Abstract
The rewilding of abandoned agricultural lands opens up opportunities for the recovery of forest ecosystem extent. Frugivorous animals not only take part in the regeneration of unaltered forests, but leave a visible footprint in restoring areas in the form of the number and spatial distribution of new trees recruited from dispersed seeds. Nevertheless, their contribution is conditioned by how environmental factors affect both the patterns of seed dispersal and the fate of post-dispersal regeneration stages throughout the whole ecosystem. Here, we evaluated the role of avian seed dispersers in tree regeneration in woodland pastures resulting from anthropic deforestation. Using an integrative approach, considering the different tree regeneration stages, we dissected the ways in which forest loss conditioned the contribution of frugivores. Habitat structure influenced bird activity, mainly restricting seed dispersal to forested areas. Tree recruitment was severely reduced during early regeneration stages, but maintained the initial forest-biased spatial distribution. However, the presence of scrub in deforested areas, which protect against grazing at late regeneration stages, drastically increased the relevance of tree recruitment outside the forest. Frugivorous birds made a significant contribution to tree regeneration in the woodland pastures under study. The interplay between seed dispersal by birds and the protective role of scrub was fundamental in facilitating the recolonization of deforested areas. If we wish to encourage this natural reforestation, we will need to preserve populations of frugivorous birds while favoring landscape configurations that encourage seed dispersal outside the forest and species that promote tree establishment (like nurse scrubs).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat degradation and biodiversity loss threaten human wellbeing (Balmford and William 2005; Hooper and others 2012) but, even as ecosystem alteration continues at the global scale, this trend is starting to be reversed in certain areas of the Northern Hemisphere (Pereira and Navarro 2015). The depopulation of areas with low productivity and remote montane rural regions throughout Europe and North America is leaving behind a large number of abandoned lands, which are open to natural forest succession (MacDonald and others 2000; Pereira and Navarro 2015). The rewilding of these abandoned lands thus represents an opportunity for the restoration of forest biodiversity and ecosystem functions and, consequently, for the recovery of the important services that forests provide (Chazdon 2008; Navarro and Pereira 2012), such as soil stabilization, flood control, CO2 storage and climate regulation, among others (Daily 1997). Nevertheless, this natural recovery is governed by many different, and potentially limiting, ecological factors. It depends not only on the kind and magnitude of the anthropogenic disturbance, but also on the resilience of the original ecosystem to that disturbance (Chazdon 2008; Cramer and others 2008). This resilience may result from the effects of forest elements still remaining in the degraded patches (the so-called internal ecological memory; Nyström and Folke 2001), the surrounding undisturbed patches which serve as sources of new propagules (external memory), and certain organisms that transfer the propagules from undisturbed to degraded patches (mobile links). Thus, to better understand forest recovery, we must study tree recruitment along gradients of ecosystem degradation, allowing us to consider those elements acting as biological legacies (for example, remnant trees), and structures which anchor recruitment (for example, nurse plants) that appear within deforested areas, as well as the remaining forest patches and the vectors of seed dispersal to deforested areas (García and others 2013).
Frugivorous animals acting as seed dispersers carry out a key ecological function by contributing to plant regeneration in natural ecosystems (Lundberg and Moeberg 2003; Howe and Miriti 2004; Farwig and Berens 2012). Furthermore, they have been recognized to be essential elements of resilience, as they favor vegetation recolonization in disturbed ecosystems when dispersing plant seeds from undisturbed areas (for example, by promoting tree recovery after deforestation; Wunderle 1997; Cavallero and others 2013). Thus, through the quantity and spatial distribution of new adult plants established from dispersed seeds, frugivorous animals leave a significant footprint in natural ecosystems. But the contribution of frugivores to plant regeneration can show important limitations. First, the regeneration potential generated by frugivores is not evenly distributed everywhere, but rather is shaped by the environmental factors that affect the distribution of frugivores and, consequently, the spatial pattern of seed deposition (that is, the seed rain; Nathan and Muller-Landau 2000). Second, although frugivores may create the initial spatial template of plant regeneration (for example, Wenny 2000; García and others 2009; Suárez-Esteban and others 2013), this template is frequently far from representative of the final distribution of adult plants (Schupp and Fuentes 1995; Rey and Alcántara 2014). This is because the spatial patterns of the seed rain are filtered by environmental factors that affect seed survival, seedling emergence and establishment, and sapling survival, and which finally dilute the initial frugivore footprint (Schupp and others 2010).
Constraints on the contribution of frugivorous animals to plant recruitment can be especially severe in human-impacted ecosystems (Duncan and Chapman 2002). The quantity and the spatial patterns of seed deposition may be restricted in these areas as a result of the detrimental influence of habitat degradation on frugivore population size and behavior (Pejchar and others 2008; Farwig and Berens 2012; Rey and Alcántara 2014). This filtering can, in addition, be exacerbated by post-dispersal seed and seedling losses, which may be higher in human-disturbed areas due to changes in abiotic conditions (for example, light, humidity and temperature; Kapos and others 1997; Uriarte and others 2010), and/or in the behavior of seed and seedling predators (García and Chacoff 2007; González-Varo 2010). Discerning the actual role of frugivorous animals on ecosystem recovery can therefore only be accomplished by simultaneously studying the different environmental factors that affect plant regeneration throughout entire landscapes, that is, by considering both pristine and degraded areas (Kremen 2005; Kremen and others 2007).
