Abstract
Ant–plant interactions have mainly been considered as a protection mutualism where ants increase plant performance through protection from herbivory. However, host plants may also benefit from nutrients deposited by ants. Nitrogen limits the plant growth in most terrestrial ecosystems and the nutrient exchange between ants and plants may be an important mechanism operating in ant–plant interactions. In this study, we quantified the exchange of macronutrients (carbon and nitrogen) between ants and plants, using the Asian weaver ant Oecophylla smaragdina as a model species in a mango agroecosystem. A method was developed with which the amount of nitrogen retrieved to their host trees could be predicted by the trail density of O. smaragdina. Ant nutrient consumption was calculated based on data on O. smaragdina abundance and per capita consumption rates obtained in laboratory colonies. On a yearly basis, the influx of nitrogen to the host tree, originating from captured prey, averaged 14.4 (range 8.0–46.4) kg N ha−1 y−1. The loss of carbon from the host tree due to ant consumption of exudates from nectaries and tended homopterans averaged 278.1 (range 149.3–939.9) kg C ha−1 y−1. O. smaragdina may provide their host plant with a significant source of nitrogen albeit a substantial amount of carbon is consumed from the host plant. This study reveals that the flow of nutrients between ants and plants may play a critical and underestimated role in ant–plant mutualisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ant–plant interactions range from facultative interactions in which plants associate with a diversity of ant species, to obligate interactions which tend to be more species specific (Heil and McKey 2003). Most commonly, the mutualistic relationship involves a provision of resources and/or services for ants that in return defend the plant against herbivory. Thus, to beckon the attention of ants, many plant species have evolved ant attractants such as extrafloral nectaries (Oliveira 1997), nutritious food nodules (Heil and others 2010) as well as specialised structures providing nesting sites for the ants (Treseder and others 1995).
Recent meta-analyses of ant–plant protection mutualism have supported the hypothesis that ant interaction leads to increased plant reproductive output (Chamberlain and Holland 2009; Rosumek and others 2009; Trager and others 2010). However, a surprisingly weak correspondence between ant-mediated reduction in herbivory and improved plant performance characteristics such as growth and reproduction (Chamberlain and Holland 2009; Trager and others 2010) suggests that other mechanisms besides protection from herbivory may increase the performance of the host plant.
Factors that contribute to this incongruence may include the exchange of nutrients between ants and plants, as ants may benefit their host plant by supplying nutrients originating from captured prey. Myrmecotrophy, the transfer of nutrients from ants to plants, has been investigated most thoroughly in plant species with domatia, specialised structures in which ant colonies may nest (Gegenbauer and others 2012; Solano and Dejean 2004; Treseder and others 1995). Through absorptive tissue in the domatia, these myrmecophytic plants absorb nutrients from ant debris and excretory deposits, with up to 80% of total plant nitrogen acquisition derived from ant deposits (Solano and Dejean 2004). However, non-myrmecophytic plants may also benefit from the ant deposits. Soil-dwelling ants have been shown to increase the nutrient content in the soil around their nests where plants can proliferate from the increased nutrient content in the soil (Finer and others 2013; Folgarait 1998; Wagner and Nicklen 2010). Also, ants nesting or foraging in the canopy may enrich their host plant with nutrients, especially in tropical areas, where ants are a prominent part of the arboreal fauna (Clay and others 2013; Hölldobler and Wilson 1990; Pinkalski and others 2015a). As central place foragers, both soil dwelling and arboreal ants may function as a nutrient sink where prey is captured, eaten and recycled to primary production. Consequently, plants hosting large ant communities may gain a nutritional benefit from the prey captured by the ants; the prey represents a nutritional value that may become available to the host plant, for example, when litter from the ants’ nests are dropped on the soil (Clay and others 2013) or as manure deposited by the ants on the leaves of the host plant (Pinkalski and others 2015a).
Benefits such as protection or fertilisation by ants, however, does not come without a cost as ant activity is fuelled by plant carbohydrates. In addition to sugar rewards obtained from plant nectaries, many ant species tend hemipteran trophobionts, such as honeydew producing aphids and scale insects, allowing the ants to maintain much higher densities that could be supported by scavenging plant-produced sugar rewards alone (Blüthgen and others 2000; Davidson 1997; Davidson and others 2003). These trophobionts impose an additional cost to the plant, which is only covered if the services by ants outweigh the resources allocated to ant-tended trophobionts (Styrsky and Eubanks 2007).
