Abstract
Transparent thin films of pure ZnO, Ca-doped ZnO (CZO), and Ga-doped ZnO (GZO) were deposited on glass by RF magnetron sputtering. The influence of calcium and gallium concentrations in zinc oxide (ZnO) films on structural, morphology, electrical, and optical properties of thin films were studied. XRD results show that the obtained films were with a hexagonal wurtzite structure and preferentially oriented perpendicular to the substrate surface. Atomic force microscopy (AFM) evidenced that the type of doping modifies the microstructure of thin films. The as-deposited films show a high transmittance in the visible range over 85%. The shift of the optical band gap of ZnO films with increasing Ca and Ga content suggests the enhancement of carrier concentration. At Ga-doped ZnO, the film has lowest resistivity of 3.8 × 10−3 cm, while the carrier concentration is highest (2.2 × 1020 cm−3).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transparent conductive oxide (TCO) thin films have been paid much attention, which can be used as transparent electrodes in various fields such as solar cells and flat panel display devices [1,2,3]. Zinc oxide (ZnO) has recently attracted much attention as a promising alternative material because of it slow material cost and relatively low deposition temperature, besides the optical and electrical properties comparable to those of ITO. In addition, ZnO films have a lower material cost and a lower deposition temperature. These advantages are of considerable interest for practical applications such as transparent electrodes, window material in display, solar cell, and various optoelectronic devices. Compared with undoped ZnO, impurity-doped ZnO has low resistivity and good stability. ZnO thin films, doped with group-III elements such as Al, B, Ga, and In, have been used as transparent and conducting films. This element gallium is the most successful and promising because the atomic radius very similar to zinc (Zn) and the lengths of covalent Ga-O and Zn-O are estimated at 1.92 and 1.97 Å, respectively; doping ZnO by the elements of the third column of the periodic table takes a lot of momentum in photovoltaic devices, because these cells may have an excellent response. Though doping enhances properties of ZnO, it also degrades some other properties. Doping of Al increases optical properties, but high reaction of Ga with oxygen deteriorates its conductivity [4, 5].
For the deposition of doped ZnO films, various techniques have been used such as thermal evaporation [6], chemical vapor deposition [7], RF magnetron sputtering [8, 9], spray pyrolysis [10], pulsed laser deposition [11], and sol–gel process [12]. However, among these techniques, sputtering and sol–gel processes offered much more advantages. Sputtering performing high deposition rate, with no toxic gas emissions, is easy to expand to large-scale glass substrate with no undesired layer formation problems [13], and is low cost and simple method. Sol–gel technology emerged within the last two decades and quickly became one of the most important and promising new material fabrication methods. Up to now, we have fabricated doped and co-doped ZnO thin films by RF magnetron sputtering from nanoparticles as target materials [14, 15]. However, among these techniques, sputtering and sol–gel processes offered much more advantages. Sputtering performs high deposition rate, with no toxic gas emissions, is easy to expand to large-scale glass substrates with no undesired layer formation problems [16], and is low cost and a simple method. On the other hand, sol–gel technique emerged within the last two decades and quickly became one of the most important and promising new material fabrication methods. Indeed, besides the fact that it is a low cost technique, it enables researchers to easily design and fabricate a wide variety of different materials, including the possibility of doping even at high level concentrations at RT. Compared with other techniques, the sol–gel method has many advantages such as low cost, simple synthesis equipment, easy fabrication of large area, easier adjustment of composition, being able to carry out doping at molecular level. Especially, they are suitable for the fabrication of oxide nanoparticles [17]. The preparation of ZnO nanoparticles high quality by the sol–gel method has become a subject of research for a comparison of the structural and optical properties of ZnO nanoparticles such as the crystallite size and the energy of the band gap. The sol–gel method prepares nanoscale particles that can be used as a source for sputtering RF magnetron [18].
In this paper, high-quality Ca-doped and Ga-doped ZnO transparent conductive films prepared by RF magnetron sputtering at low temperature were reported. The structural, electrical, and optical properties of the films were investigated in terms of the preparation conditions.