In this study, we evaluate, at a landscape scale, the contribution of avian seed dispersers to forest regeneration within the context of an ecosystem degraded by agricultural land use. For this purpose, we linked the broad-scale spatial patterns of habitat structure (distribution of forest and open pastures), bird abundance and four sequential tree regeneration stages (seed, emerged and established seedlings, and saplings) by means of an integrative approach. We took into account the influence of habitat alteration (deforestation) on tree recruitment, differentiating between effects on frugivore activity and seed dispersal and effects on post-dispersal seed fate. Specifically, we sought to answer the following questions: (1) Do the abundances of tree regeneration stages differ between forest and open pastures? (2) How do habitat characteristics (abundance of tree cover and fruit production within forest, and scrub cover in deforested areas) influence the contribution of frugivorous birds to tree regeneration? (3) To what degree do frugivorous birds contribute to tree recolonization within the deforested habitat? Answering these questions will help to develop guidelines for the management of natural forest recovery in abandoned agricultural lands.
Materials and Methods
Study System
This study was conducted on mid-elevation woodland pastures of the Cantabrian Range in northern Spain. This is a heterogeneous habitat resulting from the historical fragmentation and loss of Atlantic temperate forests exploited for timber and cleared for extensive livestock raising (mainly cattle and horses), and which is maintained by constant overbrowsing (García and others 2005a). This type of environment is commonly found, worldwide, in temperate systems influenced by historical anthropization (Darby 1956; Vera 2000; Pereira and Navarro 2015). The woodlands contain variable-sized fragments of primary forest (mainly composed of beech Fagus sylvatica) and, more frequently, secondary forest embedded in an extensive (>70% cover) human-generated matrix of open pastures composed of stony meadows and interspersed heathland (heather Erica spp., gorse Ulex europaeus). The secondary forest is dominated by fleshy-fruited trees (hawthorn Crataegus monogyna, holly Ilex aquifolium, yew Taxus baccata, rowan Sorbus aucuparia, and whitebeam Sorbus aria), which account for more than 70% of tree cover; (García and others 2013) as well as hazel (Corylus avellana). Fleshy-fruited tree species overlap in their ripening period in early autumn, with their fruits (arilated seeds on T. baccata) staying on trees until mid-winter. Their main frugivores are thrushes (Turdus spp.; Martínez and others 2008), which swallow the entire fruits, expel the intact seeds in their feces, and hence act as legitimate seed dispersers. Mammals contribute to tree seed dispersal, although to a considerably lesser extent than thrushes (Martínez and others 2008). The recruitment of these fleshy-fruited trees depends primarily on the availability of dispersed seeds (that is, demographic seed limitation) in the study system (Herrera and García 2010). The seed rain is composed of seeds dropped by frugivores and seeds from fruits fallen beneath tree canopies. Seed predation by rodents is high during the winter after seed dispersal (García and others 2005b). The seed bank is transitory and most seedlings emerge between April and June, 18 months after seed dispersal (authors’ unpublished results). Seedling mortality is high due to herbivore browsing or trampling, but recruitment may be facilitated by nurse shrubs or trees (García and Obeso 2003).
Study Site
The study site was located in the Sierra de Peña Mayor (1000 m a.s.l.; 43°17′59″N, 5°20′29″W Asturias, Northwest Spain). At this site, secondary forest is intermingled with mature forest within a dominant non-forested matrix (Figure 1A). The study was conducted within a 400 × 440 m rectangular plot (Figure 1B) chosen to represent a spatial gradient of forest loss and, thus, habitat structure characteristics: it was mainly covered (circa 70%) by pastures, heathlands, and limestone rocky outcrops, and forest cover varied from dense stands to scattered trees isolated within the non-forested matrix (Figure 1A; Martínez and García 2015). Fleshy-fruited trees (mostly holly, hawthorn and yew) are accompanied by hazel and a low number of individuals of beech and ash (Fraxinus excelsior). For the better management of spatial information, the plot was subdivided into 440, 20 × 20 m cells (Figure 1B). Previous studies have proven that these plot and cell dimensions represent a spatial scale (respectively, extent and grain size) appropriate for studying variability in habitat cover, fruit production, bird activity, seed dispersal, and seedling emergence (Herrera and García 2010; García and others 2013).
A View of the Cantabrian woodland pastures at the study site. Secondary forest patches and remnant trees appear embedded in a deforested matrix of pastures and heathlands. B Map of the study plot (400 × 440 m), subdivided into 20 × 20 m cells, showing the extent of forest cover (light green area). C Detail of the chess-board design for the sampling of tree regeneration stages, showing the distribution of seed-rain sampling stations within the 20 × 20 m cells. D Detail of a cell showing the distribution of seedling sampling stations (squares) located adjacent to seed rain stations (crosses). In both C and D, sampling stations located in open habitats are represented as dark gray squares while those in covered habitats (that is, under forest cover) are in white. Photo credits D. Martínez (Color figure online).
Habitat Characteristics
We developed a Geographic Information System of the study plot (GIS, ArcGIS9.3) based on a recent (2009) 1:5000-scale orthophotograph. We generated a layer with precise geo-referenced information related to the plot, including the grid of 440, 20 × 20 m cells (Figure 1B). Another GIS layer representing the extent and location of forest cover was generated by carefully digitizing the orthophotograph. The forest cover layer represented the canopy projection of all trees (DBH > 10 cm, height > 1.5 m), including isolated individuals within pastures.
In October of 2009 and 2010, we surveyed the entire plot, mapping all trees and identifying them at the species level. For each fruiting individual (mostly hawthorn and holly, and a small number of yew), we visually estimated the number of standing fruits using a semi-logarithmic scale [Fruit Abundance Index (FAI): 1 = 1–10 fruits; 2 = 11–100; 3 = 101–1000; 4 = 1001–10,000; 5 > 10,000]. In the system and site studied, the fruiting of all individuals of the different species is synchronous, and ripening occurs within 1–2 months (although fruits remain attached to trees for a further 1–3 months). Thus, we considered that a single sampling of fruit abundance at the beginning of the season provided an appropriate estimate of the spatial arrangement of fruit resources (García and others 2013). We also assessed the presence/absence of big trees whose tops overgrew the average forest canopy, as previous studies in the same site have evidenced that they influence bird activity in the study area (Martínez and García 2015). For further information on the big tree classification procedure, see Online Appendix A.