The Asian weaver ant Oecophylla smaragdina (F.) (Hymenoptera: Formicidae) is an arboreal predatory species that constructs nests of interwoven leaves in the canopy of a variety of trees and shrubs. The colonies can grow to a considerable size, consisting of more than a hundred nests, spanning numerous trees, and a single colony may contain hundreds of thousands of workers (Pinkalski and others 2015b). Due to their competitive dominance over many other ant species, O. smaragdina colonies play a key role in the ecosystems in which they occur by affecting the entire arthropod community (Blüthgen and Stork 2007; Floren and others 2002; Hölldobler 1983; Way and Khoo 1992). The activities of O. smaragdina are fuelled by carbohydrates supplied from nectaries as well as tended trophobionts (Blüthgen and Fiedler 2002) and as a source of protein it preys upon a wide variety of arthropods (Lokkers 1990; Lynegaard and others 2014). Although O. smaragdina bestow their host plant, the favours of reducing herbivory and supplement their host plant with nutrients originating from captured prey, the host tree as a trade-off pays the cost in terms of carbohydrates lost to an increased pressure from sap feeding trophobionts tended by these ants.
Here, we illustrate pathways and estimate the macronutrient (carbohydrate and nitrogen) exchange in the O. smaragdina-mango (Mangifera indica) interaction to assess the significance of nutrient exchange in facultative ant plant interactions. A model was developed to predict the yearly deposition of nutrients by O. smaragdina (expressed as total nitrogen originating from prey capture) in their host trees and the corresponding amount of nutrients consumed by the ants as exudates originating from the trees. Further, we discuss how this nutrient exchange may be an important mechanism operating in ant–plant interactions.
Methods
Description of Study Site
The study was performed in the wet season (Oct–Jan 2012-2013) and in the dry season (June–Aug 2013) in two mango plantations in the suburbs of Darwin, Australia (coordinates: Plantation 1: 12°23′23″S; 130°52′14″E; Plantation 2: 12°25′16″S; 130°56′19″E). Plantation 1 comprised a total of 76 mango trees and weaver ant colonies were abundant. Some of the trees in plantation 1 had been connected by strings to facilitate the movement of ants between the trees, but otherwise the ants were not managed. Plantation 2 comprised a total of 110 mango trees and had abundant and unmanaged weaver ant colonies. The trees in both plantations were of similar age (15–20 years).
Darwin has a tropical climate with distinct wet and dry seasons. The wet season is characterised by high humidity and monsoonal rain with temperatures ranging from 25 to 33°C. The temperature in the dry season is similar, ranging from 22 to 32°C, but humidity is low and very little precipitation occurs (Australian Government, Bureau of Meteorology).
Predicting Host Tree Nitrogen Acquisition from Prey Capture by O. smaragdina Colonies—Development of the Model
The conceptual framework for the following model was developed by Pinkalski and others (2015b) and forms the basis for this study. The model estimating the amount of nitrogen from prey capture acquired by O. smaragdina colonies was constructed by combining two sub-models:
(i) Prediction of nitrogen acquisition based on nest volume of O. smaragdina (this study)
(ii) Prediction of total nest volume in the host tree from O. smaragdina trail density (Pinkalski and others 2015b)
Prediction of Nitrogen Acquisition Based on Nest Volume of O. smaragdina
To predict the amount of nitrogen from captured prey retrieved to a nest of a given volume, a total of 65 nests were sampled at three occasions: sampling 1 was performed in plantation 1 in the wet season with a total of 32 nests sampled from 4 colonies. Sampling 2 was performed in the same plantation in the dry season with a total of 17 nests sampled from 3 colonies. However, due to very low abundance of ants at this time, a third sampling was performed in plantation 2 with a total of 16 nests sampled from 3 colonies. Trees affiliated with different colonies were differentiated by following worker ant trails between the trees. If in doubt of affiliation, a few workers were transferred to the relevant tree which produced an unambiguous aggressive response among workers if the tree belonged to an alien colony (Peng and Christian 2005).
Prey brought to the nests of O. smaragdina colonies was assessed by monitoring each nest for 6 min every hour from sunrise to sunset (in 24-h preliminary investigations in both the wet and dry season, nocturnal prey capture was not detected during the investigations. This was in accordance with the findings of Lokkers (1990) who assessed the circadian variation in prey capture of O. smaragdina and found that nocturnal activity was not associated with prey capture).