Experimental details
Pure ZnO, ZnO:Ga (GZO), and ZnO:Ca (CZO) nanopowders were in the first step prepared by a sol–gel method using 16 g of zinc acetate dehydrate [Zn(CH3COO)2.2H2O] as a precursor in 112 ml of methanol. After 20 min of magnetic stirring at room temperature, an adequate quantity of gallium nitrate [GaN3O9] corresponding to [Ga]/[Zn] ratios of 0.03 and of calcium chloride-6-hydrate corresponding to [Ca]/[Zn] ratios of 0.03 was added. After 30 min under magnetic stirring, the solution was placed in an autoclave and dried in the supercritical conditions of ethyl alcohol (EtOH). Then, in the second step, thin films were deposited by RF magnetron sputtering. The RF generator operated at 13.56 MHz. The sputtering chamber was evacuated to a base pressure of 6 × 10−5 Pa before introducing the sputtering argon gas with 99.9999% purity without oxygen. The sputtering targets were prepared from the aerogel powders, which had not been pressed nor sintered. During the sputtering process, the RF power was maintained at 80 W and the target-to-substrate distance was fixed at 75 mm. The substrates were not heated intentionally. The preparation conditions are summarized in Table 1. Before deposing of the ZnO:Ga3% and ZnO:Ca3% thin films, the substrate glass, with 1 mm thick, was ultrasonically cleaned in HCl, rinsed in deionized water, then subsequently in ethanol, and rinsed again. Other details on the experimental procedure used to elaborate our samples were reported in previous study [13,14,15]. The structural, morphological, electrical, and optical properties were studied in this work. The crystal structure of the aerogel powders and the thin films was determined by X-ray diffraction (XRD) using a CoKα radiation (λ = 1.78901 Å). The nanopowders as prepared in the first step were characterized using JEM-200CX transmission electron microscopy (TEM) and characterized by JEOL 5410 type scanning electron microscopy (SEM). The composition studies were done by energy-dispersive X-ray spectroscopy (EDS). The surface morphology was investigated using atomic force microscopy (AFM, Topo Metrix). The thickness of the films was measured using a Tencor profilometer. Electrical resistivity, mobility, and carrier concentration were measured at room temperature by the Van der Pauw method. The optical transmittance was determined using Shimadzu UV-3101 PC spectrophotometer in the wavelength range from 200 to 3000 nm.
Results and discussion
Structure and morphology of the aerogel nanopowder
The XRD patterns of ZnO, Ca-doped ZnO, and Ga-doped ZnO are shown in Fig. 1. Five pronounced ZnO diffraction peaks appear at 2θ = 36.66°, 39.98°, 42.03°, 55.18°, and 66.28°, corresponding to (100), (002), (101), (102), and (110) planes, respectively, which are very close to wurtzite ZnO ones [19]. The obtained XRD diffractogram matched well with the space group P63mc (No. 36–1451) of wurtzite ZnO structure [20]. This result indicates that ZnO:Ga and ZnO:Ca aerogel powder has a polycrystalline hexagonal wurtzite structure. Diffraction lines of ZnO were broad, and diffraction broadening was found to be dependent on Miller indices of the corresponding sets of crystal planes. The average grain size was calculated using Scherer’s formula [21]:
where λ is the X-ray wavelength (1.78901 Å), θ is the maximum of the Bragg diffraction peak (in radians), and β is the full width at half maximum (FWHM) of the XRD peak.
The crystallite sizes of undoped and impurity-doped ZnO were estimated using Scherer’s formula. The average grain size of the basal diameter of the cylinder-shape crystallites varies from 14 to 20 nm, whereas the height of the crystallites varies from 25 to 35 nm. These results are in a good agreement with those obtained by Mahdhi and al. [8].
To further study the morphology of these nanoparticles, TEM measurements combined with high-resolution TEM were employed. Figure 2 shows the TEM images of ZnO, CZO, and GZO nanoparticles. It is obvious that the particles are basically spherical. The TEM images also show that the particle size of undoped ZnO and doped is smaller than 40 nm. In order to confirm the chemical composition of nanoparticles, EDS analysis was performed. The results reported in Table 2 and presented in Fig. 3 confirmed the presence of gallium and calcium in the ZnO matrix with no other impurities. This means that nanoparticles were homogeneous and quasi-stoichiometric.
