Abstract
Highly efficient, PbS:Hg quantum dot–sensitized, plasmonic solar cells with TiO2 triple-layer photoanode were fabricated by successive ionic layer adsorption and reaction (SILAR) method. These nanostructured photoanodes were characterized by optical and morphological techniques and the solar cells were characterized by optical and electrical techniques. The light absorption by the photoanode was enhanced by effective light scattering process using a triple-layer TiO2 nanostructure, fabricated with a TiO2 nanofiber layer sandwiched between two TiO2 nanoparticle layers. The best plasmon-enhanced quantum dot–sensitized solar cell showed an efficiency of 5.41% with short circuit current density of 18.02 mA cm−2 and open-circuit voltage of 679.83 mV. The overall efficiency and photocurrent density of the Q-dot-sensitized solar cell are enhanced by 15.84% and 38.83% respectively due to the plasmonic effect. The enhanced efficiency appears to be due to the improved short circuit current density by increased light absorption by the triple-layered photoanode nanostructure as well as by the localized surface plasmon resonance (LSPR) effect of the plasmonic gold nanoparticles. This is the first report on plasmon-enhanced, triple-layered TiO2 photoanode sensitized with PbS:Hg Q-dots.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Semiconductor colloidal quantum dots (Q-dots) are among the promising materials for the future electronic and optoelectronic devices including solar cells and detectors [1,2,3,4,5]. During the past decade, they have been intensively studied for numerous applications due to their excellent optoelectronic properties such as the high molar extinction coefficients, tunable energy gap, and ability of multiple exciton generation [6, 7].
Q-dot-sensitized solar cells have emerged as the cost-effective third-generation solar cells in the solar energy conversion processes and also one of the main applications of colloidal Q-dots. TiO2, SnO2, and ZnO semiconductor nanoparticle-based electrodes are used as photoanodes in Q-dot-sensitized solar cells. To enhance the power conversion efficiency of Q-dot-sensitized solar cell, there are several techniques that have been reported. Improving the light harvesting by scattering using modified photoanodes, enhancing the photocurrent further by plasmonic effect, and using an efficient electrolyte and counter electrode are some of these techniques. Enhancement through the efficient light harvesting using nanostructurally modified TiO2 photoanodes based on nanofibers, nanocorals, nanotubes, nanowires, and nanohelixes has been studied [8,9,10,11,12]. Recently, TiO2 nanoparticle/nanofiber/nanoparticle triple-layered photoanode-based solar cells have been reported by us [13,14,15].
The efficiency of solar cells can be enhanced by using the plasmonic nanostructures. These nanostructures are capable of increased light trapping by plasmonic resonance effect. Plasmonic nanostructures can be placed at the top of, within, or at the base of photovoltaic devices. Free electrons on the metal surface can have strong interaction with the light. When the frequency of the incident photons matches with the frequency of these free electrons, it will lead to collective oscillations of the electrons and this oscillation is defined as localized surface plasmon resonance (LSPR) [16]. Plasmonic enhancement in photovoltaic devices occurs due to (i) the LSPR relaxation and re-emission of light acting as a secondary light source that develops the local electric field and (ii) the LSPR relaxation transferring the energy to the conduction band of the semiconductor thereby enhancing the photocurrent [17]. Wavelength of the LSPR depends on the size and shape of the plasmonic particles, inter-particle distance, volume fraction of the metal in the surrounding material, and the dielectric constant of the surrounding material such as TiO2 nanostructure [18]. Plasmonic nanoparticles are highly polarizable at their resonance frequency. At this frequency, these nanoparticles show high optical absorption and high scattering cross-sections. In the case of Au and Ag nanoparticles, the resonance frequencies are in the visible region [19]. Therefore, these plasmonic nanoparticles can be used for enhanced visible light harvesting by LSPR effect.
