Abstract
In this paper, NaSn0.02Ti1.98(PO4)3/C composite material was prepared by spray drying method combined with high-temperature calcination, which can improve the electrochemical performance of NaTi2(PO4)3. The doping of Sn4+ increases the lattice spacing of NaTi2(PO4)3, thereby accelerating the diffusion coefficient of Na+. The carbon doped on NaTi2(PO4)3 reduces the charge transfer impedance and enhances the ion diffusion coefficient. As a result, NaSn0.02Ti1.98(PO4)3/C exhibits improved electrochemical performance. The specific capacity for the initial discharge of NaSn0.02Ti1.98(PO4)3/C at 1C, 5C, 10C and 20C rates are 108 mAh/g, 86 mAh/g, 76.3 mAh/g, and 71.4 mAh/g, respectively. After 1400 cycles at 10C, the capacity of the material decreases to 64.3 mAh/g, with a capacity retention rate of 84.2%. Therefore, NaSn0.02Ti1.98(PO4)3/C exhibits fast charge/discharge performance as well as cycling stability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, with the rapid development of modern portable electronics, new energy vehicles, and grid energy storage, the demand for advanced battery energy storage systems with high energy density has increased dramatically [1,2,3]. Because of their plentiful reserves and low cost, sodium-ion batteries (SIBs) have gained a lot of attention and offer a lot of potential as a form of renewable energy storage [4,5,6,7]. SIB materials possess characteristics such as high capacity and high safety performance. Currently, the research on negative electrode materials for SIBs mainly focuses on carbon materials [8], metal selenides [9], metal sulfides [10], and phosphate compounds [11]. Among these materials, phosphate-based compounds with Na as the main constituent are the optimal choice as negative electrode materials. This is due to their strong P–O covalent bonding, which offers superior thermal and structural stabilities. Among these phosphate-based materials, NaTi2(PO4)3 with the NASICON structure has been gaining increasing attention owing to its vast theoretical potential (133 mAh/g), inexpensive cost, and good safety performance. It is considered a promising negative electrode material [12,13,14,15].
NaTi2(PO4)3 exhibits thermal stability and a high theoretical capacity, but its slow charge transfer kinetics and low electronic conductivity result in low-capacity retention and poor rate performance [16,17,18]. Attempts have been made to enhance the electrochemical performance of NaTi2(PO4)3 through carbon coating [16, 19], element doping [15, 20], or synergistic effects of carbon and element doping [5, 14, 17, 21,22,23,24]. For example, Mg-doped and carbon-doped NaTi2(PO4)3 [25, 26], Mn-doped and carbon-doped NaTi2(PO4)3 [23], Al-doped and carbon-doped NaTi2(PO4)3 [14], Zr-doped and carbon-doped NaTi2(PO4)3 [22], Fe-doped and carbon-doped NaTi2(PO4)3 [27], and Cr-doped and carbon-doped NaTi2(PO4)3 [28] have been reported. He prepared Zr-doped and carbon-coated NaTi2(PO4)3 [22], which improved the electrochemical performance of NaTi2(PO4)3, and was applied to aqueous LIBs. The capacity of discharge of NaTi1.9Zr0.1(PO4)3/C at 0.2C, 3C, and 15C were 112.5 mAh/g, 102.7 mAh/g, and 94.1 mAh/g, respectively. Wu prepared Al-doped and carbon-coated NaTi2(PO4)3 for aqueous SIBs [14], showing 102.9 mAh/g initial discharge capacity and 90.1 mAh/g reversible capacity after 200 cycles at 10C. Zhao studied the improvement of electrochemical capability of NaTi2(PO4)3 by Mg doping and carbon coating [26] and applied it to organic SIBs. The specific capacity of the material was 94.4 mAh/g at a current density of 5 A/g. After 300 cycles, the rate of capacity retention reached 99.1%, showing good cycle stability. Qu studied the improvement of Mn doping and carbon coating on the electrochemical performance of NaTi2(PO4)3 [23], which was applied to organic SIBs. It showed 116 mAh/g at 1C and 92 mAh/g after 1000 cycles at 20C (88% capacity retention).