Structure and surface morphology
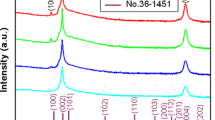
Figure 4 shows the XRD patterns of ZnO thin film and ZnO thin films doped respectively with gallium (ZnO:Ga) and calcium (ZnO:Ca). It is found that the diffraction peaks of each thin film sample match the standard diffraction pattern of ZnO crystal, that is, those as-annealed thin films are polycrystalline with a hexagonal wurtzite type structure. This figure also shows that undoped, Ga-doped, and Ca-doped ZnO thin films have growth oriented along the (002) plane. In addition, the full widths at half-maximum (FWHMs) for diffraction peaks (002) of impurity-doped thin films are wider than that of the undoped thin film. That result indicates that impurity-doped ZnO thin films have a finer crystallite size than the undoped thin film. The crystallite sizes of undoped and impurity-doped ZnO thin films were estimated using Scherer’s formula. Table 3 lists the values of crystallite size for (002) diffraction peaks. The crystallite sizes of undoped, Ga, and Ca doped ZnO thin films were 36 nm, 29.5 nm, and 24 nm, respectively (Table 3). It is found that incorporating dopants into the ZnO films reduced the average crystallite size and that films doped with the group IIA elements (Ca) exhibited finer average crystallite sizes than those doped with group III B elements (Ga).
Stress of thin films is a very important parameter for the practical application. The total stress in thin films commonly consists of two components. One is the intrinsic stress introduced by impurities, defects, and lattice distortions in the crystal, and the other is the extrinsic stress introduced by the lattice mismatch and thermal expansion coefficient mismatch between the film and substrate [22].
where c is the lattice parameter of the ZnO film calculated from (002) peak of XRD pattern and the c0 is the lattice parameter for the ZnO bulk (c0 = 0.52065 nm). The stress in the plane of the films was calculated based on the biaxial strain model, using the following formula [23]:
where C11 = 209.7 GPa, C12 = 121.1 GPa, C13 = 105.1 GPa, and C33 = 210.9 GPa are the elastic stiffness constant of bulk ZnO. The estimated values of stress r in the films grown at different samples are listed in Table 3.
The sputtering power dependence of crystallite sizes for undoped, Ga-doped, and Ca-doped ZnO films was also revealed by the AFM micrographs. Figure 5 shows the surface morphology variation of the films deposited on glass substrates. The film crystallite size depends on doping, and when the deposition begins, there are many nucleation centers on the substrate and small crystallites are produced. The root mean square (RMS) roughness for the thin films, calculated by using the AFM equipment’s software, is found to be 12, 10.20, and 9 nm for undoped, Ga-doped, and Ca-doped ZnO thin films, respectively. Obviously, the RMS values decrease with the impurity.
Optical properties
Figure 6 represents the transmission spectra and band gap plots of pure ZnO, GZO, and CZO thin films. A slight increase in transmittance is observed in the case of GZO film as compared to CZO and pure ZnO thin film, possibly due to scattering of light. All spectra show a high transparency in the visible range with a good transmission of the order of 85%. There is an intrinsic absorption below 320 nm caused by the ZnO semiconductor which leads to a transfer of electron from the valence band to the conduction band. This area is used to determine the energy of the optical gap. The transmission coefficient decreases λ > 1000 nm, and this decrease is due to absorption by free carriers (electrons in the case of ZnO:Ga is n-type) which takes place at the plasma frequency. The optical absorption coefficient (α) is calculated from the transmittance data by [24]:
where t is the thickness of the film and R and T are the reflectance and the transmittance, respectively. In the direct transition semiconductor, the optical absorption coefficient (α) and the optical energy band gap (Eg) are related by [25]:
where B is a constant for direct transition and hν is the energy of the incident photon.
The obtained results were 3.26 eV for the undoped film and 3.33 and 3.64 eV for doped samples CZO and GZO, respectively. The change in the optical band gap can be explained in terms of Burstein–Moss band gap widening [26]. According to the Burstein–Moss effect, the increase in the Fermi level in the conduction band leads to the band gap energy broadening with increasing carrier concentration. The band gap energy determined in this way is not the actual band gap of the deposit. As these are degenerate semiconductors, the Fermi level lies within the conduction band where its position depends on the density of the free electrons.
Our results agree with the literature values for the ZnO thin films [27]. It is reported that pure ZnO film has an optical band gap of 3.25–3.30 eV. We attribute the slightly higher band gap of our doped ZnO films to the Burstein-Moss effect caused by an increased free electron concentration due to the doping [26].