Habitat characteristics were quantified in a subset of 220 plot cells, selected following a chess-board design (Figure 1C). From GIS information, we calculated area of forest cover, big tree presence, and fruit abundance in each cell. Fruit abundance was obtained as the sum of the crop sizes of all the fruiting trees in a cell. Individual crop sizes were extrapolated from FAI ranks considering an allometric fit between the actual crop size and FAI (actual crop size = 1.7651.924×FAI; R 2 = 0.80; N = 136 trees; Martínez and García 2015). In addition, in the field, we quantified the cover of scrub (mostly heather, but also gorse) and the extent of pasture cover per cell. In summer 2011, we visually estimated the proportion of scrub cover in ten areas of 1-m radius randomly distributed within each cell, and extrapolated the average value of these areas to the total cell area. Scrub rarely grows under tree canopy in our study site, and thus pasture cover per cell was calculated as the surface of each cell minus forest and scrub covers.
Bird Surveys
We performed bird observations to estimate the abundance and the spatial distribution of frugivorous birds (Turdus spp.) in different cells of the study plot during two consecutive fruiting seasons (October 2009–January 2010; October 2010–January 2011). Observations of thrushes were made from five different stations in vantage positions (that is, elevated outcrops) that covered large high-visibility areas distributed along the central axis of the plot, and twelve positions within the forest to cover more reduced areas in some of the easternmost cells of the plot where bird detectability was lower (Online Appendix A, Figure A1). Observation time was balanced between the various stations throughout each season. We assigned each bird sighting to the cell of the study plot where it occurred. The abundance of birds per cell was calculated as the cumulative number of birds detected in each cell during the season divided by the total observation time for each cell, thereby calculating the number of birds per 10 h of observation. For detailed information on the methodology of bird censuses, see Online Appendix A (see also García and others 2013; Martínez and García 2015).
Sampling of Tree Regeneration Stages
We considered four consecutive tree regeneration stages: seeds (dropped by birds and in fallen fruits), emerged seedlings (that is, presenting cotyledons but no leaves, knots or lignification of the stem), established seedlings (that is, those emerged seedlings surviving after the summer), and established saplings (that is, pre-reproductive individuals ≥10 cm tall or with a stem basal diameter ≥0.5 cm). Seeds and seedlings were studied by following two consecutive yearly cohorts from fruit production to seedling establishment. The first cohort ranged from autumn 2009 (fruit stage) until late summer 2011 (established seedling stage), and the second cohort from autumn 2010 until late summer 2012. Tree sapling stage was studied through a single monitoring, covering individuals of different ages, not assignable to a given cohort.
We assessed seed availability in sampling stations across the whole plot, at the end of the fruiting season (January 2010 and 2011), when all fruits have either been consumed by birds or fallen off the trees. Ten, 50 × 50 cm sampling stations were established on the ground in each of the 220 chess-board cells (Figure 1C; see Online Appendix A for detailed methodology). In each station, we collected and counted all fleshy fruits fallen directly from trees and fleshy-fruited tree seeds deposited by birds during the fruiting season. Bird-dispersed seeds are unequivocally identifiable because they are clean of pulp remains and occur in small clusters (Martínez and others 2008). We estimated total seed density per sampling station as the sum of bird-dispersed seeds and fruit-contained seeds, expressing this density as number of seeds per square meter. We considered seeds of both origins together as previous studies have suggested that seeds from fallen fruits also account for a part of seedling emergence in the tree species studied (Herrera and García 2010).
We assessed seedling emergence and establishment in sampling stations across the whole plot in 2011 and 2012. Five, 50 × 50-cm sampling stations were placed in each of the 220 chess-board cells, each station being located next to one of the seed availability sampling stations (Figure 1D; see Online Appendix A for detailed methodology). In each station, we checked the emergence of all fleshy-fruited tree seedlings through consecutive monitoring from early April to late August. We counted the cumulative number of emerged seedlings, and the number of established seedlings as those surviving by the end of the summer. The densities of emerged and established seedlings were expressed as number of seedlings per square meter.
We determined the habitat and the microhabitat of each seed and seedling sampling station. Habitat was determined according to the major structural characteristic of the cover where the quadrat containing it was located, the categories being either covered (under woody canopy) or open (open pastures, that is, the matrix not covered by woody canopy). Microhabitats were classified according to the fine-grain structural characteristic of the cover, using the covered category for areas under trees, but distinguishing between scrub (under the canopy of scrub species) and pastures (with herbaceous vegetation) for the open habitat category.
The entire surface area of each of the 220 chess-board cells was surveyed, recording the total abundance of established saplings of fleshy-fruited tree species. We performed a single sampling (summer 2011) aimed to represent long-term, cumulative tree recruitment in the study site, as the sapling category aggregates individuals of different ages (see Online Appendix A for detailed methodology). We classified the microhabitat where each sapling stood according to the same categories used for seed and seedling sampling stations. During the 2011 sampling, we labeled 386 individuals, trying to represent an even distribution between microhabitats (covered, pasture and scrub) and species (holly, hawthorn and yew), and covering the whole extent of the study plot. Due to very low and spatially aggregated sapling recruitment in yew, we were only able to label 92 individuals of this species. We checked the survival of labeled saplings in late summer (September) 2012. Sapling survival was very high (97.15%), and mortality was widely distributed throughout the plot, and across different microhabitats and species (authors’ unpublished data). We thus considered that the success rate and the spatial template of sapling establishment represented a good proxy of long-term tree recruitment.
