and Edmonds 1999), litter composition and production (Strukelj and others 2015), soil nutrient dynamics (Hope and others 2003), and decomposer organisms (Seastedt and Crossley 1981; Kebli and others 2012). These factors have been shown to influence C and N dynamics of decaying litters (Prescott 2010; Moore and others 2011; Mäkiranta and others 2012; Rajala and others 2012). Yet, the effects of harvesting on decay rates remain uncertain, as previous studies have reported faster (Hope and others 2003; Finér and others 2016), slower (Yin and others 1989; Prescott and others 2000), or similar (Prescott and others 2000; Hope and others 2003) decay rates following harvesting. Lower critical C:N ratio has been found after clear-cutting (Berg and Ekbohm 1983) and could be associated with higher N availability in soil (Hope and others 2003) or lower C use efficiency of decomposers (Mooshammer and others 2014). These studies have mainly been conducted on foliage; hence, there is currently little information on the impacts of harvesting on wood decomposition (for example, Finér and others 2016).
The Sylviculture et Aménagement Forestier Écosystémiques (SAFE) project is a series of fully replicated silvicultural experiments initiated in 1998 in the boreal mixedwoods of Canada (Brais and others 2004). Treatments include control stands, clear-cuts, as well as a range of partial harvesting prescriptions. So far, the effects of harvesting on the initial environmental conditions and vegetation dynamics (Brais and others 2004, 2013), 9-year changes in stand C pools (Strukelj and others 2015), and wood fungal communities (Kebli and others 2012, 2014) have been documented. In the present study, we monitored changes in C and N content of foliar litter and wood of three boreal species over a period spanning 12–13 years and under a range of harvesting prescriptions. The initial (6 years) decay rates and changes in chemical composition of decaying litters have been previously reported by Strukelj and others (2012, 2015).
To our knowledge, this is the first long-term study simultaneously assessing the long-term decaying patterns of foliage and wood under different harvesting treatments. We hypothesised that: (H1) a double-exponential model would better reflect the decrease in C content during the successive phases of decomposition of foliar litter and wood; (H2) long-term (12–13 years) decay rates, and critical and stabilised C:N ratios would be higher for wood than for foliar litter; (H3) compared to natural stands, long-term decay rates, and critical and stabilised C:N ratios would decrease following clear-cutting (Berg and Ekbohm 1983; Strukelj and others 2015); and (H4) decomposition of foliar litter and wood would not be affected by partial harvesting, as little change in microclimatic conditions and in fungal communities was observed after these treatments (Brais and others 2004; Kebli and others 2012).
Materials and Methods
Study Area
The study was located in the Lake Duparquet Research and Teaching Forest (48°86′N–48°32′N, 79°19′W–79°30′W), which lies within the Abitibi region of northern Quebec, 45 km northwest of Rouyn–Noranda. Regional climate is continental with a mean annual air temperature of 1°C, and mean temperatures in the warmest and coldest months of 16.7°C (July) and −17.9°C (January), respectively. Annual precipitation is 985 mm, of which 663 mm falls as rain from April to November and the rest as snow (Environment Canada 2015). The region is located in the mixedwood sector of the boreal forest (Bergeron and others 2004), within the balsam fir (Abies balsamea [L.] Mill.)—paper birch (Betula papyrifera Marsh.) bioclimatic domain (Saucier and others 1998). Soils were formed in deep fine clayey to fine loamy-textured glacio-lacustrine deposits that were formed through sedimentation at the bottom of glacial Lake Barlow–Ojibway under fresh to moist moisture regimes (Brais and Camiré 1992). Soils are classified as Grey Luvisols (Soil Classification Working Group 1998; Boralfs, USDA classification system) and are characterised by a 5–15 cm MOR humus form with thin L and H horizons and a thicker F horizon.
Experimental Design
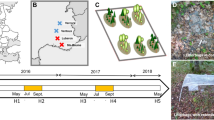
The SAFE project is set in three stand types, namely ASPEN, MIXED, and OLD (Table 1). In the ASPEN stand type (Table 1; also see Brais and others 2004; Belleau and others 2006), six treatments were applied in the winter of 1998–1999, including an unharvested control, three clear-cut, and two partial cut treatments. In the first partial cut (“1/3 PC”) and the second partial cut (“2/3 PC”), harvesting of stems greater than 9 cm diameter at breast height (dbh, 1.3 m) removed, respectively, 31 and 62% of basal area compared to controls, according to a dispersed and regular pattern (Brais and others 2004). In clear-cut stands, one of the three following treatments was applied: a slash treatment consisting of a stem-only harvesting treatment with stems being delimbed on site, with no additional slash manipulation (“CC-SOH”), a whole-tree harvesting treatment, where debris with diameters between 3 and 7 cm were removed (“CC-WTH”), and a slash–burn treatment, which was applied in August 1999 (“CC-SB”; for information on burn conditions and slash consumption, see Belleau and others 2006).
In the MIXED stand type (Table 1; also see Brais and others 2013), treatments were established in the winter of 2000–2001 and included a control, a clear-cut (stem-only harvesting, “CC-SOH”), and two partial cuts. In both partial cut treatments, about 40% of the total basal area (stems > 9 cm dbh) was removed. The dispersed partial cut treatment (“PC-D”) created a regular harvesting pattern, similar to those of partial cuts conducted in the ASPEN stand type. In the gap partial cut treatment (“PC-G”), all trees were removed along harvesting trails and in gaps (400 m2), resulting in light conditions and forest regeneration patterns closer to those observed in clear-cuts than in dispersed harvesting (Beaudet and others 2011; Brais and others 2013).
The OLD stand type (Table 1) was affected by the 1970–1987 outbreak of spruce budworm (Choristoneura fumiferana; Morin and others 1993). Two intensities of harvesting were applied in the winter of 1999–2000, a control and a clear-cut treatment (stem-only harvesting, “CC-SOH”).
A randomised complete block design with three replicates per treatment was applied in all stand types. Experimental units (EU) ranged from 1 to 3 ha in area. There were 18 EU (3 × 6 treatments) in the ASPEN stand type, 12 EU (3 × 4 treatments) in the MIXED stand type, and 6 EU (3 × 2 treatments) in the OLD stand type. Within each experimental unit, five permanent sampling plots (400 m2) were evenly distributed over each EU at the onset of the study.
Litterbag Decomposition Experiment
Foliar and wood litters from one of the common species in each stand type (see Table 1) were chosen to prepare litterbags (ca. 10 × 8 cm, 1-mm mesh size). Freshly fallen foliage of trembling aspen (Populus tremuloides Michx.) was collected in the autumn. Foliage of balsam fir and white spruce (Picea glauca [Moench] Voss) represents a small fraction of litterfall in these stands, and fresh foliage from all needle age classes was sampled directly from trees. Wood blocks approximately 5 × 5 × 10 cm of each species were cut from dry dimensional lumber from a local mill (for more details on litter collection and the initial litter characterisation, see Strukelj and others 2012). Five litterbags containing about 8 g foliage and five containing about 114 g wood blocks were positioned in each permanent sampling plot and left in situ to decompose. Of a total of 1800 litterbags that had been placed in the field, 1748 litterbags were retrieved 1–13 years after the start of each experiment (Table 1).