Electrical properties
The variation of the resistivity (ρ), carrier concentration (n), and Hall mobility (μ) of the films undoped, CZO, and GZO, are shown in Fig. 7. All our Hall measurements confirmed the n-type nature of the films and that they exhibited a semiconducting behavior. It is seen from the figure that the resistivity decreased with doping, which is mainly related to the increase of the carrier concentration (n) and of mobility of electrons in the films. The charge carrier concentration, estimated from Hall measurements, increased from 4 × 1019 cm−3 in the case of undoped ZnO thin film up to 3 × 1020 cm−3 for GZO thin film. Gallium doping in ZnO acts as an effective donor due to the contribution from the Ga ions at the substitutional occupancy of Zn ions and also due to the changes in local lattice distortions, deviations in the nature, and symmetry of Zn-O bonds surrounding these dopants; it is clear that the charge carrier density in the GZO thin films is well above the Mott density of ZnO viz. 4.0 × 1018 cm−3 thereby making the semiconductors degenerate. As the incorporation of donors or acceptors shifts the Fermi energy, spontaneous formation of these charged defects acts to compensate the prevailing conductivity in ZnO. The formation energy of Zni across most of the Fermi level range is high making them an unlikely self-compensating species in n-type material.
The electrical conductivity in ZnO:Ga film is higher than that in pure ZnO films due to the contribution of Ga3+ ions on substitutional sites of Zn2+ [28]. A minimum resistivity of 0.38 × 10−2 Ω cm, at this doping, also displayed a higher preferential c-axis orientation, due to the reduction in the scattering of the carriers at the grain boundaries and crystal defects, which increased the apparent carrier mobility [29]. The increase of μ is mainly due to the improvement of crystallinity because the sputtering particles have more sufficient surface diffusion when their energy increased. This conversion can be interpreted by the calcium oxide/gallium oxide-related precipitates which form at the grain boundaries that may act as scattering centers.
Conclusion
Transparent and high conductive undoped, Ga-doped, and Ca-doped ZnO thin films on glass substrates have been obtained at room temperature using RF magnetron sputtering from aerogel nanoparticles prepared by sol–gel technique as a target material. The films crystallize in the hexagonal wurtzite structure and are highly oriented with their crystallographic c-axis perpendicular to the substrate. The height of unit cell slightly increases with doping, with Ca and Ga. Along the c-axis, the compressive stress is differently influenced by the nature of element (Ga or Ca). The decrease of the grain size by doping was attributed to the increasing of nucleation centers leading to the formation of small grains (from XRD and AFM measurements). All films exhibit a transmittance higher than 85% in the visible region and a sharp fundamental absorption edge. The optical band gap of ZnO is smaller than the bulk Eg = 3.26 eV, while the band gap energy of CZO and GZO films deposited is larger than that of the bulk ZnO, which is believed to result from the Burstein–Moss effect. The electrical properties improved with doping. The lowest obtained resistivity was 3.8 × 10−3 Ω cm.
References
Tonooka K, Bando H, Aiura Y (2003) Proceedings of the 3rd international symposium on transparent oxide thin films for electronics and optics. Thin Solid Films 445(2):155–392
Yao KX, Zeng HC (2007) ZnO/PVP nanocomposite spheres with two hemispheres. J Phys Chem C 111(36):13301–13308
Mei ZX, Du XL (2005) Controlled growth of Zn-polar ZnO epitaxial film by nitridation of sapphire substrate. Appl Phys Lett 86(11):112111
Gu XQ, Zhu LP, Cao L, Ye ZZ, He HP, Chu PK (2011) Optical and electrical properties of ZnO:Al thin films synthesized by low-pressure pulsed laser deposition. Mater Sci Semicond Process 14(1):48–51
Lung C, Marconi D, Pop A (2016) Characterization of the aluminum concentration upon the properties of aluminum zinc oxide thin films. Anal Lett 49(8):1278–1288
Minami T, Ida S, Miyata T, Minamino Y (2003) Transparent conducting ZnO thin films deposited by vacuum arc plasma evaporation. Thin Solid Films 445(2):268–273
Prasada Rao T, Santhosh MC (2010) Physical properties of Ga-doped ZnO thin films by spray pyrolysis. J Alloys Compd 502(2):788–793