For each cell and cohort (2009 and 2010), we calculated the average density of seeds and emerged and established seedlings per square meter. Sapling density per cell was estimated as the number of individuals per square meter. Also, for each cell and cohort, we estimated the densities of seeds, emerged seedlings, established seedlings, and saplings in deforested habitat by exclusively considering the data from those sampling stations (or individuals in the case of saplings) in open microhabitats (Figure 1C, D).
Statistical Analyses
We were interested in taking into account the influence of habitat degradation on tree recruitment while studying the contribution of frugivorous birds to forest regeneration. For this purpose, we first evaluated the influence of habitat and microhabitat structure on the abundance of the different tree regeneration stages. Between-habitat (covered vs. open) density differences were tested for the different regeneration stages by means of independent Generalized Linear Models (GLMs) for each stage, considering quasi-Poisson error distributions and log link functions. Analyses concerning seed availability, seedling emergence, and seedling establishment were based on densities at the sampling station scale, whereas those concerning sapling establishment used density estimated on a per-cell basis. We compared the distribution of individuals across microhabitats (covered, scrub, and pastures) between different stages by means of contingency tables and Chi square tests (for paired stages). We used the same methodology to compare the distribution of the individuals of each stage with that of the microhabitats within the study plot (from the total frequencies of microhabitats across all sampling stations; hereafter referred to as microhabitat availability).
Our objective was to evaluate the role of avian seed dispersers in forest regeneration, explicitly taking into account the influence of habitat characteristics on both the activity of birds and the different tree regeneration stages. To this end, we used Structural Equation Modeling (SEM), which allows the evaluation of complex (that is, multivariate) relationships, including cascading effects, across parameters representing causal processes and consecutive patterns (for example, habitat effects and regeneration stages; Grace 2006). Structural equation models are initially built as saturated causal schemes, or path diagrams, representing all hypothesized causal links between different predictors and response variables, based on previous knowledge of the ecological system. Path analysis enables both direct and indirect effects to be measured between variables across the whole scheme. Direct effects are represented by links between consecutive predictor and response variables, and measured by standardized partial regression coefficients between them. Indirect effects are calculated as the sum of the products of all standardized partial regression coefficients over all paths between non-consecutive predictor and response variables.
Our main aim was to represent the effect of avian seed dispersal on forest regeneration across all habitat types in the landscape, both forest remnant patches and the deforested habitat matrix. For this purpose, we constructed independent models to study the factors influencing, on the one hand, the emergence of seedlings from different cohorts and, on the other, the recruitment of saplings (as saplings were not assignable to a particular cohort). In the first case, the path model of each cohort (2009 and 2010) included emerged seedling and seed densities, abundance of birds, and habitat characteristics (forest cover, big tree presence, fruit abundance, and scrub cover) on a cell basis (220 cell chess-board subset). In the second case, the path model included densities of emerged seedlings (averaged between cohorts), established seedlings (averaged between cohorts), and established saplings and habitat characteristics (forest cover, big tree presence, fruit abundance, and scrub cover) on this same cell basis (N = 220).
Moreover, we were interested in evaluating the role of avian seed dispersal in forest recolonization (that is, tree recruitment in the deforested matrix). For this purpose, we repeated the above models, but only considering the data for the tree regeneration stages obtained from stations in open pastures. As such, only those cells with a minimum of four stations in open microhabitats for seed sampling, and of two such stations for seedling sampling, were incorporated in the models (N = 183 cells). No big trees appeared within these cells, and thus this variable was excluded from these models.
We considered possible alternatives to the saturated model by constructing nested models sharing the same causal structure. The final models were chosen on the basis of the Akaike Information Criterion (AIC). The fit of each model to the data was assessed using a likelihood χ 2 value, with non-significant values indicating fit between model and the data. To avoid the possibility that deviations from multivariate normality could be influencing both parameter estimations and overall model χ 2 value, we applied Satorra–Bentler corrections (Satorra and Bentler 1994).
Due to the configuration of the sampling framework (adjacent cells of the study plot), the estimation of the effects of habitat characteristics and bird abundance on the different tree regeneration stages may have been affected by potential spatial autocorrelation in the studied variables (Keitt and others 2002). Thus, to check for the consequences of spatial constraints in the previous SEMs, we fitted simultaneous autoregressive models (SAR; Keitt and others 2002; see Online Appendix B). We considered that the partial regression coefficients provided by SAR models represented the direct effects of habitat characteristics and bird abundance on the different regeneration stages, free of autocorrelation constraints (see García and others 2010 for a similar procedure).
All the analyses were performed using R 2.15, with the lavaan package for SEMs (Rosseel 2012).
Results
Habitat Heterogeneity
Habitat characteristics showed strong spatial heterogeneity across the whole plot (Figure 2). Namely, forest cover, big trees (only appearing within forest), and fruits presented highly overlapped distributions, whereas scrub cover showed the opposite pattern to that of forest cover. The spatial pattern of bird abundance and the densities of seeds, emerged seedlings, and established seedlings (almost identical to that of emerged seedlings) strongly matched the gradient of forest cover. In contrast, saplings were spread widely across the whole study plot, and their establishment pattern emerged as a combination of both forest and scrub covers.
Distribution of forest and scrub cover, abundance of fleshy fruits, abundance of thrushes, and densities of seeds, emerged seedlings, and established saplings in the study plot. The average value of the two study years is represented for fruits, thrushes, seeds, and emerged seedlings. A contoured scale (indicated by shades of gray) represents the percentage of the total sum of each variable in the plot accounted for by each cell. Dashed lines surrounding the minimum value considered (0.1%) and the scale used are shown. Vertical and horizontal axes of the panels represent the dimensions (in meters) and the boundaries of the plot.