After conducting research, it has been found that only a few researchers have utilized Sn-doped NaTi2(PO4)3. The low cost of tin as a dopant makes it favorable for industrial applications. In this study, we prepared NaSn0.02Ti1.98(PO4)3/C and applied it in organic SIBs. The electrochemical performance of the material was systematically investigated. The results indicate that the doping of Sn and carbon significantly improved performance of NaTi2(PO4)3 electrochemically.
Experimental
Material synthesis
Firstly, 2.5 mmol of C6H5Na3O7 (0.645 g), 14.7 mmol of C8H20O4Ti (3.35 g), 0.3 mmol of C4H10O2Sn (0.063 g), and 22.5 mmol of NH4H2PO4 (2.59 g) are added to a ball mill containing 40 g of water. The mixture is then ball-milled for 5 h at a constant temperature of 20 °C and a rotation speed of 500 rpm. After ball milling, adjust the inlet temperature of the spray dryer to 220 °C and the outlet temperature to around 80 °C. Once the conditions are met, start the feeding process with a peristaltic pump operating at a rate of 20 rpm to obtain the precursor. The dried solid powder was placed in a corundum crucible and heated at 900 °C for 10 h in an N2 atmosphere at a rate of 5 °C/min to obtain the NaSn0.02Ti1.98(PO4)3/C. The NaTi2(PO4)3/C was obtained by removing the tin source C4H10O2Sn, while other experimental conditions remained unchanged. The NaTi2(PO4)3 was obtained by removing the tin source C4H10O2Sn and replacing C6H5Na3O7 with Na2CO3, while other experimental conditions remained unchanged.
Characterizations
X-ray diffractometer (X’Pert Powder XRD) analysis was performed to determine the purity of the produced material. A thermogravimetric analyzer (STA25000 TG) was used, and mass loss was recorded to quantify the carbon content of the material. Micromorphology of different composites observed by scanning electron microscope (S-3400N SEM) and EDS analyzed the distribution of elements in the composites. Inductively coupled plasma atomic emission spectrometry was utilized to measure the Sn content using the Thermo ICP6300 ICP-AES..
Electrode preparation and electrochemical testing
The working electrode consisting of 70% active material, 20% acetylene black, and 10% sodium carboxymethyl cellulose (adhesive) was prepared by placing these materials in a 50 ml agate spherule tank with N-methylpyrrolidone (NMP), ball milling for 1 h. The mixture is applied to the copper foil. After vacuum drying at 110 ℃ for 12 h, the electrode material was cut into small circles with an area of 1.13 cm2, where the mass load of the electrode was about 1 mg/cm2. A CR2032-type coin cell was assembled in an argon-filled glove box with water and oxygen concentrations of less than 0.1 ppm. The battery is mainly composed of working electrode (prepared electrode), electrode level (sodium metal sheet), diaphragm (glass fiber), and electrolyte (1 M NaClO4 dissolved in a mixture solution of ethyl carbonate and dimethyl carbonate (volume ratio, 1:1)). Constant current/charge test was conducted using Land-2001A. An electrochemical workstation (CHI660E) was used for cyclic voltammetry (CV) measurements and EIS investigations. Constant discharge/charge test and CV test voltage range are consistent; both are 1.5–3 V. The range of EIS frequency is 200 kHz to 10 MHz.