Mahdhi H, Ben Ayadi Z, Alaya S, Gauffier JL, Djessas K (2014) The effects of dopant concentration and deposition temperature on the structural, optical and electrical properties of Ga-doped ZnO thin films. Superlattice Microst 72:60–71
Mahdhi H, Gauffier JL, Djessas K, Ben Ayadi Z (2017) Structural, optical and electrical properties of transparent conductive ZnO:Ca films prepared by rf magnetron sputtering from aerogel nanopowders. Optik – Int J Light Electron Optics 137:156–166
Bougrine A, El Hichou A, Addou M, Ebothe J, Kachouane A, Troyon M (2003) Structural, optical and cathodoluminescence characteristics of undoped and tin-doped ZnO thin films prepared by spray pyrolysis. Mater Chem Phys 80(2):438–445
Villanueva YY, Ren Liu D, Cheng PT (2006) Pulsed laser deposition of zinc oxide. Thin Solid Films 501(1–2):366–369
Brien SO, Koh LHK, Crean GM (2008) ZnO thin films prepared by a single step sol–gel process. Thin Solid Films 516(7):1391–1395
Mahdhi H, Djessas K, Ben Ayadi Z (2018) Synthesis and characteristics of Ca-doped ZnO thin films by rf magnetron sputtering at low temperature. Mater Lett 214:10–14
Mahdhi H, Ben Ayadi Z, Gauffier JL, Djessas K, Alaya S (2015) Effect of sputtering power on the electrical and optical properties of Ca-doped ZnO thin films sputtered from nanopowders compacted target. Opt Mater 45:97–103
El Mir L, Ben Ayadi Z, Saadoun M, Djessas K, Alaya S (2007) Preparation and characterization of n-type conductive (Al, Co) co-doped ZnO thin films deposited by sputtering from aerogel nanopowders. Appl Surf Sci 254(2):570–573
Aygun G, Yildiz I (2009) Interfacial and structural properties of sputtered HfO2 layers. J Appl Phys 106(1):014312
Bououdina M, Omri K, El-Hilo M, El Amiri A, Lemine OM, El Mir L (2014) Structural and magnetic properties of Mn-doped ZnO nanocrystals. Phys E Low-dimensional Syst Nanostruct 56:107–112
Ben Ayadi Z, El Mir L, Djessas K, Alaya S (2007) Electrical and optical properties of aluminum-doped zinc oxide sputtered from an aerogel nanopowder target. Nanotechnology 18(44):445702
Omri K, Najeh I, El Mir L (2014) Effects of temperature on the optical and electrical properties of ZnO nanoparticles synthesized by sol–gel method. Microelectron Eng 128:53–58
Slama R, Ghribi F, Houas A, El Mir L (2011) Visible photocatalytic properties of vanadium doped zinc oxide aerogel nanopowder. Thin Solid Films 519(17):5792–5795
MCS T, Rao P (2009) Effect of thickness on structural, optical and electrical properties of nanostructured ZnO thin films by spray pyrolysis. Appl Surf Sci 255:4579–4584
Puchert MK, Timbrell PY, Lamb RN (1996) Post deposition annealing of radio frequency magnetron sputtered ZnO films. J Vac Sci Technol A 14(4):2220–2230
Cebulla R, Wendt R, Ellmer K (1998) Al-doped zinc oxide films deposited by simultaneous rf and dc excitation of a magnetron plasma: relationships between plasma parameters and structural and electrical film properties. J Appl Phys 83:1087
Mahdhi H, Ben Ayadi Z, El Mir L, Djessas K, Alaya S (2011) Elaboration and characterization of Ca doped ZnO films prepared by rf-magnetron sputtering at room temperature. J Sens Lett 9(6):2150–2153
Mandal S, Singha RK, Dhar A, Ray SK (2008) Optical and structural characteristics of ZnO thin films grown by rf magnetron sputtering. Mater Res Bull 43(2):244–250
Suchea M, Christoulakis S, Katsarakis N, Kitsopoulos T, Kiriakidis G (2007) Comparative study of zinc oxide and aluminum doped zinc oxide transparent thin films grown by direct current magnetron sputtering. Thin Solid Films 515(16):6562–6566
Song D, Aberle AG, Xia J (2002) Optimization of ZnO: Al films by change of sputter gas pressure for solar cell application. Appl Surf Sci 195(1-4):291–296
I gasaki Y, Saito H (1991) Substrate temperature dependence of electrical properties of ZnO:Al epitaxial films on sapphire (12̄10). J Appl Phys 69(4):2190–2195
Musat V, Teixeira B, Fortunato E, Monteiro RCC, Vilarinho P (2004) Al-doped ZnO thin films by sol–gel method. Surf Coat Technol 180-181:659–662
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Ivo Alexandre Hümmelgen
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahdhi, H., Ayadi, Z.B. & Djessas, K. Physical properties of metal-doped ZnO thin films prepared by RF magnetron sputtering at room temperature. J Solid State Electrochem 23, 3217–3224 (2019). https://doi.org/10.1007/s10008-019-04312-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-019-04312-7