Following collection of litterbags, material from individual litterbags was oven-dried at 65°C to constant mass (≥48 h) and weighed. A composite sample was obtained by combining the material of the five litterbags collected in each EU, for each litter type and collection date. The resulting 360 samples (36 EU × 2 litter types × 5 collection dates) were analysed by high-temperature dry combustion to determine C and N concentrations (TruMac CNS analyser, LECO Corporation, St. Joseph, MI, USA). The C (or N) mass of each decomposition litterbag was estimated from its dried mass and C (or N) concentration of the composite sample at the time of sampling. Residual C (or N) content in litterbags is the proportion of the initial C (C i ; or initial N, N i ) mass that remains at the time of sampling, and was expressed in g C g C −1 i (or g N g N −1 i ).
Decomposition Models
Four different and plausible decomposition models were applied to litterbag residual C content as a function of time of incubation. We constrained the initial proportion of residual C mass to 1. The first equation (one parameter) is the “single negative exponential” model (Olson 1963), with a constant relative decay rate (Wieder and Lang 1982). This model assumes an initially uniform pool of C, which progressively increases in recalcitrance with decomposition, while the remaining mass fraction tends towards 0.
where P t (g C g C −1 i ) is the residual C content (0 < P t < 1) at time t (years) and k E (year−1) is the decay rate.
The second equation (two parameters), an “asymptotic” model (Wieder and Lang 1982), seeks to better account for the large decline in decay rates that was found in long-term decomposition trials, when litters enter a slow phase of decay (Berg 2000). This model implies that a recalcitrant fraction of the material will undergo very little decomposition over time.
where D (g C g C −1 i ) is the maximum decomposition limit corresponding to the initial proportion (0 < D < 1) of the material subject to loss at a decay rate k D (year−1) and A (g C g C −1 i ) is the asymptote (0 < A < 1). The sum of D and A is 1 g C g C −1 i .
The third equation (three parameters) is a double-exponential model, which recognises two contrasting fractions within decaying litters, a labile fast decomposing one and a more recalcitrant slowly decomposing fraction. The two fractions undergo decomposition simultaneously (“double-exponential—simultaneous”; Wieder and Lang 1982; Harmon and others 2009; Manzoni and others 2012). Hence, different decay rates are applied to the relative proportion of these fractions.
where L (g C g C −1 i ) is the initial labile fraction (0 < L < 1), with fast decay rate k L (year−1), and R (g C g C −1 i ) is the initial recalcitrant fraction (0 < R < 1), with a slow decay rate k R (year−1). The sum of L and R is 1 g C g C −1 i .
The fourth equation (three parameters) is also a double-exponential model, but assumes the initial fresh litter mineralises first and gives rise to a more stable fraction (Manzoni and others 2012). The two decomposing fractions are assumed to decompose successively or in series (“double-exponential—successive”).
where k F (year−1) is the decay rate of fresh litter, [1 − exp(−k F t)] is the decayed material, and S (g C g C −1 i ) is the fraction of the decaying material (0 < S < 1) that forms stable material decomposing at a decay rate k S (year−1).
We can note that the single-exponential model can be considered a special case of the three other models, in which the fractions A (model 2), L (or R, model 3), or S (model 4) would be undetectable.
Statistical Analyses
We used linear and nonlinear mixed models to analyse changes in residual C and N contents with decomposition (see below). As C and N dynamics could differ among litter types (Harmon and others 2009; Moore and others 2011), all analyses were conducted separately for the six litter types using the nlme package of R (α = 0.05; Pinheiro and Bates 2000; R Development Core Team 2015). In all models, experimental block was included as a random factor. Nesting of sampling plots (five litterbags) within each EU and nesting of EU (harvesting treatments) within each experimental block were removed from the random effects, because the variance associated with these factors was generally negligible. Fixed factors differed between models of C and N dynamics and are presented in detail below.
Decomposition Models of Foliar Litters and Wood (Hypotheses H1, and H2)
To analyse changes in residual C content, the parameters of the four aforementioned decomposition models were estimated by nonlinear mixed-effects models using the nlme function (Pinheiro and Bates 2000). Harvesting treatment was included as a fixed factor in all models to estimate parameters simultaneously for control and harvested stands. To limit the number of estimated parameters and facilitate convergence of the models (3) and (4), we tested the effect of harvesting and the random factor on only two parameters [that is, k L and k R in model (3), and k F and k S in model (4)]. Therefore, we assumed that, for each litter type, the initial L (and S) fractions were similar in all harvesting treatments and all experimental blocks. For aspen foliage, we nevertheless included a random effect for the L (and S) fractions to consider the higher C mass loss in the second block of replication compared to the others (L estimate for the second block was double that of the others; see Figure 1; Table 3). For both coniferous woods (see Table 2), two of the models did not allow the convergence criteria of the iterative procedure to be reached or led to a solution hindering interpretation (that is, parameter <0 or inversion of k L and k R , or of k F and k S , between treatments).
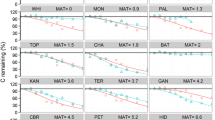
Residual carbon (C) content of decaying foliage and wood of trembling aspen, white spruce, and balsam fir as a function of time of decomposition and harvesting treatment. Curves correspond to predictions based on the best supported nonlinear mixed models (see Tables 2, 3). For explanations of harvesting treatments, see the note in Table 1. Random effect: first (square), second (circle), and third (triangle) blocks of replication.
We conducted model selection based on the second-order Akaike’s Information Criterion (AICc) to determine which model, within the set of a priori statistical models, had the most support (Burnham and Anderson 2002). Models were ranked according to their respective AICc values, with the lowest value corresponding to the model that was best supported by the data. Comparison among models was based on delta AICc (Δ i , that is, difference in AICc relative to the top model) and Akaike weights (w i , that is, probability that the model is the best among candidate models) that were implemented in the AICcmodavg package (Mazerolle 2015). We used the model with the greatest w i to interpret the decomposition parameters and to estimate long-term decay rates.