Results and discussion
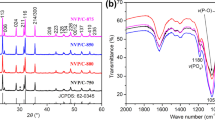
Figure 1a is the XRD of NaTi2(PO4)3/C and NaSn0.02Ti1.98(PO4)3/C. The results indicate that all sharp diffraction peaks of the two samples correspond to the NaTi2(PO4)3 standard card (PDF#84-2009). In the patterns, there were no impurity peaks to be seen. Figure 1b shows the magnification of diffraction peaks (104), (110), (113), and (202) of NaTi2(PO4)3/C and NaSn0.02Ti1.98(PO4)3/C in the range of 20 to 30°. Figure 1c shows the magnification of diffraction peaks (116) and (300) of NaTi2(PO4)3/C and NaSn0.02Ti1.98(PO4)3/C in the range of 30 to 40°. It can be observed that the diffraction peaks of NaSn0.02Ti1.98(PO4)3/C have shifted to the left, indicating a decrease in angle. According to Bragg formula, the diffraction peak position of the NaSn0.02Ti1.98(PO4)3/C shifts to a smaller angle, indicating that the lattice expansion and the ion diffusion channel becoming wider, consistent with previously reported results [14, 29, 30]. The doping of Sn4+ can increase the lattice spacing of NaTi2(PO4)3, and the ionic radius of Sn4+ (69 pm) is larger than that of Ti4+ (60.5 pm). Increasing the cell size will widen the transfer path of Na+ within the crystal structure, thereby accelerating the diffusion coefficient of Na+. The crystal cell parameters of NaTi2(PO4)3 and NaSn0.02Ti1.98(PO4)3/C were obtained after refining the XRD data using Rietveld (Fig. 1e, f). It can be seen from Table 1 that for NaSn0.02Ti1.98(PO4)3/C, the lattice parameter c = 21.7796 Å which is an increases of 0.40% compared to NaTi2(PO4)3. The unit cell volume of NaTi2(PO4)3 is 1340.5 Å3, and the unit cell volume of NaSn0.02Ti1.98(PO4)3/C is 1341.4 Å3, which is 0.06% larger than that of NaTi2(PO4)3. And expanded cell will result in larger sodium ion diffusion channel.
a XRD of NaTi2(PO4)3/C and NaSn0.02Ti1.98(PO4)3/C, b amplification of diffraction peaks (104), (110), (113), and (202) of NaTi2(PO4)3/C and NaSn0.02Ti1.98(PO4)3/C, c amplification of diffraction peaks (116) and (300) of NaTi2(PO4)3/C and NaSn0.02Ti1.98(PO4)3/C, d TGA of NaSn0.02Ti1.98(PO4)3/C, e refined XRD of NaTi2(PO4)3, f refined XRD of NaSn0.02Ti1.98(PO4)3/C
The TG test results (Fig. 1d) show that the weight decreases in the temperature range of 450–750 °C, which is due to the reaction and pyrolysis of carbon in sodium citrate in the material with air (it has been studied that the tin source will not decompose within 750 °C). Doping a small amount of carbon in the material can improve the electronic conductivity of the material, thereby enhancing the electrode material’s performance. According to the curve in Fig. 1d, the carbon content in the NaSn0.02Ti1.98(PO4)3/C sample is about 1.54%.
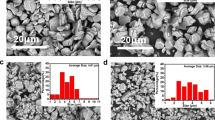
Figure 2a–d are the SEM images of NaTi2(PO4)3/C and NaSn0.02Ti1.98(PO4)3/C, respectively. From Fig. 2a, c, it can be seen that both NaTi2(PO4)3/C and NaSn0.02Ti1.98(PO4)3/C are microspheres, and the distribution of microspheres is relatively uniform, without great difference. The magnified images in Fig. 2b, d show NaTi2(PO4)3/C particles and NaSn0.02Ti1.98(PO4)3/C particles, respectively. It is evident that the micro-sized particles of both materials are composed of smaller particles, having a particle size distribution that ranges from 1 to 3 μm. In addition, the EDS mapping of C, O, Ti, P, Na, and Sn are shown in Fig. 2e for NaSn0.02Ti1.98(PO4)3/C. Sn and C are equally distributed and successfully doped in the NaSn0.02Ti1.98(PO4)3/C sample, as evidenced by the fact that the distribution of Sn and C are consistent with the distribution of O, Ti, P, and Na. The content of Sn in the sample was measured by ICP. The mass percentage of Sn element in NaSn0.02Ti1.98(PO4)3/C is about 1.42%.