Nests subjectively determined as small, medium or large nests were sampled haphazardly to cover a range from small to large nests. Nests located above 4 m could not be sampled as they were out of reach and nests with more than one ant trail were avoided to ensure that all ants entering the nest could be monitored. Prey items carried to the nests by worker ants were sampled with a pair of tweezers and identified to taxonomic order. As workers of O. smaragdina regularly transport other colony members in their mandibles, only dead ants were counted as prey items. Subsequently, the nests were cut down, falling directly into a cardboard box that was immediately closed. Upon returning to the laboratory, the ants were killed in a freezer and nest dimensions were measured. Based on the cylindrical appearance of the nests, the dimensions measured was nest length (defined as the longest side of the nest) and nest circumference (measured at the centre of the nest perpendicular to nest length). Nest volume was calculated as \( V = 0.75 \times \pi \times \left( {\frac{\text{circumference}}{2\pi }} \right)^{2} \times \,{\text{lenght}} \), where the constant of 0.75 corrects for the bias of assuming cylindrically shaped nests (Pinkalski and others 2015b). The nests were dissected by hand to measure the ant biomasses (wet weight) within the nests. Prey items sampled during the experiment were freeze dried for 24 h, dry weight determined and nitrogen content was analysed with an elemental analyser (VARIO EL III, Elementar).
Prediction of Total Nest Volume in the Host Tree from O. smaragdina Trail Density
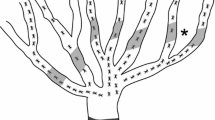
The ant trail density (hereafter referred to as trail score) was assessed on a total of 16 trees with 8 trees sampled in the wet season and 8 trees in the dry season. The assessments were performed in the afternoon between 16.00 and sunset, as this is the period of highest ant activity in both the wet and dry season (Peng and others 2012). Trail scores were determined on two successive days by counting the number of O. smaragdina trails on all major branches in the tree (Figure 1). The assessment of ant trails was initiated about half a metre from the branching point on all branches in the tree, as ants occasionally aggregated at the junctions. If a trail divided into two trails at the successive division of the branch (see asterisk on Figure 1), the trail score assessments were made on the individual branches. The presence of ants was followed 2–3 m along the branch to ensure it was an active ant trail. Each trail was observed for a few seconds and graded on a six-point scale, according to the ant density. Thus, trails were assigned a score of 1 if there were 1–10 ants m−1, 2 if there were 10–20 ants m−1, 4 if there were 20–40 ants m−1 and 6 if there were >40 ants m−1. The total trail score of the tree was given as the sum of trail scores divided by 6.
The relation between trail score and total ant nest volume in the tree was obtained by a subsequent measurement of the volumes of all ant nests in the tree (see Supplementary Section S1 for an in-depth description of determining the relation between trail score and host tree nest volume or see Pinkalski and others (2015b)).
Constructing the Model for Host Tree Nitrogen Acquisition Assessment
By combining the relation between nest volume and nitrogen acquisition from prey capture with the relation between trail score and total host tree nest volume, the nitrogen retrieved to a host tree inhabited by O. smaragdina could be predicted from the trail score. To assess the uncertainty of the prediction, a Bayesian latent variable model (Clark 2007; Gelman and others 2003) was fitted to the data (see Supplementary Section S2 for development of the model).
Application of the Model: Ant–Plant Nutrient Exchange Assessment in Plantation 1
In the wet season before the sampling of nests began, trail scores were determined on two successive days on all trees in Plantation 1 harbouring O. smaragdina colonies. Furthermore, the diameters of the tree crowns were measured allowing for calculation of the projected surface area of the tree. Using the model developed in this study, the amount of nitrogen from prey capture retrieved to each tree was predicted.
Furthermore, the amount of ant biomass in each tree was predicted from the trail score using the method developed by Pinkalski and others (2015b), where the biomass of O. smaragdina in a tree could be predicted by
Calculation of carbon consumption by the inhabiting colonies was based on data from Pinkalski (2016a, unpublished data). In this study, food consumption rates of 18 queenright O. smaragdina colonies containing both workers and brood was assessed in a laboratory experiment. With the reservation that laboratory experiments may underestimate field conditions due to shorter feeding routes, lower temperature, and so on, the consumption rate of carbohydrates was found to be 23.8 mg sucrose g wet weight ant biomass−1 day−1 (assuming a fresh weight:dry weight ratio of 3:1).
The sucrose consumption was converted into carbon equivalents by multiplying with 0.42 which is the molar fraction of carbon in sucrose.
Calculation of nitrogen consumption via plant-derived exudates (mango trees provide exudates via the honeydew excreted by homopterans trophobionts and from extrafloral nectaries situated on developing leaves) was based on the estimated carbon consumption above. With the assumption of a carbohydrate concentration in plant-derived exudates of 300 g L−1, an amino acid concentration of 3.3 g L−1 (Blüthgen and others 2004) and an amino acid nitrogen content of 16%, the consumption of nitrogen from plant derived exudates was calculated as
Statistics
Total amount of nitrogen brought to each nest per day were estimated by multiplying the amount of prey sampled by 10, as the sampling was performed hourly for 6 min (1/10 of an hour) from sunrise to sunset. Data were normalised to nitrogen acquisition per litre of nest, to correct for differences in nest volume between the sampling occasions, and log transformed to achieve normality of residuals. Comparing nitrogen acquisition between the three sampling occasions, the effect of sampling was tested using a likelihood ratio test by comparing hierarchical linear mixed models (sampling as a fixed factor and colony as a random factor) with 2 degrees of freedom. All Analyses were carried out using R software version 3.0.2 (R Core Team 2013).