Distribution of Tree Regeneration Stages
The density of available seeds was an order of magnitude higher than those of emerged and established seedlings, and three orders of magnitude higher than the density of established saplings for the two different one-year cohorts studied (Table 1). Bird-dispersed seeds accounted for more than 60% of total seed availability in both fruiting seasons, even for covered habitat (Table 1). The density of bird-dispersed seeds and that of seeds from fallen fruits were highly correlated (Spearman ρ > 0.75, P < 0.001, N = 220 cells, for both fruiting seasons).
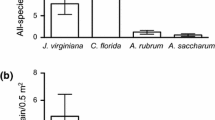
The density of the tree regeneration stages studied was different between habitats (GLM, |t| > 5 and P < 0.0001 in all cases), with all stages presenting higher densities in covered than in open habitat (Table 1). Furthermore, distribution of the different stages across microhabitats differed significantly from that of microhabitat availability in the study plot (χ 2 > 5.99, P < 0.05 in all cases). We also found different microhabitat distributions between stages, except when comparing those of dispersed seeds and established seedlings (χ 2 = 3.39, P = 0.18; χ 2 > 5.99; P < 0.05 in all other cases). The distributions of seeds, emerged seedlings, and established seedlings were strongly biased toward covered microhabitats, whereas half the saplings appeared in open areas, with most of them standing within scrub (Figure 3), even though covered and scrub were the least abundant microhabitats.
Forest Regeneration
The best-fit SEMs for seedling emergence included all the predictors considered except scrub cover, while those for sapling establishment included all predictors except fruit abundance, with respect to both forest regeneration and recolonization models (Figures 4, 5).
Path diagrams of the best-fit structural equation models showing the effects of habitat characteristics and the abundance of avian seed dispersers (thrushes) on tree regeneration stages for the forest regeneration model (N = 220 cells). Values of standardized partial regression coefficients are shown. A Models for habitat and bird influences on the densities of seeds and emerged seedlings, for the cohorts studied. B Model for habitat influence on the densities of established seedlings and saplings. All arrows represent effects that are significant at P ≤ 0.05, except for the dashed-line arrow (P = 0.1).
Path diagrams of the best-fit structural equation models showing the effects of habitat characteristics and the abundance of avian seed dispersers (thrushes) on tree regeneration stages, for the forest recolonization model (N = 183 cells). Values of standardized partial regression coefficients are shown. A Models for habitat and bird influences on the densities of seeds and emerged seedlings, for the studied cohorts. B Model for habitat influence on the densities of established seedlings and saplings. All arrows represent effects that are significant at P ≤ 0.05.
SEMs representing forest regeneration (that is, those that incorporated the whole dataset) showed that the density of seeds was directly affected by the habitat characteristics of the cell (Figure 4). That is, forest cover and fruit abundance both had a direct positive influence on seed density in both cohorts, while big trees only showed direct, and negative, effects in the 2009 cohort (Online Appendix C, Table C1). These models also indicated that the density of emerged seedlings was, in turn, directly influenced by both the density of seeds and forest cover. The density of established seedlings was not directly influenced by habitat characteristics, and was only related to the density of emerged seedlings (Figure 4). In contrast, the density of sapling establishment was unrelated to previous regeneration stages, but positively influenced by forest and scrub cover. As judged by the values of standardized path coefficients, forest cover was the habitat characteristic that most influenced densities of established saplings (Online Appendix C, Table C1).
Forest Recolonization in Open Pastures
SEMs representing forest recolonization (that is, those including the data from open sampling stations) revealed that, as well as forest cover and fruit abundance, abundance of thrushes had a significant positive direct effect on the density of seeds. This effect cascaded into the density of emerged seedlings in open habitats, for both cohorts, as this response variable in itself was unaffected by any environmental predictor (Figure 5). Path structure for sapling establishment in open habitats was similar to that in the regeneration model, but with scrub cover as the main predictor of sapling densities in these areas (Online Appendix C, Table C2).
The SAR models relating the different regeneration stages to habitat characteristics and bird abundance corroborated the effects suggested by previous SEMs, free of spatial autocorrelation constraints (see Online Appendix B, Tables B1 and B2).
Discussion
In this study, we evaluated the role of frugivorous birds to forest regeneration and recolonization under a context of anthropogenic deforestation and agricultural land use. In contrast to previous microhabitat-centered studies (García and others 2005b; González-Varo and others 2012; Rey and Alcántara 2014), here we used a landscape-scale approximation, enabling us to reveal the footprint that frugivore activity left on a degraded ecosystem. In addition, using an integrative approach allowed us to uncover the different ways through which habitat characteristics influence the contribution of birds to landscape-scale patterns of tree recruitment.
Forest Regeneration
Our observational results suggest that habitat structure strongly influenced forest regeneration when considered through the whole study plot. Even when most of the landscape was deforested, tree regeneration stages appeared heavily biased toward forest areas. This is probably because forest cover directly favors recruitment across the different regeneration stages, but also because the presence of forest could indirectly affect the role of other components of the system in the regeneration process (Figure 4; Online Appendix C, Table C1).
As has been found for other temperate and tropical ecosystems (Holl and others 2000; García and others 2010; Bustamante-Sánchez and Armesto 2012), the availability of seeds for further recruitment was concentrated under forest canopy. As judged by the analysis of seed rain, which showed most seeds being found in bird droppings, this pattern was mostly the result of birds being concentrated within forests, but fruit fall under source trees also contributed. Nonetheless, path analysis showed that changes in bird density did not affect the broad-scale patterns of seed availability for tree regeneration. Two facts could explain this lack of a relationship: (1) birds may stay longer in areas with high forest cover, thus creating large seed clumps even at low bird densities (as suggested by the direct effect of forest cover on seed availability; see also García and others 2010; Martínez and García 2015); or (2) the variability in seed availability is also capturing the variation in fruit fall (as suggested by the direct effect of fruit abundance).