We characterized its electrochemical performance. From Fig. 3a, it can be observed that at 1C, the initial overpotential of NaTi2(PO4)3/C is 252.6 mV. At 2C, 5C, 10C, and 20C, the potential differences are 389 mV, 718.9 mV, 719 mV, and 994.4 mV, respectively. The capacities at 1C, 2C, 5C, 10C, and 20C are 100.4 mAh/g, 91.6 mAh/g, 76.9 mAh/g, 65.8 mAh/g, and 55.8 mAh/g, respectively. From Fig. 3b, it can be observed that at 1C, the initial overpotential of NaSn0.02Ti1.98(PO4)3/C is 185.3 mV. At 2C, 5C, 10C, and 20C, the potential differences are 317.5 mV, 531.7 mV, 598.9 mV, and 789.6 mV, respectively. The capacities at 1C, 2C, 5C, 10C, and 20C are 108 mAh/g, 102.3 mAh/g, 91.6 mAh/g, 78.4 mAh/g, and 71.4 mAh/g, respectively. It can be seen that the overpotential of NaSn0.02Ti1.98(PO4)3/C becomes smaller and the capacity becomes larger. To further investigate the electrochemical performance of the material, we conducted rate performance tests (Fig. 3c, d), which clearly show the material’s ability for rapid charge and discharge. As shown in Fig. 3d, the initial discharge-specific capacities of NaSn0.02Ti1.98(PO4)3/C at 1C, 5C, 10C, and 20C are 108mAh/g, 86mAh/g, 76.3mAh/g, and 71.4mAh/g, respectively, all higher than those of NaTi2(PO4)3/C (Fig. 3c). Figure 3e is the cycling performance of NaTi2(PO4)3/C and NaSn0.02Ti1.98(PO4)3/C at 10C. The initial discharge-specific capacity of NaTi2(PO4)3/C is 79.9 mAh/g, which decays to 49.5 mAh/g after 1400 cycles, and the capacity retention rate is 61.9%. The initial discharge-specific capacity of NaSn0.02Ti1.98(PO4)3/C is 76.3 mAh/g, which decays to 64.3 mAh/g after 1400 cycles, and the capacity retention rate is 84.2%. It has good fast charge-discharge performance and cycle stability.
a Discharge/charge curves of NaTi2(PO4)3/C at different rates, b discharge/charge curves of NaSn0.02Ti1.98(PO4)3/C at different rates, c rate performance of NaTi2(PO4)3/C at different rates, d rate performance of NaSn0.02Ti1.98(PO4)3/C at different rates, e long-term cycling performance of NaTi2(PO4)3/C and NaSn0.02Ti1.98(PO4)3/C at 10C
Figure 4a–c is the CV curves of working electrode NaTi2(PO4)3, NaTi2(PO4)3/C, and NaSn0.02Ti1.98(PO4)3/C in the voltage range of 1.5 ~ 3.0 V and the scan rate of 0.1 mV/s. In Fig. 4a, NaTi2(PO4)3 shows two significant redox peaks at about 2.23 V/1.92 V, and the potential interval is about 340 mV. In Fig. 4b, NaTi2(PO4)3/C shows two significant redox peaks at about 2.23 V/2.05 V, with potential intervals of about 253 mV. In Fig. 4c, NaSn0.02Ti1.98(PO4)3/C shows two significant redox peaks at about 2.23 V/2.05 V, with potential intervals of about 185 mV. In the CV curve, the ∆E between the peak potential difference of the cathode and anode reflects the polarization and dynamic characteristics of the electrodes. As is well known, the electron conduction resistance and ion diffusion resistance from materials are the main reason for polarization. Compared to NaTi2(PO4)3, NaTi2(PO4)3/C increases the electronic conductivity of the material due to carbon coating, which can effectively reduce the polarization of the electrode, thus exhibiting a smaller ∆E. Furthermore, NaSn0.02Ti1.98(PO4)3/C exhibits a smaller ∆E than NaTi2(PO4)3/C, indicating that NaSn0.02Ti1.98(PO4)3/C has a smaller polarization, which is mainly attributed to the increase in Na+ expansion coefficient caused by Sn4+ doping. Having the minimum polarization gives NaSn0.02Ti1.98(PO4)3/C the best rate performance.