Results
Prey Capture in Relation to O. smaragdina Nest Volume
The prey retrieved to the nests of O. smaragdina consisted of a wide variety of arthropods (Table 1). The vast majority of prey items, in terms weight and nitrogen content, was constituted by other ants accounting for nearly half the nitrogen intake and hemipterans (almost exclusively leafhoppers) constituting roughly a quarter. In total, guilds that are predominantly non-predatory composed roughly 40 and 60% of the prey intake in terms of weight and numbers, respectively. Prey items were predominantly transported whole. The fragments consisted of various body parts, such as limbs torn from prey during capture or scavenged carcasses from dead arthropods, from which the order could not be readily identified. The relation between nest volume and prey nitrogen acquisition (Figure 2) showed large variation although prey intake increased with nest volume. There was a trend towards a lower prey intake in Sample 2 (plantation 1 in the dry season), however, the effect of sampling occasion was not significant (Likelihood ratio = 4.72, df = 2, P = 0.09) and all data were included in the model.
Host Tree Nitrogen Acquisition as a Function of Trail Score—The Model
The prediction model for nitrogen acquisition (Figure 3) showed a linear increase as a function of trail score. To allow for a general application, the prediction model was approximated by a linear model which would allow the prediction of nitrogen acquisition in a tree directly from the trail score:
and the linear model approximated the original model with an R 2 of 0.997
Ant–Plant Nutrient Exchange Assessment in Plantation 1
A total of 40 trees in the plantation (roughly 50% of the trees) were inhabited by O. smaragdina (Table 2). Five colonies were identified with territories ranging from 2 to 14 trees. Trail scores ranged from approximately 0.5 on trees where there were only few and low density ant trails, to 3.9 on the tree with the highest trail score. On such trees, most of the major branches were occupied by ant trails. The average tree crown area (projected surface) was 27.7 m2 (SD = 16.0 m2).
From equation (1) and (2) above, the total ant biomass and daily amount of nitrogen retrieved to each tree were predicted and carbohydrate consumption by O. smaragdina was calculated based on the per capita consumption rate found by Pinkalski (2016a, unpublished data) (Table 3). Extrapolated to a yearly basis, the average nitrogen acquisition per tree was 39.9 g N tree−1 y−1 (ranging from 22.6 to 128.4 g N tree−1 y−1 in the trees with the lowest and highest trail score, respectively). The corresponding within-tree rate of nitrogen acquisition, calculated as the average nitrogen acquisition per tree divided by the average projected surface area per tree, was 14.4 (range 8.0–46.4) kg N ha−1 y−1. The average consumption of carbon per tree was 0.71 (range 0.38–2.39) kg C tree−1 y−1, corresponding to a within-tree rate of 278.1 (range 149.3–939.9) kg C ha−1 y−1. The corresponding consumption of nitrogen from plant-derived exudates was on average 1.1 (range 0.6–3.8) kg N ha−1 y−1.
The ratio between and nitrogen acquisition and carbon consumption was 1:19, meaning that for each gram of nitrogen acquired the ants consumed 19 g of carbon.
Discussion
Here, we quantified the macronutrient dynamics between the dominant arboreal ant O. smaragdina and their mango host plants. We showed that O. smaragdina colonies retrieve a significant amount of nitrogen from captured prey and that their foraging activity is fuelled by a considerable amount of carbon derived from their host plant. These quantifications demonstrate that nutrient exchange may play an important role in facultative symbiotic relationship between canopy ants and their host plants.
Deposition of ant refuse has been shown to increase the nutrient content in the soil around ant nests (Clay and others 2013; Folgarait 1998; Shukla and others 2013; Wagner 1997) where soil nutrients originating from ant deposits can be assimilated by nearby plants (Wagner and Nicklen 2010). Correspondingly, O. smaragdina may fertilise their host plants, however, due to their arboreal foraging, it may be more efficient than exclusively soil foraging ants. Ant plants may function as nutrient sinks, where nutrients from captured prey are retained within the plants boundaries and may become locally available over time. In addition, O. smaragdina may increase the accessibility of these nutrients by delivering them directly to the tree canopy as manure (Offenberg 2007; Pinkalski and others 2015a). Ant manure contain N-rich compounds such as amino acids (Vidkjær 2016, unpublished data) and urea (Vidkjær and others 2015) which can be taken up by leaves of their host plants (Vidkjær and others 2016; Pinkalski 2016b, unpublished data) thus bypassing competition from neighbouring plants. Ant manure is mainly deposited on the growing parts of the plants where ant activity is highest (Offenberg 2007) due to higher availability of trophobionts, prey and leaves suitable for nest construction. Therefore, not only does O. smaragdina increase the availability of nitrogen to their host plant, they also deliver it where needed most.