Long-Term Decay Rates (Hypotheses H2, H3, and H4)
Direct comparison of decay rates among litter types and harvesting treatments was problematic because the selected decomposition models had different numbers of parameters. As suggested by Harmon and others (2009), a long-term (12–13 years) integrated average decay rate constant was calculated according to the following formula:
where k LT corresponds to the long-term decay rate and M cs to the estimated residual C mass of litter under steady-state conditions. M cs was calculated over a period of 100 years in all stand types by assuming constant annual C inputs of litter of 1 unit and C outputs that were estimated based on the most probable decomposition model. Confidence intervals (95%) of the long-term decay rates were estimated using a Monte Carlo approach consisting of 5000 iterations. At each iteration, we simulated a new data set from a normal distribution (rnorm function) with a mean equal to the fitted values of the best supported model and standard deviation equal to the residual standard deviation of the model. Using this data set, we ran a model that was identical to the best supported model. We obtained new estimates of parameters, from which we calculated a new estimate of k LT. Calculations of confidence intervals were based on the 2000 first models that converged, after having removed models yielding nonsensical values (that is, parameter <0, or inversion of k L and k R , or of k F and k S ). To help readers interpret differences in means, we provided a z-test as supplementary information.
Critical and Stabilised C:N Ratios (Hypotheses H2, H3, and H4)
Critical C:N ratios were assessed by analysing changes in residual N content as a function of residual C content (Moore and others 2006), using linear mixed models (lme function, Pinheiro and Bates 2000). We included the squared value of residual C content as an explanatory variable to estimate the quadratic relationship between residual N content and residual C content (Moore and others 2006). Effects of harvesting, and their interactions with residual C content and its squared value were also included as fixed factors, but interactions were removed when their p values were >0.10. Normality and homogeneity of variance were verified by visual assessment of residuals. When patterns of residuals deviated from the expected pattern consistent with a normal distribution, we applied square root transformations to the response variables or added variance stabilising functions with the weights argument, which allows the variance to differ between groups (Pinheiro and Bates 2000). Confidence intervals of critical C:N ratios were estimated by Monte Carlo simulations, with 2000 iterations. The approach was similar to that used in the previous section. Finally, the stabilised C:N ratio (sCN) of decayed litters was assessed with an asymptotic function:
where the parameters y, z, and sCN were estimated by the nlme function in a manner similar to the method that was used for decomposition models. The asymptote, sCN, was the only parameter represented in Figure 4B.
Results
Decomposition Models of Foliar Litter and Wood
Despite their higher numbers of estimated parameters (K, Table 2), double-exponential Eqs. (3) and (4) were the most plausible models for foliage decomposition of all species and for aspen wood, as indicated by their lower AICc and higher w i . Given their very low w i , single-exponential models were very unlikely fits for these four litter types. For aspen, evidence ratios (Burnham and Anderson 2002) between the double-exponential simultaneous model and the double-exponential successive model were 1/2.96 × 10−22 = 3.4 × 1021 in foliage and 0.87/0.13 = 6.69 in wood (see Table 2). This indicates that the simultaneous model had 3.4 × 1021 and 6.69 times more support than the successive model in aspen foliage and wood, respectively. For fir and spruce foliage, the double-exponential successive model attained support (w i ) comparable to the double-exponential simultaneous model.
The labile fraction of foliage, which corresponded to the L value in Eq. (3), was 0.57, 0.51, and 0.71 g C g C −1 i for aspen, for spruce, and for fir, respectively (Table 3; Figure 1). The decay rates of the labile fractions of foliage ranged from 0.61 to 0.84 year−1 in control stands, that is, these labile fractions became a minor fraction of residual C content (<1%) and had almost disappeared within 6.2–8.8 years. Decay rates of the corresponding recalcitrant fractions ranged from 0.067 to 0.076 year−1. For aspen wood, the labile fraction amounts to 0.27 g C g C −1 i and decomposed in less than 4 years at a decay rate of 1.13 year−1 in control stands, whereas the recalcitrant fraction had a decay rate of 0.14 year−1. For spruce and fir foliage, the double-exponential successive model yielded respective decay rates of 0.71 and 0.80 year−1 for fresh litter in control stands, while 0.51 and 0.30 g C g C −1 i of this decaying material became more stable, decomposing at 0.079 and 0.076 year−1, respectively (Table 3; Figure 1).
For coniferous wood decomposition, variability was higher than for the other litter types (Figure 1), and two of the four models did not converge with biologically interpretable values (Table 2). The best of the two remaining models for spruce and fir wood was the single-exponential model, as indicated by the lower AICc and w i ≥ 0.82. The model implies a single decomposing fraction with a decomposition rate of 0.094 year−1 in balsam fir wood and 0.138 year−1 in white spruce wood.
Based on estimates and their 95% confidence intervals (95% CI) obtained from the most plausible model for each litter type (Table 3), we found that the size of the labile fraction was higher in foliage (0.51–0.71 g C g C −1 i ) than in wood (0.28 g C g C −1 i in aspen, and undetected according to the single-exponential model in spruce and fir). The 95% CI of the decomposition rates of the labile fraction of aspen wood and foliage slightly overlapped, suggesting weak differences between them (Table 3). The decomposition rate of the recalcitrant fraction of aspen wood (0.138 year−1, 95% CI [0.114–0.162]) was higher than that of aspen foliage (0.067 year−1, 95% CI [0.049–0.084], Table 3). Similarly, the decomposition rate of the single spruce wood fraction (0.138 year−1, 95% CI [0.121; 0.154]) was higher than that of the recalcitrant fraction of its foliage (0.076 year−1; 95% CI [0.047; 0.105], Table 3). For fir, the decay rate of the single decomposing fraction of wood (0.094 year−1, 95% CI [0.077; 0.111]) and the decay rate of the recalcitrant fraction of foliage (0.075 year−1, 95% CI [0.040; 0.110]) did not differ.
Average Long-Term Decay Rates
When considering only control stands, the long-term average decay rate (k LT) of foliage was faster for fir than for either aspen (z = 2.21, p = 0.03) or spruce (z = 2.39, p = 0.02; Figure 2). In contrast, balsam fir wood had the slowest k LT, while aspen wood had the fastest. Long-term decay rates were similar for spruce foliage and wood, whereas they were marginally significantly slower in aspen foliage than in wood (z = 1.92, p = 0.06) and faster for fir foliage than for wood (z = 4.77, p < 0.01). Consistent with long-term decay rates, residual C content after 12–13 years of decomposition averaged 0.22, 0.22, and 0.11 g C g C −1 i in foliage and 0.14, 0.19, 0.27 g C g C −1 i in wood of aspen, spruce, and fir, respectively (Figure 1).
Long-term decay rates k LT (mean ± 95% CI) of foliar litter and wood blocks of trembling aspen, white spruce, and balsam fir following partial and clear-cut harvesting treatments. Confidence intervals were estimated using a Monte Carlo approach (see “Materials and Methods” section). For explanations of the harvesting treatments, see the note in Table 1.
Following clear-cutting, the long-term decay rates of aspen foliage increased under the CC-WTH (z = 2.54, p = 0.01) and CC-SB (z = 3.99, p < 0.01) treatments (Figures 1, 2). However, the long-term decay rates did not differ between the control and CC-SOH treatments in foliage of aspen (z = 0.52, p = 0.60), spruce (z = 0.52, p = 0.60), and fir (z = 1.29, p = 0.20), despite slower decay rates of the labile fraction in several cases (Table 5 in Online Appendix). Clear-cut harvesting increased the long-term decay rate of fir wood (z = 2.76, p < 0.01), but had no effect on aspen and spruce wood decomposition (Figures 1, 2).