Even when a high degree of demographic filtering took place during early regeneration stages (that is, low probability of seedling establishment), the spatial pattern of tree regeneration determined by seed availability was strongly retained. Certain minor alterations of spatial patterns occurred during the seed-to-seedling transition, but these changes were reversed during seedling establishment and the initial pattern of seed rain restored. This high concordance between the spatial patterns of early regeneration stages suggests that the environmental constraints affecting them, even when considering severe demographic losses, were spatially more homogeneous than seed availability (Schupp and Fuentes 1995; García and others 2005b).
In contrast, seedling-to-sapling transition meant not only a drastic decrease in the number of individuals, but also an alteration of the forest-biased pattern of early regeneration, with most saplings appearing in open areas (Figure 3). The fact that the saplings sampled did not belong to the same cohorts as the early regeneration stages studied here could explain this mismatch. Nevertheless, the large between-stages differences in the distribution of individuals across microhabitats suggest the existence of strong environmental filtering during late recruitment which imposed a new spatial pattern and decoupled the spatial pattern of saplings from that of previous stages (Schupp and Fuentes 1995; García and others 2005b). This was the only regeneration stage correlated with scrub cover, and, in fact, most saplings standing in deforested areas grew within scrub bushes, suggesting some kind of facilitation effect for saplings. As summer drought is rare under the Atlantic climatic conditions of our study site, we suggest that scrub had a positive effect on sampling establishment by providing protection from ungulate browsing in the study site (see also García and Obeso 2003; Padilla and Pugnaire 2006; Smit and others 2015).
Recolonization of Deforested Areas
The influence of habitat structure on forest recolonization seemed to be quite similar to that found for regeneration across the whole plot, with tree recruitment in open areas being favored in the vicinity of forest remnants. Frugivorous birds visited deforested areas more frequently when these were surrounded by abundant forest cover and fruits, and the larger number of birds visiting these deforested areas led to increased seed deposition there. In other open habitats, birds use isolated trees as perches, resulting in an increase in the seed rain in the area surrounding them (Cardoso da Silva and others 1996; Duncan and Chapman 2002; Martínez and García 2015). Again, the spatial patterns of the different early regeneration stages were highly concordant, while the distribution of saplings was unrelated to that of the previous stages, being shaped by habitat structure. Indeed, scrub cover was the most important predictor of sapling occurrence in the deforested areas (Figure 5), and around 75% of individuals standing in these areas were found within scrub bushes. Both facts provide evidence of the importance of plant–plant facilitation for forest recolonization.
Contribution of Frugivorous Birds
Our field samplings show that dispersal by birds provided most of the seeds available for recruitment, both under forest canopy and within pastures (Table 1). It is likely that this quantitative contribution would be maintained, or even reinforced, during the seedling and sapling stages, as bird-dispersed seeds usually show higher survival and germination rates than seeds from fallen fruits (Traveset 1998; García and others 2007). However, as stated above, our correlational analysis demonstrated that seed and seedling distributions across the whole landscape (that is, the forest and the non-forest matrix taken together) depended on landscape structure, rather than on variations in bird abundance. In other words, birds would not erase, through seed dispersal, the patterns of tree regeneration molded by forest cover and fruit abundance. In fact, due to their spatial tracking of forest cover and fruits, they would actually reinforce the recruitment template established by adult trees (as judged by the positive correlation between seed deposition by birds and seeds in fallen fruits). Nonetheless, our results show that the contribution of birds to shaping broad-scale patterns of tree recruitment only emerged when considering recolonization of open pastures. Moreover, the initial influence of birds on forest recruitment became blurred in the long term, as the spatial patterns of recruitment drastically changed in the seedling-to-sapling transition. Even when birds dispersed few seeds to open pastures, browsing by ungulates biased the survival of saplings toward those growing under the protective canopy of scrub species, severely increasing the relevance of tree recruitment in the deforested matrix.
Conclusions
Our landscape-scale study shows that habitat structure influences the role of frugivorous birds not only by influencing their spatial distribution, and hence seed-rain patterns, but also by determining the fate of post-dispersal regeneration stages. Frugivores help reinforce the spatial patterns of forest self-regeneration at the broad scale, but, at the same time, they foster the recolonization of deforested pastures by delivering seeds into safe microhabitats. Birds are essential for tree recruitment in deforested areas, but scrub is equally necessary to ensure that bird-promoted recruitment effectively leads to ecosystem recovery.
Management programs aiming to sustain, or even encourage, the rewilding of semi-abandoned montane pastures in the Cantabrian range should focus on the main species involved, together with the configuration of the habitat structure across the landscape. Conserving populations of frugivorous birds is fundamental if the aim is to trigger forest recovery. This is especially important in our study system, where some of the main seed-disperser species show population declines (Rivalan and others 2007; SEO/BirdLife 2013). It would also be necessary to favor those landscape configurations that contribute to reducing limitations on seed dispersal in open pastures by maintaining, or even planting, small forest patches and scattered fleshy-fruited trees (Holl and others 2000; Duncan and Chapman 2002; Cavallero and others 2013). Finally, measures aimed at overcoming restrictions to tree establishment, which would reduce the negative impact of ungulates, are also needed. Maintaining nurse scrub within deforested areas, together with establishing small, short-term (that is, 10–15 years) ungulate exclosures (compatible with the exploitation of pastures by cattle raisers), would be particularly useful for this purpose (Gómez-Aparicio and others 2004; Padilla and Pugnaire 2006; Swanson and others 2011; Smit and others 2015). We consider that these guidelines can be easily extrapolated to many other temperate areas where natural ecosystems have been impacted by a similar process of degradation by human land use (Darby 1956; Vera 2000; Pereira and Navarro 2015).