a CV curve of NaTi2(PO4)3 at 0.1 mV/s, b CV curve of NaTi2(PO4)3/C at 0.1 mV/s, c CV curve of NaSn0.02Ti1.98(PO4)3/C at 0.1 mV/s, d Nyquist plots of NaTi2(PO4)3, NaTi2(PO4)3/C, NaSn0.02Ti1.98(PO4)3/C, e GITT curves and DNa+ of NaTi2(PO4)3/C charge/discharge process, f GITT curves and DNa+ of NaSn0.02Ti1.98(PO4)3/C charge/discharge process
Electrical conductivity and Na+ diffusion are widely regarded as the primary determining factors for electrode performance. Electrochemical impedance spectroscopy (EIS) measurements were conducted to elucidate the kinetic processes of the NaTi2(PO4)3 electrode (Fig. 4d). As shown in Fig. 4d, the charge transfer resistance of NaSn0.02Ti1.98(PO4)3/C is approximately 91 Ω, significantly lower than that of NaTi2(PO4)3/C (173 Ω) and NaTi2(PO4)3 (305 Ω). The decreased resistance of NaSn0.02Ti1.98(PO4)3/C contributes to its improved electrochemical performance.
In order to better verify the promotion of Sn doping on the electrochemical reaction kinetics of the material, the sodium ion diffusion rate of the material was tested by the GITT method using LANDdt. The GITT curves and Na+ diffusion coefficients for NaTi2(PO4)3/C and NaSn0.02Ti1.98(PO4)3/C are shown in Fig. 4e, f. The Na+ diffusion coefficient (DNa+) of the electrode can be calculated from GITT data using Eq. (1):
In the equation, MB and mB are the molar mass and the active mass of NaTi2(PO4)3/C and NaSn0.02Ti1.98(PO4)3/C electrode (1.96 mg/cm2), Vm is the molar volume of NaTi2(PO4)3, A is the surface area of the electrode piece (1.13 cm2), τ is the interval time, ΔEs is the voltage change of steady-state after a discharge pulse and open circuit standing, and ΔEτ is the cell voltage change during a discharge pulse process. According to the Na+ diffusion coefficient calculated from the GITT curve, the average value of DNa+ in the discharge process of NaTi2(PO4)3/C electrode is about 10−11cm2 s−1, and the average value of DNa+ in the charging process is about 10−10cm2 s−1. The average value of DNa+ in the discharge process of NaSn0.02Ti1.98(PO4)3/C electrode is about 10−10cm2 s−1, and the average value of DNa+ in the charging process is about 10−9cm2 s−1. It can be seen that NaSn0.02Ti1.98(PO4)3/C has a greater DNa+. The sodium ion rate diffusion of NaSn0.02Ti1.98(PO4)3/C is faster because of the substitution of Sn2+, which enlarges the channel for Na+ diffusion.
Conclusions
In summary, we prepared NaSn0.02Ti1.98 (PO4)3/C for organic SIBs to improve electrochemical performance. The doping of Sn4+ can increase the lattice spacing of the NaTi2(PO4)3, thus accelerating the diffusion coefficient of Na+. ICP analysis revealed that the mass percentage of Sn in NaSn0.02Ti1.98(PO4)3/C is approximately 1.42%. The results of the TG test show that the carbon content of the sample is around 1.54%. The initial discharge-specific capacities of NaSn0.02Ti1.98(PO4)3/C at 1C, 5C, 10C, and 20C rates are 108 mAh/g, 86 mAh/g, 76.3 mAh/g, and 71.4 mAh/g, respectively. After 1400 cycles at 10C, the capacity of the material decreases to 64.3 mAh/g, with a capacity retention rate of 84.2%. EIS results confirm that carbon coating leads to lower charge transfer impedance and increased ion diffusion coefficient in NaTi2(PO4)3. Therefore, NaSn0.02Ti1.98(PO4)3/C has better electrochemical performance and fast charge/discharge performance.
Data availability
The datasets generated during and/or analyzed the current study are available from the corresponding author on reasonable request.