A marginally significant faster long-term decay rate of spruce foliage was observed following the PC-D treatment (z = 1.85, p = 0.06). But partial harvesting had no effect on decay rates of aspen foliage, and aspen and spruce wood (Figures 1, 2).
Critical and Stabilised C:N Ratios
Successive increase and decrease in residual N content with decreasing residual C content (as a measure of decay state) were observed in the six litter types (Figure 3; Table 6 in Online Appendix). Foliage residual N content increased to a maximum of 1.1–1.3 times the initial values. These maxima were reached following 0.6–2.0 years of decomposition. Residual N content of decaying wood increased by factors of 2.0–2.7, and these maxima were reached following 3.2–4.5 years of decomposition (Figure 3; Table 7 in Online Appendix). At maximum residual N content, the residual C content ranged from 0.57 to 0.71 g C g C −1 i (decomposed fraction of the initial C of 29–43%) for foliage and from 0.44 to 0.66 g C g C −1 i in wood (decomposed fraction of the initial C of 34–46%). The critical C:N ratio ranged from 28 to 30 for foliage and from 130 to 275 for wood (Figure 4A), whereas stabilised C:N ratios ranged from 16 to 23 for foliage and from 14 to 83 for wood (Figure 4B). The observed C:N ratios in control stands at the end of the experiment ranged from 20.0 to 22.8 for foliar litters and from 46.4 to 95.6 for wood blocks (Table 8 in Online Appendix).
Residual nitrogen (N) content as a function of residual C content and harvesting treatments in decaying foliage and wood of trembling aspen, white spruce, and balsam fir. For the sake of clarity, only the control and clear-cut with stem-only harvesting (CC-SOH) treatments are presented (see Tables 6, 7 in Online Appendix). Residual N content was square root transformed and was analysed as a function of residual C content and squared value of residual C content using linear mixed models. Time of decomposition at maximum N content (t, years, calculated using the model with the greatest support, see Tables 2, 3; Figure 1) is indicated in figures.
A Critical C:N ratio and B stabilised C:N ratio (mean ± 95% CI) for decaying foliage and wood of trembling aspen, white spruce, and balsam fir following partial and clear-cut harvesting treatments. Critical C:N ratios were assessed by linear mixed models of square root of residual N content as a function of residual C content and squared value of residual C content. Confidence intervals of critical C:N ratios were estimated by Monte Carlo simulations (see “Materials and Methods” section). Stabilised C:N ratios were estimated by nonlinear mixed-effects models using an asymptotic function. The asymptote, corresponding to the stabilised C:N ratio, is presented. For explanations of the harvesting treatments, see the note in Table 1.
The critical C:N ratio decreased under the CC-SOH treatment in foliage of spruce (z = 3.14, p < 0.01) and fir (z = 8.06, p < 0.01; Figure 4A). The decrease was marginally significant in wood of spruce (z = 1.67, p = 0.10) and fir (z = 1.64, p = 0.10). The critical C:N ratio of aspen foliage and wood did not differ between the control and the treatments of clear-cutting (CC-SOH, CC-WTH, and CC-SB). The critical C:N ratio was generally reached after a longer time of decomposition under the clear-cutting treatments, compared to the control (Figure 3; Table 7 in Online Appendix). The stabilised C:N ratio of aspen and spruce foliar litters also decreased under the CC-SOH treatment (p < 0.01, and p < 0.01, respectively; Figure 4B). We found no differences among treatments for the stabilised C:N ratio of wood due to the high degree of variability in the data (Table 8 in Online Appendix). Differences between control and CC-SOH treatments can also be found in the final values of C:N ratio (Table 8 in Online Appendix).
Relative to the control treatment, the PC-G treatment had significant effects on the residual N content of foliar litter and wood of spruce over the course of the experiment (Table 6 in Online Appendix). The PC-G treatment decreased the stabilised C:N ratio of spruce foliage (p = 0.02) and marginally decreased the critical C:N ratio of spruce wood (z = 1.67, p = 0.10; Figure 4). But other partial harvesting treatments had no effect on N dynamics.
Discussion
Long-term (over 10 years) decomposition experiments in forests are rare. The extensive CIDET (up to 12 years; Moore and others 2011) and LIDET (up to 10 years; Harmon and others 2009) studies are notable exceptions. The long-term patterns in C losses reported here lend empirical support to alternatives to the single-exponential decomposition model that would better account for long-term mass losses and C retention within the forest floor. The results also question the assumption that wood material, because of its low nutrient and high lignin content, is more recalcitrant to decomposition than foliar litter over the long term. Finally, the study provides new information regarding the effects of harvesting on long-term average decomposition rates and N release of both foliar litter and wood in boreal ecosystems.
Most Probable Decomposition Models (Hypothesis H1)
The double-exponential simultaneous model, selected for four of the six litter types (trembling aspen, balsam fir, and white spruce foliage, and aspen wood), underscores the presence of two groups of components that decompose simultaneously, but at different rates. The initial chemical composition of these four litter types, analysed by 13C nuclear magnetic resonance (NMR), was dominated by O/N-alkyl C structures (51–83%, Table 4). Over the first 5–6 years of decomposition, concentrations of these structures decreased as the mineralisation of holocelluloses and leaching of soluble carbohydrates proceeded (Strukelj and others 2012). The presence in early stages of decomposition of endophyte or/and opportunistic ascomycetes, with high levels of cellulolytic enzyme activity, could account for this initial rapid C loss (Šnajdr and others 2011; Štursová and others 2012; Foudyl-Bey and others 2016). In addition, this phase probably involves the leaching of proteins, tannins and other polyphenols, and of newly formed microbial products (Šnajdr and others 2011; Strukelj and others 2012; Cotrufo and others 2013). Also, some lignin components could form a part of the labile fraction (Thevenot and others 2010). Fioretto and others (2005) observed a rapid mineralisation of lignin in a 3-year decomposition study of foliar litter, and Foudyl-Bey and others (2016) found significant ligninolytic activity in fresh decaying foliage and wood of trembling aspen from close by similar stands.
Again, according to the simultaneous model, the recalcitrant components of litters are present both initially and in advanced stages of decomposition (Fukasawa and others 2009; Strukelj and others 2012, 2013). This recalcitrant fraction likely includes aliphatic (alkyl C) molecules such as fatty acids, waxes and resins (Lorenz and others 2007), and holocelluloses (O/N-alkyl C) and lignin (aromatic C) that are protected from decomposition by their covalent linkages to each other (Talbot and others 2012).