According to Huett and Dirou (2000), the yearly nitrogen removal from a 10 t ha−1 production of mango crop is 11 kg N ha−1. As O. smaragdina is presently being used as bio-control agents in orchards (Offenberg 2015; Offenberg and others 2013; Van Mele 2008), the average rate of 14.4 kg N ha−1 y−1 retrieved from prey capture may potentially compensate for the nitrogen removed from fruit production. In an ant–plant system, however, a proportion of the nitrogen from captured prey may not represent an actual influx. For instance, prey nitrogen may be derived from sources within the system, for example, herbivores feeding on the mango tree or omnivores feeding from soil detritus beneath the tree. As opposed to plants without O. smaragdina where nutrients are lost when herbivores leave the canopy, nutrients in plants with ants may be retained within the plants’ boundaries, although only prey of external origin represents a net influx. Also, a proportion of the captured prey will be incorporated into ant biomass. According to Pinkalski and others (2016a, unpublished data), the incorporation efficiency of dietary nitrogen by O. smaragdina is at least 50%, with which 7.2 kg N ha−1 y−1 will be built into ant biomass. Although nutrients invested in worker ant biomass eventually may become available through decomposition of dead ants in the soil beneath the plant, nutrients allocated into production of sexuals confer a loss as these individuals leave the colony. In O. smaragdina colonies, sexuals may compose up to 50% of the colony biomass (Pinkalski and others 2015b). With an average colony biomass of 25.3 kg dry weight ha−1 (Table 2, using an average canopy area of 27.7 m2 and a fresh weight:dry weight ratio of 3:1), this corresponds to 12.7 kg dry weight sexuals ha−1. Assuming a nitrogen content of 7% (Pinkalski 2016a, unpublished data), the average amount of nitrogen lost due to investment in sexuals equals 0.9 kg N ha−1 y−1. In addition, O. smaragdina consume nitrogen from the host tree itself through honeydew produced by trophobionts, which was estimated to be 1.1 kg N ha−1 y−1. These intricate nitrogen dynamics between O. smaragdina and their host tree is illustrated in Figure 4.
Quantification of nitrogen dynamics in the ant–plant system consisting of O. smaragdina and their mango host tree given as estimated yearly fluxes of nitrogen per hectare. Green lines represent an influx of nitrogen and red lines represent a loss of nitrogen from the system. The broken red lines represent the unknown amount of nutrients from captured prey that are derived from the host tree itself and the amount of nutrients that are lost when herbivores feed and leave the canopy. Black lines represent redistribution of nitrogen within the system. The amount of nitrogen deposited as ant manure was taken from Pinkalski and others (2015a). Of the nitrogen consumed from plant derived exudates 50% was assumed to be assimilated into ant biomass (Pinkalski and others 2015c, unpublished data) and the remaining nitrogen deposited as ant manure (Color figure online).
The host plant pays the cost of an increased accessibility of nitrogen in terms of carbohydrates consumed by O. smaragdina. This consumption confers a loss to the host plant as the carbohydrates are derived from the host plant itself through either extrafloral nectaries or ant-tended trophobionts. The loss of carbohydrates through extrafloral nectaries can, at least partly, be controlled by the host plant, for example, by increasing or decreasing the amount of nectar produced in response to herbivory (Wackers and others 2001; Wackers and Bonifay 2004). However, ants may have by passed plant control by tapping the host plant of resources by means of tended trophobionts. Accordingly, carbohydrates derived from the host plant may originate from honeydew production rather than from extrafloral nectaries (Blüthgen and Fiedler 2002), giving the ants access to an excessive consumption of carbohydrates.