References
Wang Z, Ni J, Li L (2022) Gradient designs for efficient sodium batteries. ACS Energy Lett 7(11):4106–4117
Shen X, Qian T, Chen P, Liu J, Wang M, Yan C (2018) Bioinspired polysulfiphobic artificial interphase layer on lithium metal anodes for lithium sulfur batteries. ACS Appl Mater Interfaces 10(36):30058–30064
Wang J, Wang Z, Ni J, Li L (2022) Electrospinning for flexible sodium-ion batteries. Energy Stor Mater 45:704–719
Liao W, Hung T, Abdelaal MM, Chao C, Fang C, Mohamed SG, Yang C (2022) Highly efficient sodium-ion capacitor enabled by mesoporous NaTi2(PO4)3/C anode and hydrogel-derived hierarchical porous activated carbon cathode. J Energy Storage 55:105719
Zheng W, Wu M, Yang C, Tang Z, Hu H (2020) Sn substitution endows NaTi2(PO4)3/C with remarkable sodium storage performances. Ceram Int 46(9):12921–12927
He H, Gan Y, Mu M, Yuan J, Zhang C, Zhang X, Li X, Ma X, Yu H, Mou J, Liu J (2023) Fabrication of nano-solid spherical Nb2O5/nitrogen-doped carbon composite for high-performance sodium-ion battery anodes. J Solid State Electrochem 27(9):2337–2345
Wu S, Bashir T, Zhang Y and Gao L (2023) Scalable single-step synthesis of α-Nb2O5@C@Ti3C2 composite as high power and long life anode material for sodium-ion batteries. J Solid State Electrochem
Yang C, Sun X, Zhang YR, Liu Y, Zhang QA, Yuan CZ (2019) Facile synthesis of hierarchical NaTi2(PO4)3/Ti3C2 nanocomposites with superior sodium storage performance. Mater Lett 236:408–411
Liu R, Liu H, Sheng T, Zheng S, Zhong G, Zheng G, Liang Z, Ortiz G F, Zhao W, Mi J, Yang Y (2018) Novel 3.9 V Layered Na3V3(PO4)4 Cathode material for sodium ion batteries. ACS Appl Energy
Kitajou A, Yamashita M, Kobayashi W, Okada M, Nanami T, Muto S (2022) Anode properties of NaTi2(PO4)3 prepared by adding excess Na2CO3 for aqueous sodium-ion batteries. ACS Appl Energy Mater 1(8):3603–3606
Xu T, Zhao M, Li Z, Su Z, Ren W, Yang S, Pol VG (2022) A high rate and long cycling performance NaTi2(PO4)3 core–shell porous nanosphere anode for aqueous sodium-ion batteries. Energy Technol-ger 10(11):2200970
Wei P, Liu Y, Su Y, Miao L, Huang Y, Liu Y, Qiu Y, Li Y, Zhang X, Xu Y, Sun X, Fang C, Li Q, Han J, Huang Y (2019) F-Doped NaTi2(PO4)3/C Nanocomposite as a high-performance anode for sodium-ion batteries. ACS Appl Mater Interfaces 11(3):3116–3124
Lei P, Li S, Luo D, Huang Y, Tian G, Xiang X (2019) Fabricating a carbon-encapsulated NaTi2(PO4)3 framework as a robust anode material for aqueous sodium-ion batteries. J Electroanal Chem 847:113180
Wu J, Yang L, Liu H, Bu H, Wang W, Zeng C, Zhu S (2022) Effect of Al doping on electrochemical performance of NaTi2(PO4)3/C anode for aqueous sodium ion battery. J Appl Electrochem 52(11):1563–1572
Kavitha KR, Babu BM, Vadivel S, Shkir M, Hakami J, Minnam Reddy VR (2022) Promoting the performances of NaTi2(PO4)3@C porous composite as novel anode materials for application in sodium ion battery. Ceram Int 48(19, Part B):29514–29522
Cao X, Yang Y (2018) Facile synthesis of NaTi2(PO4)3-carbon composite through solid state method and its application in aqueous sodium ion battery. Mater Lett 231:183–186
Nian Z, Zhang J, Du Y, Jiang Z, Chen Z, Li Y, Han C, He Z, Meng W, Dai L, Wang L (2021) Chlorine doping enables NaTi2(PO4)3/C excellent lithium ion storage performance in aqueous lithium ion batteries. J Electroanal Chem 880:114941