For balsam fir and white spruce foliar litter, the double-exponential successive model (4) was as probable as was the simultaneous model (3). This model assumes that the stable fraction results from the decomposition of labile components. As decomposition proceeds, decaying litters become enriched in microbial products (Cotrufo and others 2013) and in aromatic by-products from lignin decomposition (Prescott 2010). These structures provide secondary recalcitrance to decaying organic matter (Von Lützow and others 2006). Manzoni and others (2012) also found the two models equally likely when modelling foliar litter and soil organic matter decomposition. Simultaneous and successive decompositions are not mutually exclusive as biological mechanisms can be provided for both models (see discussion above). Modelling mass losses of foliage and roots over 27 different sites and over a 10-year period, Harmon and others (2009) reported that the double-exponential model (simultaneous) provided a good fit in 59% of cases. Simultaneous and successive decompositions are not mutually exclusive as biological mechanisms can be provided for both models (see discussion above). As highlighted by Wieder and Lang (1982), double-exponential models “are a compromise between a single-exponential model and a model that could track several fractions of fresh litter decomposing exponentially”. This conception of decomposition may be more consistent with recent advances in fungal ecology and enzymology such as the Guild Decomposition Model (Baldrian and Lindahl 2011).
In contrast, single-exponential models, found to be the most plausible for spruce and fir wood, imply the decomposition of single fractions at constant relative rates. These rates (0.094 year−1 in balsam fir wood and 0.138 year−1 in white spruce wood in control stands) were similar to those of the recalcitrant fraction of aspen wood (0.138 year−1). Although a faster decaying fraction must be present in spruce and fir wood, it was likely too small to be reliably detected given the time interval between measurements and given the large variations in C loss values in conifer wood samples (Figure 1). The limited changes observed in the relative proportions of chemical structures for spruce and fir wood over the first 5–6 years of decomposition (Strukelj and others 2012) were consistent with decay patterns exhibited by nonselective white-rot fungi (Boddy and Watkinson 1995). The variability in the decay patterns of coniferous wood was greater than that of their foliage (Figure 1) despite being incubated under identical field conditions. This may be related to variations in fungal communities at various scales, from pieces of wood to the landscape (Boddy and Watkinson 1995; Wickings and others 2012). It may also have been caused by variability in wood density and chemical composition within wood blocks.
The majority of empirical studies relating decomposition rates to environmental factors or to litter properties are still based on short-term data (<5–6 years) and use the single-exponential model (for example, Zhang and others 2008). While inferences can be drawn regarding which factors control the initial phase of decomposition, inferences to longer term C dynamics must be questioned. Aside from a better representation and understanding of mass loss patterns, selecting an appropriate model has implications when modelling ecosystem C balance. The single-exponential model could underestimate C sequestration from decomposing litters by as much as 34% (Harmon and others 2009).
Comparison Between Foliar Litter and Wood (Hypothesis H2)
It is assumed the low nutrient and high lignin concentrations of wood hinder its decomposition (Cornwell and others 2009; Cornelissen and others 2012). However, we hypothesised that because foliage is richer in N and alkyl C compounds than wood (Strukelj and others 2012, 2013), foliage could generate more stable compounds resulting in slower long-term decay rates. A faster average long-term decay rate in wood than in foliage was only found for aspen. However, for all three species, we found no evidence that the recalcitrant fraction of wood decomposed at a slower rate than the recalcitrant fraction of foliage. As mentioned earlier, the higher concentrations of N and alkyl C compounds in foliage than in wood may account for the slow decay rates of the recalcitrant fraction of the former. Low molecular N compounds may repress the synthesis of lignin-degrading enzymes (Berg 2000; Prescott 2010), whereas alkyl C includes recalcitrant waxes and other lipids (Lorenz and others 2007). On the other hand, holocelluloses and lignin of wood can be efficiently decomposed by white-rot fungi, such as Bjerkandera adusta and Phlebia centrifuga. Both species were found on fresh aspen and balsam fir wood blocks within 6 months of being set on the forest floor of the study stands (Kebli and others 2014). B. adusta can cause high weight losses in fresh wood (Fukasawa and others 2011), whereas some strains of P. centrifuga degrade lignin more efficiently than other wood components (Hakala and others 2004).
Apart from chemical factors, the physical accessibility of litter components to microorganisms (surface area to volume ratio, cell structure; Cornwell and others 2009; Cornelissen and others 2012) likely explains differences in decomposition between foliar litter and wood. The small size of the wood blocks used for the study increased wood accessibility to fungal attack and increased wood decay rates when compared to estimates from log chronosequences (Brais and others 2006), allowing differences in chemical properties between foliage and wood to supplant differences in litter size.
As hypothesised, the critical C:N ratios of wood were higher than those of foliage. Critical C:N ratios of 33–68 were reported for the foliage of the seven forest tree species included in the CIDET experiment (Moore and others 2011), while a value of 165 was reported for western hemlock wood blocks (Smyth and others 2016), the single wood type included in the experiment. Differences in critical C:N ratios reflect differences in the initial C:N ratios and presumably differences in the saprotrophic community C:N ratio and C use efficiency (Manzoni and others 2008; Ågren and others 2013). Net N release is assumed to result from mycelium senescence due to energy limitations (Boddy and Watkinson 1995; Hart 1999; Philpott and others 2014). Foliage and wood reached critical C:N ratios at comparable decomposed fractions of the initial C (29–56%). This fraction might be a threshold where litters become C-limited, inducing filamentous fungi to forage in order to maintain their growth and activity. Fungal mycelial extension away from C-limited litters to C-rich litters may also explain the decrease in N content observed in decayed litters (Lindahl and others 2002). Although wood samples reached their critical C:N ratio during the study period, most samples collected over the whole 13-year experiment had residual N content above 1 g N g N −1 i , highlighting wood N sequestration potential. In contrast, foliage reached a residual N content below 1 g N g N −1 i within 15 months to 6 years of decomposition.
The critical C:N ratios were higher than the observed C:N ratios at the end of the experiment, indicating that decomposition still proceeds in spite of periodic energy limitations. The final C:N ratios were consistent with C:N values reported for the forest floor originating from foliage decomposition (16–24) and with C:N ratios from decaying wood found buried within the forest floor (30–80; Strukelj and others 2013; Foudyl-Bey and others 2016). Hence, despite limiting macrofauna access to the incubating material, litterbag incubations allow decomposition to proceed, although at different rate, to end products comparable in chemical composition to the material forming the forest floor. This may be particularly true for the forest floor of boreal forests where fungi are the main decomposers (Lindahl and others 2002).
Effects of Clear-Cutting Versus Partial Harvesting (Hypotheses H3, and H4)
Clear-cut harvesting effects on decomposition rate differed among litter material, tree species, and slash treatment. Contrary to our third hypothesis, the average long-term decay rates following clear-cutting were either similar or faster than those observed in control stands Also, as observed here for aspen and fir foliage (Table 5 in Online Appendix), harvesting can have opposite effects on the decomposition of the labile and recalcitrant fractions of litters, leading to different conclusions depending on the study duration. This unpredictability is consistent with the range of effects reported from various studies (Yin and others 1989; Prescott and others 2000; Finér and others 2016).