Total leaf consumption by folivorous insects in a Bornean rainforest has been estimated to be 185 kg C ha−1 y−1 (Suzuki and others 2013). In comparison, the average rate of carbon consumption by O. smaragdina colonies was 278.1 kg C ha−1 y−1. However, caution should be taken when comparing a natural forest with a mango agroecosystem and in that respect the carbon consumption by O. smaragdina may represent an overestimate. For instance, the estimated average rate of carbon consumption per tree was 0.71 kg C tree−1 y−1. Assuming a mango plantation with 100 trees ha−1, this corresponds to a yearly consumption of only 71 kg C ha−1. Nevertheless, the carbon consumption by this single ant species is in the same order of magnitude as carbon consumed by all folivorous insects combined. The extensive intake of carbohydrates by O. smaragdina is by no means confined to this species. For instance, in colonies of Formica aquilonia, honeydew composed 78–92% of their diet on a dry weight basis (Domisch and others 2009) and an average sized colony of Formica polyctena may consume 155 l of honeydew over the season (Horstman 1974). Assuming that honeydew contains 30% sucrose (Blüthgen and others 2004) and the density of Formica polyctena is 8 colonies ha−1 (Chen and Robinson 2013), this corresponds to a consumption of 156 kg C ha−1 y−1. Consequently, ants may be much more dependent on a carbohydrate based diet than previously expected, and our findings lend support to the hypothesis of Tobin (1994) that ants may effectively function as “cryptic herbivores”. Although O. smaragdina may reduce direct herbivory on their host plant by predation and potentially through non-consumptive effects such as induced behavioural changes of herbivores (Offenberg and others 2004; Rudgers and others 2003), the effect in terms of carbon lost from the plant may be marginal. However, carbohydrates lost from tissue feeding herbivores originate from structures in which the plant already have invested resources and may thus represent a much higher value to the host plant than simple carbohydrates lost via sap feeding trophobionts.
The acquisition of nitrogen in exchange for carbohydrates in ant–plant interactions resembles one of the most abundant terrestrial mutualisms on Earth: The symbiosis between plants and arbuscular mycorrhizal fungi (Read 1991). Here the plant receives nutrients and water from symbiotic fungi which in turn receive carbohydrates from the infected plant. Although the stoichiometric ratios of C/N exchange between fungi and plants are unknown (Correa and others 2015), it appears that when nitrogen is limiting, carbon is an excess, rather than costly resource that can be allocated to support the growth of the symbiotic fungi (Correa and others 2012). Correspondingly, the increase in plant performance experienced by ant–plants may partly arise from an enhanced acquisition of nutrients by the host plant. For instance, Wagner (1997) compared the seed production in Acasia constricta with and without Formica perpilosa ants nesting at the base of the plants. Despite no significant difference in herbivory, plants associated with Formica perpilosa produced 1.9 as many seeds compared to plants not associated with an ant nest. However, although ant protection or provision of nutrients may be beneficial, the host plant has only limited control of the amount of carbohydrates consumed by the ants; in the absence of herbivory or nutrient limitations, ants may impose a significant cost to the host plant due to the maintenance of ant colonies (Palmer and Brody 2013).
In conclusion, this study revealed that colonies of O. smaragdina may provide their host plant with a significant amount of nitrogen while feeding extensively on carbohydrates derived from the host plant. Clearly, the interactions between ants and plants stretch beyond ant mediated reductions in herbivory, and a more comprehensive understanding of the nutrient exchange between ants and plants is needed in order to further elucidate the mechanisms operating in ant–plant interactions.
References
Blüthgen N, Fiedler K. 2002. Interactions between weaver ants Oecophylla smaragdina, homopterans, trees and lianas in an australian rain forest canopy. J Anim Ecol 71(5):793–801.
Blüthgen N, Gottsberger G, Fiedler K. 2004. Sugar and amino acid composition of ant-attended nectar and honeydew sources from an australian rainforest. Austral Ecol 29(4):418–29.
Blüthgen N, Verhaagh M, Goitia W, Jaffe K, Morawetz W, Barthlott W. 2000. How plants shape the ant community in the amazonian rainforest canopy: the key role of extrafloral nectaries and homopteran honeydew. Oecologia 125(2):229–40.
Blüthgen N, Stork NE. 2007. Ant mosaics in a tropical rainforest in australia and elsewhere: a critical review. Austral Ecol 32(1):93–104.
Chamberlain SA, Holland JN. 2009. Quantitative synthesis of context dependency in ant-plant protection mutualisms. Ecology 90(9):2384–92.
Chen Y, Robinson EJH. 2013. A comparison of mark-release-recapture methods for estimating colony size in the wood ant formica lugubris. Insectes Soc 60(3):351–9.
Clay NA, Lucas J, Kaspari M, Kay AD. 2013. Manna from heaven: refuse from an arboreal ant links aboveground and belowground processes in a lowland tropical forest. Ecosphere 4(11):1–15.
Correa A, Cruz C, Ferrol N. 2015. Nitrogen and carbon/nitrogen dynamics in arbuscular mycorrhiza: the great unknown. Mycorrhiza 25(7):499–515.