References
Balmford A, William B. 2005. Trends in state of nature and their implications for human well-being. Ecology Letters 8:1218–34.
Bustamante-Sánchez MA, Armesto JJ. 2012. Seed limitations during early forest succession in a rural landscape on Chiloé Island, Chile: Implications for temperate forest restoration. Journal of Applied Ecology 49:1103–12.
Cardoso da Silva JMC, Uhl C, Murray G. 1996. Plant succession, landscapes management, and the ecology of frugivorous birds in abandoned Amazonian pastures. Conservation Biology 10:491–503.
Cavallero L, Raffaele E, Aizen MA. 2013. Birds as mediators of passive restoration during early post-fire recovery. Biological Conservation 158:342–50.
Chazdon RL. 2008. Beyond deforestation: Restoring forest and ecosystem services on degraded lands. Science 320:1458–60.
Cramer VA, Hobbs RJ, Standish RJ. 2008. What’s new about all fields? Land abandonment and ecosystem assembly. Trends in Ecology and Evolution 23:104–12.
Daily GC. 1997. Nature’s services. Societal dependence on natural ecosystems. Washington: Island Press.
Darby HC. 1956. The clearing of the woodland in Europe. In: Thomas WL Jr, Ed. Man’s role in changing the face of the Earth. Chicago: University of Chicago Press. p 183–216.
Duncan RS, Chapman CA. 2002. Limitations of animal seed dispersal for enhancing forest succession on degraded. In: Levey DJ, Silva WR, Galetti M, Eds. Seed dispersal and frugivory. Ecology, evolution and conservation. Wallingford: CAB International. p 437–50.
Farwig N, Berens D. 2012. Imagine a world without seed dispersers: A review of threats, consequences and future directions. Basic and Applied Ecology 13:109–15.
García D, Chacoff NP. 2007. Scale-dependent effects of habitat fragmentation on hawthorn pollination, frugivory and seed predation. Conservation Biology 21:400–11.
García D, Obeso JR. 2003. Facilitation by herbivore-mediated nurse plants in a threatened tree, Taxus baccata: Local effects and landscape level consistency. Ecography 26:739–50.
García D, Martínez I, Obeso JR. 2007. Seed transfer among bird-dispersed trees and its consequences for post-dispersal seed fate. Basic and Applied Ecology 8:533–43.
García D, Obeso JR, Martínez I. 2005a. Spatial concordance between seed rain and seedling establishment in bird-dispersed trees: Does the scale matter? Journal of Ecology 93:693–704.
García D, Rodríguez-Cabal M, Amico GC. 2009. Seed dispersal by a frugivorous marsupial shapes the spatial scale of a mistletoe population. Journal of Ecology 97:217–29.
García D, Zamora R, Amico GC. 2010. Birds as suppliers of seed dispersal in temperate ecosystems: Conservation guidelines from real-world landscapes. Conservation Biology 24:1070–9.
García D, Martínez D, Herrera JM, Morales JM. 2013. Functional heterogeneity in a plant-frugivore assemblage enhances seed dispersal resilience to habitat loss. Ecography 36:197–208.
García D, Quevedo M, Obeso JR, Abajo A. 2005b. Fragmentation patterns and protection of montane forests in the Cantabrian range (NW Spain). Forest Ecology and Management 208:29–43.
Gómez-Aparicio L, Zamora R, Gómez JM, Hodar JA, Castro J, Baraza E. 2004. Applying plant facilitation to forest restoration: A meta-analysis of the use of nurse plants. Ecological Applications 14:1128–38.
González-Varo JP. 2010. Fragmentation, habitat composition and the dispersal/predation balance in interactions between the Mediterranean myrtle and avian frugivores. Ecography 33:185–97.
González-Varo JP, Nora S, Aparicio A. 2012. Bottlenecks for plant recruitment in woodland remnants: An ornithochorous shrub in a Mediterranean “relictual” landscape. Perspectives in Plant Ecology, Evolution and Systematics 14:111–22.
Grace JB. 2006. Structural equation modeling in natural systems. Cambridge: Cambridge University Press.
Herrera JM, García D. 2010. Effects of forest fragmentation on dispersal and seedling establishment in ornithochorous trees. Conservation Biology 24:1089–98.
Holl KD, Loik ME, Lin EHV, Samuels IA. 2000. Tropical montane forest restoration in Costa Rica: Overcoming barriers to dispersal and establishment. Restoration Ecology 8:339–49.
Hooper DU, Adair EC, Cardinales BJ, Byrnes JEK, Hungate BA, Matulich KL, González A, Duffy JE, Gamfeldt L, O’Connor MI. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486:105–8.
Howe HF, Miriti MA. 2004. When seed dispersal matters. Bioscience 54:651–60.
Kapos V, Wandelli E, Camargo JL, Granade G. 1997. Edge-related changes in environment and plant responses due to forest fragmentation in central Amazonia. In: Laurence WF Jr, Bierregaard RO, Eds. Tropical forest remnants. Chicago: Chicago University Press. p 33–44.
Keitt TH, Bjornstad ON, Nixon PM, Citron-Pousty S. 2002. Accounting for the spatial pattern when modeling organism-environment relationships. Ecography 25:616–25.
Kremen C. 2005. Managing ecosystem services: What do we need to know about their ecology. Ecology Letters 8:468–79.
Kremen C, Williams NM, Aizen MA, Gemmill-Herren B, LeBuhn G, Minckley R, Packer L, Potts SG, Roulston T, Steffan-Dewenter I, Vázquez DP, Winfree R, Adams L, Crone EE, Greenleaf SS, Keitt TH, Klein AM, Regetz J, Ricketts TH. 2007. Pollination and other ecosystem services produced by mobile organisms: A conceptual framework for the effects of land-use change. Ecology Letters 10:299–314.