Despite decomposing side by side, wood and foliage of aspen and fir were affected in different ways by clear-cutting. Saprotrophic fungal communities of fresh wood and foliage are recognised as different functional or ecophysiological groups (Eichlerová and others 2015). Their respective enzyme synthesis systems may respond differently to changes in environmental conditions induced by harvesting. Although many studies have quantified the impacts of harvesting on deadwood volume (Brassard and Chen 2008; Hagemann and others 2010; Strukelj and others 2015), little information regarding harvesting effects on wood decomposition is available (for example, Marra and Edmonds 1996; Strukelj and others 2015). This general lack of information regarding deadwood decomposition compromises the adequacy of broad-scale estimates of heterotrophic CO2 in managed forests (Harmon and others 2011). Moreover, inferring results of short-term experiments conducted on foliage litters to other dead organic matter pools could be misleading.
The most consistent effect of clear-cutting observed in the study was the decrease in critical C:N ratios of foliage and wood of spruce and fir under the CC-SOH treatment when compared with control stands. The stabilised C:N ratio of aspen foliage also decreased under the CC-SOH treatment. A decrease in the critical C:N ratio following clear-cut harvesting was reported for Scott pine needles (Berg and Ekbohm 1983), but not for ponderosa pine needles (Hart and others 1992). Although strongly dependent on the litter initial characteristics, immobilisation and release of N during decomposition are also conditioned by the surrounding environment of decaying litters (Hart and Firestone 1991; Hart and others 1992; Moore and others 2011).
Higher amounts of fresh harvesting slash were found in stands immediately after stem-only harvesting when compared with controls (52 Mg ha−1 in CC-SOH vs. 14 Mg ha−1 in controls of ASPEN stands, Belleau and others 2006; 90 Mg ha−1 in CC-SOH versus 32 Mg ha−1 in controls of MIXED stands, Brais and others 2013). Responses of the microbial communities to large inputs of fresh organic matter could include the redistribution of fungal biomass and N away from well-decomposed organic matter towards fresh litter via mycelial growth and translocation by cord-forming fungi (Schimel and Hättenschwiler 2007; Boberg and others 2014; Philpott and others 2014; Foudyl-Bey and others 2016). Large inputs of fresh organic matter can also change microbial use efficiencies of N and C (Boberg and others 2014; Mooshammer and others 2014) and increase retention of N in mycelial biomass (Boddy and Watkinson 1995). Such responses could delay fungal biomass senescence and N mineralisation in litters (Philpott and others 2014; Foudyl-Bey and others 2016) resulting in lower critical C:N ratio and increased N retention following clear-cut harvesting.
Partial harvesting had minor effects on long-term decay rates and critical C:N ratios as forecast in our fourth hypothesis, probably because of its minor effects on soil temperature and moisture (Brais and others 2004), foliar litter and deadwood inputs (Strukelj and others 2015), and decomposer communities (Kebli and others 2014). The effects of partial harvesting on decomposition have not been investigated to the same extent as those of clear-cutting, and require further investigation (Yin and others 1989; Prescott 1997; Prescott and others 2000; Hope and others 2003).
Conclusion
Based on long-term litterbag incubations of wood and foliage, our study lends empirical support to alternatives to the single-exponential decay model that better explain long-term litter C losses in forest ecosystems. Double-exponential models, when plausible, highlight the presence of labile and recalcitrant fractions decomposing at different rates within a given litter type. Comparing wood and foliage C losses, we found no evidence for differences between litter origins in the decomposition rate of their respective recalcitrant fraction. However, the critical C:N ratio appears to be substrate specific and affected by the amount of fresh organic matter surrounding the decaying litters. The results challenge the use of short-term decomposition outcomes to long-term C balance estimates in managed and unmanaged forest ecosystems. With recent developments in genetic, enzymatic, and chemical analyses, it is now possible to conduct litterbag experiments that advance our understanding of saprotrophic communities and processes involved in litter decomposition. Long-term comprehensive empirical data on the patterns and controls of decomposition gained from these experiments could support more theoretical approaches and improve assessments of C and N fluxes in forest ecosystems.
References
Ågren GI, Hyvönen R, Berglund SL, Hobbie SE. 2013. Estimating the critical N:C from litter decomposition data and its relation to soil organic matter stoichiometry. Soil Biol Biochem 67:312–18.
Angers VA, Drapeau P, Bergeron Y. 2012. Mineralization rates and factors influencing snag decay in four North American boreal tree species. Can J For Res 42:157–66.
Baldrian P, Lindahl B. 2011. Decomposition in forest ecosystems: after decades of research still novel findings. Fungal Ecol 4:359–61.
Barg AK, Edmonds RL. 1999. Influence of partial cutting on site microclimate, soil nitrogen dynamics, and microbial biomass in Douglas-fir stands in western Washington. Can J For Res 29:705–13.
Beaudet M, Harvey BD, Messier C, Coates KD, Poulin J, Kneeshaw DD, Brais S, Bergeron Y. 2011. Managing understory light conditions in boreal mixedwoods through variation in the intensity and spatial pattern of harvest: a modelling approach. For Ecol Manag 261:84–94.
Belleau A, Brais S, Paré D. 2006. Soil nutrient dynamics after harvesting and slash treatments in boreal aspen stands. Soil Sci Soc Am J 70:1189–99.
Berg B. 2000. Litter decomposition and organic matter turnover in northern forest soils. For Ecol Manag 133:13–22.
Berg B, Ekbohm G. 1983. Nitrogen immobilization in decomposing needle litter at variable carbon:nitrogen ratios. Ecology 64:63–7.
Bergeron Y, Gauthier S, Flannigan M, Kafka V. 2004. Fire regimes at the transition between mixedwood and coniferous boreal forest in Northwestern Quebec. Ecology 85:1916–32.
Boberg JB, Finlay RD, Stenlid J, Ekblad A, Lindahl BD. 2014. Nitrogen and carbon reallocation in fungal mycelia during decomposition of boreal forest litter. PLoS ONE 9:e92897.
Boddy L, Watkinson SC. 1995. Wood decomposition, higher fungi, and their role in nutrient redistribution. Can J Bot 73:1377–83.
Brais S, Camiré C. 1992. Keys for soil moisture regime evaluation for northwestern Quebec. Can J For Res 22:718–24.
Brais S, Harvey BD, Bergeron Y, Messier C, Greene D, Belleau A, Paré D. 2004. Testing forest ecosystem management in boreal mixedwoods of northwestern Quebec: initial response of aspen stands to different levels of harvesting. Can J For Res 34:431–46.
Brais S, Paré D, Lierman C. 2006. Tree bole mineralization rates of four species of the Canadian eastern boreal forest: implications for nutrient dynamics following stand-replacing disturbances. Can J For Res 36:2331–40.