Correa A, Gurevitch J, Martins-Loucao MA, Cruz C. 2012. C allocation to the fungus is not a cost to the plant in ectomycorrhizae. Oikos 121(3):449–63.
Davidson D. 1997. The role of resource imbalances in the evolutionary ecology of tropical arboreal ants. Biol J Linn Soc 61(2):153–81.
Davidson D, Cook S, Snelling R, Chua T. 2003. Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300(5621):969–72.
Domisch T, Finer L, Neuvonen S, Niemela P, Risch AC, Kilpelainen J, Ohashi M, Jurgensen MF. 2009. Foraging activity and dietary spectrum of wood ants (formica rufa group) and their role in nutrient fluxes in boreal forests. Ecol Entomol 34(3):369–77.
Finer L, Jurgensen MF, Domisch T, Kilpelainen J, Neuvonen S, Punttila P, Risch AC, Ohashi M, Niemela P. 2013. The role of wood ants (formica rufa group) in carbon and nutrient dynamics of a boreal norway spruce forest ecosystem. Ecosystems 16(2):196–208.
Floren A, Biun A, Linsenmair K. 2002. Arboreal ants as key predators in tropical lowland rainforest trees. Oecologia 131(1):137–44.
Folgarait P. 1998. Ant biodiversity and its relationship to ecosystem functioning: a review. Biodivers Conserv 7(9):1221–44.
Gegenbauer C, Mayer VE, Zotz G, Richter A. 2012. Uptake of ant-derived nitrogen in the myrmecophytic orchid caularthron bilamellatum. Ann Bot 110(4):757–65.
Heil M, McKey D. 2003. Protective ant-plant interactions as model systems in ecological and evolutionary research. Annu Rev Ecol Evol Syst 34:425–53.
Heil M, Orona-Tamayo D, Eilmus S, Kautz S, Gonzalez-Teuber M. 2010. Chemical communication and coevolution in an ant-plant mutualism. Chemoecology 20(2):63–74.
Hölldobler B, Wilson EO. 1990. The ants. Cambridge: Belknap Press of Harvard University Press.
Hölldobler B. 1983. Territorial behavior in the green tree ant (Oecophylla-smaragdina). Biotropica 15(4):241–50.
Horstman K. 1974. Investigations on food-consumption of red wood ants (formica-polyctena foerster) in an oak forest.3. Annual turnover. Oecologia 15(2):187–204.
Huett DO, Dirou JF. 2000. An evaluation of the rationale for fertiliser management of tropical fruit crops. Aust J Exp Agric 40:1137–43.
Lokkers C. 1990. Colony dynamics of the green tree ant (Oecophylla smaragdina fab.) in a seasonal tropical climate. PhD thesis, James Cook University. http://eprints.jcu.edu.au/24114/.
Lynegaard GK, Offenberg J, Fast TS, Axelsen JA, Mwatawala MW, Rwegasira GM. 2014. Using insect traps to increase weaver ant (Oecophylla longinoda) prey capture. J Appl Entomol 138(7):539–46.
Offenberg J. 2015. Ants as tools in sustainable agriculture: the case of weaver ants and beyond. J Appl Ecol 52:1197–205.
Offenberg J. 2007. The distribution of weaver ant pheromones on host trees. Insectes Soc 54(3):248–50.
Offenberg J, Nielsen M, MacIntosh D, Havanon S, Aksornkoae S. 2004. Evidence that insect herbivores are deterred by ant pheromones. Proc R Soc B Biol Sci 271:433–5.
Offenberg J, Cuc Nguyen Thi Thu, Wiwatwitaya D. 2013. The effectiveness of weaver ant (Oecophylla smaragdina) biocontrol in southeast asian citrus and mango. Asian Myrmecol 5:139–49.
Oliveira P. 1997. The ecological function of extrafloral nectaries: herbivore deterrence by visiting ants and reproductive output in Caryocar brasiliense (caryocaraceae). Funct Ecol 11(3):323–30.
Palmer TM, Brody AK. 2013. Enough is enough: the effects of symbiotic ant abundance on herbivory, growth, and reproduction in an african acacia. Ecology 94(3):683–91.
Peng R, Christian K. 2005. Integrated pest management for mango orchards using green ants as a major component. A manual for conventional and organic mango growers in Australia. School of Science and Primary Industries, Charles Darwin University, Darwin.
Peng R, Christian K, Gibb K. 2012. The best time of day to monitor and manipulate weaver ant colonies in biological control. J Appl Entomol 136(1–2):155–60.