Metallic nanoparticles such as Au, Ag, and Zn have been studied due to their exceptional optical properties in various fields including photovoltaics. In addition to the metallic nanoparticles, different shapes of metallic plasmonic materials such as nanoclusters, hemisphere, and core-shell sphere can also be used. In dye-sensitized solar cells, metallic nanoparticles have been used to enhance the light trapping [20,21,22,23,24]. PbS/ZnO nanowire bulk-heterojunction Q-dot-sensitized solar cell with plasmonic silver nanocubes has been reported with an overall efficiency of 6.03% [25]. More recently, TiO2/Au nanoparticle-based CdS/CdSe core-shell Q-dot-sensitized solar cells have been fabricated with an efficiency of 6.00% [26]. In another study, Ag plasmonic nanostructure–incorporated TiO2/CdS Q-dot-sensitized solar cell with 6.00% efficiency has been reported [27]. Tokuhisa Kawawaki et al. [28] recently reported a significant efficiency enhancement due to plasmonic gold nanoparticles in solar cells sensitized with PbS quantum dots. PbS quantum dots have gained a great attention in various studies due to their excellent optoelectronic properties. PbS has a high absorption coefficient in the order of 105 cm−1 and wide range of tunable energy gap [29]. Moreover, they have relatively large exciton Bohr radius of 18 nm that allows tuning their band gap in the range from 0.50 to 5.50 eV [30, 31]. According to the quantum confinement effect, by controlling the size of the PbS Q-dots, the absorption wavelength of the first exciton peak can easily be shifted towards the infrared region to harvest the near infrared and infrared photons for the photovoltaic applications.
In this study, Hg-doped PbS Q-dots were deposited using SILAR method on plasmonic Au nanoparticle–incorporated TiO2 tri-layer photoanode structure and the solar cells were fabricated and characterized. Jin-Wook Lee et al. [29] using extended X-ray absorption fine structure (EXAFS) have established the nature and the presence of Hg in the photoanode. Further, it has been revealed that the Pb-S bond distance is decreased by the Hg doping leading to bond reinforcement and reduced structural disorder enhanced electron injection. The overall performance of the solar cells has been enhanced due to the plasmonic effect. To the best of our knowledge, this is the first report on plasmon-enhanced triple-layered TiO2 electrode sensitized with PbS:Hg Q-dots.
Experimental
Materials
Fluorine-doped tin oxide (FTO) coated glass (8 Ω cm−2, Solarnoix), titanium (IV) isopropoxide (97%, Fluka), propan-1-ol (99.9%, Fisher), glacial acetic acid (99%, Fisher), titanium dioxide P-90 powder (Evonik), titanium dioxide powder P-25 (Degussa), sulfur (99%, Daejng), triethanolamine (99%, Fluka), ethanol (96%, BDH) and hydrogen tetrachloroaurate (III) (99.9%), trisodium citrate dihydrate (99%), mercury (II) chloride (99.5%), sodium sulfide hydrate (> 60%), N,N-dimethyl formamide (99%), potassium chloride (99%), lead (II) nitrate (99%), poly ethylene glycol (99.8%), Triton X-100, hydrochloric acid (37%), and methanol (99.8%) all from Sigma-Aldrich were used as received.
Preparation of the Au nanoparticles
Au nanoparticle solution was synthesized using the citrate reduction method as described by Huang et al. [32]. 0.1 g of Na3C6H5O7 was dissolved in 10 ml of de-ionized water and 1 mM of HAuCl4 solution was prepared with 20 ml de-ionized water. This solution was boiled under continuous stirring and 2 ml of Na3C6H5O7 solution was added to the boiling HAuCl4 solution. When the color of the mixture became deep red, the hotplate was turned off and the solution was allowed to cool.
Preparation of the TiO2 triple-layer nanostructure
TiO2 compact layer solution was prepared with 8 ml of ethanol, 1 ml of propan-1-ol, 1 ml of glacial acetic acid, 1 ml of titanium (IV) isopropoxide, and 1 drop of conc. HNO3. This solution was used to spin coat the first TiO2 compact layer on pre-cleaned FTO glass substrate at 3000 rpm for 1 min and the layer was sintered at 120 °C for 5 min. Again, the same spin coating process was repeated to form the second TiO2 compact layer on the first compact layer and both layers were subsequently sintered at 450 °C for 45 min. A paste was prepared by grinding 0.25 g of TiO2 P-90 powder with 1 ml of 0.1 M HNO3 and it was spin-coated on the TiO2 compact bi-layer structure at 3000 rpm for 1 min and the resulting photoanode was subsequently sintered at 450 °C for 45 min. 0.25 g of TiO2 P-25 powder was added to 10 drops of 0.1 M HNO3 and one drop of triton X-100 and the mixture was ground for 15 min. Then, 0.05 g of polyethylene glycol was added to the mixture and appropriate amount of 0.1 M HNO3 was added to the mixture and creamy paste was obtained. In order to study the plasmonic effect, different concentrations of Au nanoparticle colloidal were added and the paste was ground further 15 min to get a homogeneous distribution of Au nanoparticles in the TiO2 P-25 paste. The TiO2 P-25 nanoparticle paste containing Au nanoparticles was spin-coated on TiO2 P-90 layer at 1000 rpm for 1 min for each Au nanoparticle concentration. Finally, the electrodes were sintered for 45 min at 450 °C. The Q-dot-sensitized solar cells fabricated with FTO/compact layer/TiO2 P-90/TiO2 P-25 photoanode incorporating 0.45 ml of Au NP colloidal solution were found to exhibit the highest solar cell efficiency.