Brais S, Work TT, Robert É, O’Connor CD, Strukelj M, Bose AK, Celentano D, Harvey BD. 2013. Ecosystem responses to partial harvesting in eastern boreal mixedwood stands. Forests 4:364–85.
Brassard BW, Chen HYH. 2008. Effects of forest type and disturbance on diversity of coarse woody debris in boreal forest. Ecosystems 11:1078–90.
Burnham KP, Anderson DR. 2002. Model selection and multimodel inference. A practical information-theoretic approach. New York: Springer.
Cornelissen JHC, Sass-Klaassen U, Poorter L, Van Geffen K, Van Logtestijn RSP, Van Hal J, Goudzwaard L, Sterck FJ, Klaassen RKWM, Freschet GT, Van der Wal A, Eshuis H, Zuo J, De Boer W, Lamers T, Weemstra M, Cretin V, Martin R, Den Ouden J, Berg MP, Aerts R, Mohren GMJ, Hefting MM. 2012. Controls on coarse wood decay in temperate tree species: birth of the LOGLIFE experiment. AMBIO 41:231–45.
Cornwell WK, Cornelissen JHC, Allison SD, Bauhus J, Eggleton P, Preston CM, Scarff F, Weedon JT, Wirth C, Zanne AE. 2009. Plant traits and wood fates across the globe: rotted, burned, or consumed? Glob Chang Biol 15:2431–49.
Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E. 2013. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Chang Biol 19:988–95.
Eichlerová I, Homolka L, Žifčáková L, Lisá L, Dobiášová P, Baldrian P. 2015. Enzymatic systems involved in decomposition reflects the ecology and taxonomy of saprotrophic fungi. Fungal Ecol 13:10–22.
Environment Canada. 2015. Canadian climate normals or averages 1981–2010. Available from http://climate.weatheroffice.gc.ca/climate_normals/index_e.html2015.
Finér L, Jurgensen M, Palviainen M, Piirainen S, Page-Dumroese D. 2016. Does clear-cut harvesting accelerate initial wood decomposition? A five-year study with standard wood material. For Ecol Manag 372:10–18.
Fioretto A, Di Nardo C, Papa S, Fuggi A. 2005. Lignin and cellulose degradation and nitrogen dynamics during decomposition of three leaf litter species in a Mediterranean ecosystem. Soil Biol Biochem 37:1083–91.
Foudyl-Bey S, Brais S, Drouin P. 2016. Litter heterogeneity modulates fungal activity, C mineralization and N retention in the boreal forest floor. Soil Biol Biochem 100:264–75.
Fukasawa Y, Osono T, Takeda H. 2009. Dynamics of physicochemical properties and occurrence of fungal fruit bodies during decomposition of coarse woody debris of Fagus crenata. J For Res 14:20–9.
Fukasawa Y, Osono T, Takeda H. 2011. Wood decomposing abilities of diverse lignicolous fungi on nondecayed and decayed beech wood. Mycologia 103:474–82.
Hagemann U, Moroni MT, Gleißner J, Makeschin F. 2010. Disturbance history influences downed woody debris and soil respiration. For Ecol Manag 260:1762–72.
Hakala TK, Maijala P, Konn J, Hatakka A. 2004. Evaluation of novel wood-rotting polypores and corticioid fungi for the decay and biopulping of Norway spruce (Picea abies) wood. Enzyme Microb Technol 34:255–63.
Harmon ME, Bond-Lamberty B, Tang J, Vargas R. 2011. Heterotrophic respiration in disturbed forests: a review with examples from North America. J Geophys Res 116:1–17.
Harmon ME, Franklin JF, Swanson FJ, Sollins P, Gregory SV, Lattin JD, Anderson NH, Cline SP, Aumen NG, Sedell JR, Lienkaemper GW, Cromack JRK, Cummins KW. 1986. Ecology of coarse woody debris in temperate ecosystems. In: Caswell H, Ed. Advances in ecological research. Orlando: Academic Press. p 133–302.
Harmon ME, Silver WL, Fasth B, Chen H, Burke IC, Parton WJ, Hart SC, Currie WS, LIDET. 2009. Long-term patterns of mass loss during the decomposition of leaf and fine root litter: an intersite comparison. Glob Chang Biol 15:1320–38.
Hart SC. 1999. Nitrogen transformations in fallen tree boles and mineral soil of an old-growth forest. Ecology 80:1385–94.
Hart SC, Firestone MK. 1991. Forest floor-mineral soil interactions in the internal nitrogen cycle of an old-growth forest. Biogeochemistry 12:103–27.
Hart SC, Firestone MK, Paul EA. 1992. Decomposition and nutrient dynamics of ponderosa pine needles in a Mediterranean-type climate. Can J For Res 22:306–14.
Hope GD, Prescott CE, Blevins LL. 2003. Responses of available soil nitrogen and litter decomposition to openings of different sizes in dry interior Douglas-fir forests in British Columbia. For Ecol Manag 186:33–46.
Kebli H, Brais S, Kernaghan G, Drouin P. 2012. Impact of harvesting intensity on wood-inhabiting fungi in boreal aspen forests of Eastern Canada. For Ecol Manag 279:45–54.
Kebli H, Kernaghan G, Drouin P, Brais S. 2014. Development and activity of early saproxylic fungal communities in harvested and unmanaged boreal mixedwood stands. Eur J For Res 133:905–18.
Laiho R, Prescott CE. 2004. Decay and nutrient dynamics of coarse woody debris in northern coniferous forests: a synthesis. Can J For Res 34:763–77.
Lindahl BD, Taylor AFS, Finlay RD. 2002. Defining nutritional constraints on carbon cycling in boreal forests—towards a less `phytocentric’ perspective. Plant Soil 242:123–35.
Lorenz K, Lal R, Preston CM, Nierop KGJ. 2007. Strengthening the soil organic carbon pool by increasing contributions from recalcitrant aliphatic bio(macro)molecules. Geoderma 142:1–10.
Mäkiranta P, Laiho R, Penttilä T, Minkkinen K. 2012. The impact of logging residue on soil GHG fluxes in a drained peatland forest. Soil Biol Biochem 48:1–9.
Manzoni S, Jackson RB, Trofymow JA, Porporato A. 2008. The global stoichiometry of litter nitrogen mineralization. Science 321:684–6.
Manzoni S, Piñeiro G, Jackson RB, Jobbágy EG, Kim JH, Porporato A. 2012. Analytical models of soil and litter decomposition: solutions for mass loss and time-dependent decay rates. Soil Biol Biochem 50:66–76.
Marra JL, Edmonds RL. 1996. Coarse woody debris and soil respiration in a clearcut on the Olympic Peninsula, Washington, U.S.A. Can J For Res 26:1337–45.