Pinkalski C, Damgaard C, Jensen KV, Peng R, Offenberg J. 2015a. Quantification of ant manure deposition in a tropical agroecosystem: implications for host plant nitrogen acquisition. Ecosystems 18:1373–82.
Pinkalski C, Damgaard C, Jensen KV, Gislum R, Peng R, Offenberg J. 2015b. Non-destructive biomass estimation of Oecophylla smaragdina colonies: a model species for the ecological impact of ants. Insect Conserv. Divers. 8(5):464–73.
Read D. 1991. Mycorrhizas in ecosystems. Experientia 47(4):376–91.
Rosumek FB, Silveira FAO, Neves FdS, Barbosa NPdU, Diniz L, Oki Y, Pezzini F, Fernandes GW, Cornelissen T. 2009. Ants on plants: a meta-analysis of the role of ants as plant biotic defenses. Oecologia 160(3):537–49.
Rudgers JA, Hodgen JG, White JW. 2003. Behavioral mechanisms underlie an ant-plant mutualism. Oecologia 135(1):51–9.
Shukla RK, Singh H, Rastogi N, Agarwal VM. 2013. Impact of abundant pheidole ant species on soil nutrients in relation to the food biology of the species. Appl Soil Ecol 71:15–23.
Solano P, Dejean A. 2004. Ant-fed plants: comparison between three geophytic myrmecophytes. Biol J Linn Soc 83(4):433–9.
Styrsky JD, Eubanks MD. 2007. Ecological consequences of interactions between ants and honeydew-producing insects. Proc R Soc B Biol Sci 274(1607):151–64.
Suzuki S, Kitayama K, Aiba S, Takyu M, Kikuzawa K. 2013. Annual leaf loss caused by folivorous insects in tropical rain forests on mt. kinabalu, borneo. J Forest Res 18(4):353–60.
Tobin J. 1994. Ant as primary consumers: diet and abundance in the formicidae. In: Hunt JH, Nepala CA, Eds. Nourishment and evolution in insect societies. Westview: Boulder.
Trager MD, Bhotika S, Hostetler JA, Andrade GV, Rodriguez-Cabal MA, McKeon CS, Osenberg CW, Bolker BM. 2010. Benefits for plants in ant-plant protective mutualisms: a meta-analysis. PLoS ONE 5(12):e14308.
Treseder K, Davidson D, Ehleringer J. 1995. Absorption of ant-provided carbon-dioxide and nitrogen by a tropical epiphyte. Nature 375(6527):137–9.
Van Mele P. 2008. A historical review of research on the weaver ant oecophylla in biological control. Agric Entomol 10(1):13–22.
Vidkjær NH, Wollenweber B, Jensen KMV, Ambus PL, Offenberg J, Fomsgaard IS. 2016. Urea in weaver ant feces: quantification and investigation of the uptake and translocation of urea in Coffea arabica. J Plant Growth Regul. doi:10.1007/s00344-016-9586-1.
Vidkjær NH, Wollenweber B, Gislum R, Jensen KV, Fomsgaard IS. 2015. Are ant feces nutrients for plants? A metabolomics approach to elucidate the nutritional effects on plants hosting weaver ants. Metabolomics 11(4):1013–28.
Wackers F, Bonifay C. 2004. How to be sweet? Extrafloral nectar allocation by Gossypium hirsutum fits optimal defense theory predictions. Ecology 85(6):1512–18.
Wackers F, Zuber D, Wunderlin R, Keller F. 2001. The effect of herbivory on temporal and spatial dynamics of foliar nectar production in cotton and castor. Ann Bot 87(3):365–70.
Wagner D. 1997. The influence of ant nests on acacia seed production, herbivory and soil nutrients. J Ecol 85(1):83–93.
Wagner D, Nicklen EF. 2010. Ant nest location, soil nutrients and nutrient uptake by ant-associated plants: does extrafloral nectar attract ant nests and thereby enhance plant nutrition? J Ecol 98(3):614–24.
Way M, Khoo K. 1992. Role of ants in pest-management. Annu Rev Entomol 37:479–503.
Acknowledgements
This study was funded by the Danish Council for Independent Research (11-106824)
Conflict of interest
All authors declare that they have no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
CP, KMVJ, RP, and JO conceived the work. CP carried out the field work with assistance from RP. CP and CD analysed the data and constructed the model. CP wrote the paper under supervision from KMVJ and JO.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pinkalski, C., Damgaard, C., Jensen, KM.V. et al. Macronutrient Exchange Between the Asian Weaver Ant Oecophylla smaragdina and Their Host Plant. Ecosystems 19, 1418–1428 (2016). https://doi.org/10.1007/s10021-016-0013-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-016-0013-z