Lundberg J, Moeberg F. 2003. Mobile link organisms and ecosystem functioning: implications for ecosystem resilience and management. Ecosystems 6:87–98.
MacDonald D, Crabtree JR, Wiesinger G, Dax T, Stamou N, Fleury P, Gutierrez Lazpita J, Gibon A. 2000. Agricultural abandonment in mountain areas of Europe: Environmental consequences and policy response. Journal of Environmental Management 59:47–69.
Martínez D, García D. 2015. Disentangling habitat use by frugivorous birds: constant interactive effects of forest cover and fruit availability. Basic and Applied Ecology 16:460–8.
Martínez I, García D, Obeso JR. 2008. Differential seed dispersal patterns generated by a common assemblage of vertebrate frugivores in three fleshy-fruited trees. Ecoscience 15:189–99.
Nathan R, Muller-Landau HC. 2000. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends in Ecology and Evolution 15:278–85.
Navarro LM, Pereira HM. 2012. Rewilding abandoned landscapes in Europe. Ecosystems 15:900–12.
Nyström M, Folke C. 2001. Spatial resilience of coral reefs. Ecosystems 4:406–17.
Padilla FM, Pugnaire FI. 2006. The role of nurse plants in the restoration of degraded environments. Frontiers in Ecology and the Environment 4:196–202.
Pejchar L, Pringle RM, Ranganathan J, Zook JR, Duran G, Oviedo F, Daily GC. 2008. Birds as agents of seed dispersal in a human-dominated landscape in southern Costa Rica. Conservation Biology 141:536–44.
Pereira HM, Navarro LM. 2015. Rewilding European landscapes. New York: Springer.
Rey P, Alcántara JM. 2014. Effects of habitat alteration on the effectiveness of plant-avian seed dispersal mutualisms: Consequences for plant regeneration. Perspectives in Plant Ecology, Evolution and Systematics 16:21–31.
Rivalan P, Frderiksen M, Loïs G, Julliard R. 2007. Contrasting responses of migration strategies in two European thrushes to climate change. Global Change Biology 13:275–87.
Rosseel Y. 2012. lavaan: An R package for structural equation modeling. Journal of Statistical Software 48:1–36.
Satorra A, Bentler PM. 1994. Corrections to test statistics and standard errors in covariance structure analyses. In: Von Eye A, Clogg CC, Eds. Latent variables analyses: Applications for developmental research. California: SAGE Publications. p 399–419.
Schupp EW, Fuentes M. 1995. Spatial patterns of seed dispersal and the unification of plant population ecology. Écoscience 2:267–75.
Schupp EW, Jordano P, Gómez JM. 2010. Seed dispersal effectiveness revisited: A conceptual review. New Phytologist 188:333–53.
SEO/BirdLife. 2013. Programas de seguimiento de avifauna de SEO/BirdLife en 2012. Spain: SEO/BirdLife.
Smit C, Ruifrok JL, van Klink R, Olff H. 2015. Rewilding with large herbivores: The importance of grazing refuges for sapling establishment and wood-pasture formation. Biological Conservation 182:134–42.
Suárez-Esteban A, Delibes M, Fedriani JM. 2013. Unpaved road verges as hotspots of fleshy-fruited shrub recruitment and establishment. Biological Conservation 167:50–6.
Swanson ME, Franklin JF, Beschta RL, Crisafulli CM, DellaSala DA, Hutto RL, Lindenmayer DB, Swanson FJ. 2011. The forgotten stage of forest succession: early-successional ecosystems on forest sites. Frontiers in Ecology and the Environment 9:117–25.
Traveset A. 1998. Effect of seed passage through vertebrates’ guts on germination: A review. Perspectives in Plant Ecology, Evolution and Systematics 9:151–90.
Uriarte M, Bruna EM, Rubim P, Anciães M, Jonckheere I. 2010. Effects of forest fragmentation on the seedling recruitment of a tropical herb: assessing seed vs. safe-site limitation. Ecology 91:1317–28.
Vera FWM. 2000. Grazing ecology and forest history. Wallingford: CABI Publishing.
Wenny DG. 2000. Seed dispersal, seed predation and seedling recruitment of a neotropical montane tree. Ecological Monographs 70:331–51.
Wunderle JM. 1997. The role of animal seed dispersal in accelerating native forest regeneration on degraded tropical lands. Forest Ecology and Management 99:223–35.
Acknowledgements
We thank C. Guardado, J. Rodríguez, R. Pérez, and D. O. Pastene for technical support and R. Lendrum for linguistic advice. We are also grateful to K. With and three anonymous reviewers who provided helpful suggestions to improve the manuscript. Field work was conducted with the permission of the Wildlife Service of Asturias. Funding was provided by the Spanish Government and the European Social Fund (FPI BES2009-25093 Grant to DM and CGL2008-01275, CGL2011-28430, and CGL2015-68963-C2-2-R Grants to DG).
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contribution
DM and DG contributed equally to the conception and the design of the study. DM performed the research and the analysis of the data, and wrote the manuscript. DG contributed substantially to the research and the writing.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Appendix A
Additional information on sampling methodologies (DOC 910 kb)
Online Appendix B
Spatial Simultaneous Autoregressive models (DOC 88 kb)
Online Appendix C
Direct, indirect and total effects estimated in Structural Equation Models (DOC 69 kb)
Rights and permissions
About this article
Cite this article
Martínez, D., García, D. Role of Avian Seed Dispersers in Tree Recruitment in Woodland Pastures. Ecosystems 20, 616–629 (2017). https://doi.org/10.1007/s10021-016-0043-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-016-0043-6