Mazerolle MJ. 2015. AICcmodavg: model selection and multimodel inference based on (Q)AIC(c). R package version 2.0-3. Available from https://cran.r-project.org/web/packages/AICcmodavg/AICcmodavg.pdf2015.
Moore TR, Trofymow JA, Prescott CE, Fyles J, Titus BD, group CW. 2006. Patterns of carbon, nitrogen and phosphorus dynamics in decomposing foliar litter in Canadian forests. Ecosystems 9:46–62.
Moore TR, Trofymow JA, Prescott CE, Titus BD, Group CW. 2011. Nature and nurture in the dynamics of C, N and P during litter decomposition in Canadian forests. Plant Soil 339:163–75.
Mooshammer M, Wanek W, Zechmeister-Boltenstern S, Richter A. 2014. Stoichiometric imbalances between terrestrial decomposer communities and their resources: mechanisms and implications of microbial adaptations to their resources. Front Microbiol 5:1–10.
Morin H, Laprise D, Bergeron Y. 1993. Chronology of spruce budworm outbreaks near Lake Duparquet, Abitibi region, Quebec. Can J For Res 23:1497–506.
Olson JS. 1963. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–31.
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Hart SC, Fasth B. 2007. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–4.
Philpott TJ, Prescott CE, Chapman WK, Grayston SJ. 2014. Nitrogen translocation and accumulation by a cord-forming fungus (Hypholoma fasciculare) into simulated woody debris. For Ecol Manag 315:121–8.
Pinheiro JC, Bates DM. 2000. Mixed-effects models in S and S-PLUS. New York: Springer.
Prescott CE. 1997. Effects of clearcutting and alternative silvicultural systems on rates of decomposition and nitrogen mineralization in a coastal montane coniferous forest. For Ecol Manag 95:253–60.
Prescott CE. 2010. Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–49.
Prescott CE, Blevins LL, Staley CL. 2000. Effects of clear-cutting on decomposition rates of litter and forest floor in forests of British Columbia. Can J For Res 30:1751–7.
R Development Core Team. 2015. R: a language and environment for statistical computing. Version 2.15.2. Vienna: R Foundation for Statistical Computing. Available from http://www.r-project.org/2012.
Rajala T, Peltoniemi M, Pennanen T, Mäkipää R. 2012. Fungal community dynamics in relation to substrate quality of decaying Norway spruce (Picea abies [L.] Karst.) logs in boreal forests. FEMS Microbiol Ecol 81:494–505.
Saucier J-P, Bergeron J-F, Grondin P, Robitaille A. 1998. Les régions écologiques du Québec méridional (3rd version). L’Aubelle 124:S1–12.
Schimel JP, Hättenschwiler S. 2007. Nitrogen transfer between decomposing leaves of different N status. Soil Biol Biochem 39:1428–36.
Seastedt TR, Crossley DAJ. 1981. Microarthropod response following cable logging and clear-cutting in the southern Appalachians. Ecology 62:126–35.
Smyth CE, Titus B, Trofymow JA, Moore TR, Preston CM, Prescott CE, the CIDET Working Group. 2016. Patterns of carbon, nitrogen and phosphorus dynamics in decomposing wood blocks in Canadian forests. Plant Soil 409:459–77.
Šnajdr J, Cajthaml T, Valášková V, Merhautová V, Petránková M, Spetz P, Leppänen K, Baldrian P. 2011. Transformation of Quercus petraea litter: successive changes in litter chemistry are reflected in differential enzyme activity and changes in the microbial community composition. FEMS Microbiol Ecol 75:291–303.
Soil Classification Working Group. 1998. The Canadian system of soil classification. Ottawa: National Research Council of Canada, Agriculture and Agri-Food Canada.
Strukelj M, Brais S, Paré D. 2015. Nine-year changes in carbon dynamics following different intensities of harvesting in boreal aspen stands. Eur J For Res 134:737–54.
Strukelj M, Brais S, Quideau SA, Angers VA, Kebli H, Drapeau P, Oh S-W. 2013. Chemical transformations in downed logs and snags of mixed boreal species during decomposition. Can J For Res 43:785–98.
Strukelj M, Brais S, Quideau SA, Oh S-W. 2012. Chemical transformations of deadwood and foliar litter of mixed boreal species during decomposition. Can J For Res 42:772–88.
Štursová M, Žifčáková L, Leigh MB, Burgess R, Baldrian P. 2012. Cellulose utilization in forest litter and soil: identification of bacterial and fungal decomposers. FEMS Microbiol Ecol 80:735–46.
Talbot JM, Yelle DJ, Nowick J, Treseder KK. 2012. Litter decay rates are determined by lignin chemistry. Biogeochemistry 108:279–95.
Thevenot M, Dignac M-F, Rumpel C. 2010. Fate of lignins in soils: a review. Soil Biol Biochem 42:1200–11.
Trofymow JA, Preston CM, Prescott CE. 1995. Litter quality and its potential effect on decay rates of materials from Canadian forests. Water Air Soil Pollut 82:215–26.
Von Lützow M, Kögel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H. 2006. Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review. Eur J Soil Sci 57:426–45.
Wickings K, Grandy AS, Reed SC, Cleveland CC. 2012. The origin of litter chemical complexity during decomposition. Ecol Lett 15:1180–8.
Wieder RK, Lang GE. 1982. A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 63:1636–42.
Yin X, Perry JA, Dixon RK. 1989. Influence of canopy removal on oak forest floor decomposition. Can J For Res 19:204–14.
Zhang D, Hui D, Luo Y, Zhou G. 2008. Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 1:85–93.
Acknowledgements
We are grateful to Josée Frenette, Mylène Bélanger, Émilie Robert, Alfred Coulombe, and Mario Major for field assistance, Serge Rousseau for laboratory analyses, and William F. J. Parsons for English revision. We are also thankful to two anonymous reviewers and Dr. Stephen Hart for their comprehensive review of the manuscript. This study was supported by grants from the Programme de financement de la recherche et du développement en aménagement forestier of the Ministère des Forêts, de la Faune et des Parcs du Québec, from the Cooperative Research Development Program to Drapeau and collaborators (NSERC, RDC475301-14), and Discovery Grant to Brais (NSERCs 217118) of the Natural Sciences and Engineering Research Council of Canada, by the Lake Duparquet Research and Teaching Forest, and by the industrial partner Tembec Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
MS performed field and laboratory work and is the lead researcher on decomposition models and the first author of the manuscript. SB designed the study and is the principal investigator for the Safe project. MJM contributed to data analyses. DP contributed to laboratory analyses. PD coordinated the overall project. All authors contributed ideas and commented on the multiple drafts of the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Strukelj, M., Brais, S., Mazerolle, M.J. et al. Decomposition Patterns of Foliar Litter and Deadwood in Managed and Unmanaged Stands: A 13-Year Experiment in Boreal Mixedwoods. Ecosystems 21, 68–84 (2018). https://doi.org/10.1007/s10021-017-0135-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-017-0135-y