Jiang Z, Li Y, Han C, Huang Z, Wu X, He Z, Meng W, Dai L, Wang L (2020) Raising Lithium Storage Performances of NaTi2(PO4)3 by Nitrogen and Sulfur Dual-Doped Carbon Layer. J Electrochem Soc 167(2):020550
Mao W, Zhang S, Cao F, Pan J, Ding Y, Ma C, Li M, Hou Z, Bao K, Qian Y (2020) Synthesis of NaTi2(PO4)3@C microspheres by an in situ process and their electrochemical properties. J Alloy Compd 842:155300
Zhang L, Zhou Y, Li Y, Ma W, Wu P, Zhu X, Wei S, Zhou Y (2022) Achieving in-situ hybridization of NaTi2(PO4)3 and N-doped carbon through a one-pot solid state reaction for high performance sodium-ion batteries. J Solid State Chem 310:123036
Yan H, Zhang B, Fu Y, Wang Y, Dong J, Li Y, Li Z (2022) Gd3+-doped NaTi2(PO4)3@C negative material with superior Na-storage property for sodium-ion batteries. J Alloy Compd 892:162068
He X, Zou Q, Wu L (2021) Zr doping and carbon coating endow NaTi2(PO4)3 electrode with enhanced performances. J Alloy Compd 859:157836
Qu D, Chen Z, Xu G, Liu X, Wei X, Yang L (2020) Mesoporous Mn-doped and carbon-coated NaTi2(PO4)3 nanocrystals as an anode material for improved performance of sodium-ion hybrid capacitors. J Mater Sci-Mater El 31(20):17550–17562
Voronina N, Jo JH, Choi JU, Jo CH, Kim J, Myung ST (2019) Nb-Doped titanium phosphate for sodium storage: electrochemical performance and structural insights. J Mater Chem A 7(10):5748–5759
Yu ZE, Lyu YC, Zou ZY, Su N, He B, Wang SF, Shi SQ, Guo BK (2021) Understanding the structural evolution and storage mechanism of NASICON-structure Mg0.5Ti2(PO4)3 for Li-ion and Na-ion batteries. ACS Sustain Chem Eng 9(40):13414–13423
Zhao Y, Wei Z, Pang Q, Wei Y, Cai Y, Fu Q, Du F, Sarapulova A, Ehrenberg H, Liu B, Chen G (2017) NASICON-Type Mg0.5Ti2(PO4)3 Negative electrode material exhibits different electrochemical energy storage mechanisms in Na-Ion and Li-ion batteries. ACS Appl Mater Interfaces 9(5):4709–4718
Difi S, Saadoune I, Sougrati MT, Hakkou R, Edstrom K, Lippens PE (2015) Mechanisms and performances of Na1.5Fe0.5Ti1.5(PO4)3/C composite as electrode material for Na-ion batteries. J Phys Chem C 119(45):25220–25234
Wang QY, Luo YX, Gu FP, Shui M, Shu J (2020) The preparation, characterization, electro-chemical performance and transporting mechanism of Na1.25Cr0.25Ti1.75(PO4)3/C as anode material for SIBs. Solid State Ion 352
Zhao Y, Zhang P, Liang J, Xia X, Ren L, Song L, Liu W, Sun X (2022) Uncovering sulfur doping effect in MnO2 nanosheets as an efficient cathode for aqueous zinc ion battery. Energy Stor Mater 47:424–433
Deng S, Zhu H, Liu B, Yang L, Wang X, Shen S, Zhang Y, Wang J, Ai C, Ren Y, Liu Q, Lin S, Lu Y, Pan G, Wu J, Xia X, Tu J (2020) Synergy of ion doping and spiral array architecture on Ti2Nb10O29: a new way to achieve high-power electrodes. Adv Funct Mater 30(25):2002665
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (Grants No. BK20201 472) and Changzhou Science and Technology Bureau (CM20223017).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gu, J., Zhang, S., Zhang, X. et al. NaSn0.02Ti1.98(PO4)3/C as a promising anode material with high performance for sodium-ion batteries. J Solid State Electrochem 28, 2093–2101 (2024). https://doi.org/10.1007/s10008-023-05722-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05722-4