TiO2 nanofiber layer was prepared by the following method described by us in an earlier report [13]. Initially, 9.5 ml of N, N-dimethyl formamide and 0.5 ml of glacial acetic acid were thoroughly mixed. Subsequently, 1.5 ml of titanium (IV) isopropoxide was added to the mixture which was subjected to magnetic stirring for 20 min. Finally, 0.75 g of poly (vinylacetate) was added to the mixture and magnetically stirred for 4 h. A TiO2 nanofiber layer was deposited for 20 min with a solution flow rate of 2 ml h−1 on the Au nanoparticle–incorporated TiO2 P-25 layer by electrospinning (NaBond Electrospinner, NaBond Technologies, Hong Kong). During the electrospinning, the voltage difference and the distance between the spinneret and photoanode were kept at 15 kV and 6.5 cm respectively. The electrodes FTO/TiO2 compact layer/TiO2 P-90/TiO2 P-25 covered with Au nanoparticles/TiO2 layer were sintered at 450 °C for 45 min. In order to fabricate the tri-layer photoanode, another Au-incorporated TiO2 P-25 layer was deposited on the TiO2 nanofiber layer using a spin coater with rpm of 1000 for 1 min. Finally, the electrodes were sintered 450 °C for 45 min.
Preparation of PbS:Hg Q-dot-sensitized TiO2 photoanodes
PbS:Hg quantum dots were incorporated to each layer of TiO2 triple-layer photoanode by SILAR method [13]. For the preparation of cationic precursor solution, 0.1 M Pb(NO3)2, 0.8 M triethanolamine, and 6 mM HgCl2 were dissolved in de-ionized water. 0.1 M Na2S was dissolved in de-ionized water for anionic precursor solution. Based on previous trails, 6 SILAR cycles were found to give the highest efficiency solar cells and therefore 6 SILAR cycles were used to deposit PbS:Hg Q-dots on each TiO2 layer in the composite photoanode structure [13]. In each SILAR cycle, TiO2 electrode was dipped for 1 min in the cationic precursor and for 1.5 min in the anionic precursor solution. Between each dipping process, the composite photoanode was washed with de-ionized water. Finally, PbS:Hg Q-dot-sensitized photoanode was sintered at 120 °C for 10 min and immersed in a solution of 0.1 M Na2S for 1 min at room temperature. Then, the photoanode was washed with de-ionized water and dried. A Q-dot-sensitized solar cell with an identical TiO2 triple-layer photoanode nanostructure (nanoparticle/nanofiber/nanoparticle) sensitized with PbS:Hg Q-dots, but without colloidal Au nanoparticles, was also fabricated and used as the control device.
Optical absorption measurements
Optical absorption spectra of Au nanoparticle colloidal, TiO2 triple-layer, TiO2 triple-layer with Au nanoparticles, and PbS:Hg quantum dots were taken using a Shimadzu 2450 spectrophotometer in the 350–1100-nm wavelength range.
Preparation of the polysulfide electrolyte
Polysulfide electrolyte was prepared as described previously [13]. 2 M Na2S, 2 M S, and 0.2 M KCl were dissolved in a 7:3 (v/v) mixture of methanol and water. The mixture was magnetically stirred at room temperature until the solution became clear deep-orange color.
Preparation of the counter electrode
A brass plate of size 2 cm × 1 cm was cleaned with concentrated HCl at 80 °C. A mask with 0.12 cm2 hole was fixed on the cleaned brass plate and the unmasked area was covered with the polysulfide electrolyte. The Cu2S layer formed on this brass plate was used as a counter electrode.
Current–voltage characterization
Current–voltage characterization of each type of PbS:Hg Q-dot-sensitized solar cells with an active area of 0.12 cm2 was measured under illumination of 100 mW cm−2 with AM 1.5 filter using a computer-controlled setup consisting of a multi-meter (Keithley 2000) connected to a potentiostat/galvanostat unit (HA-301).
Electrochemical impedance measurements
Electrochemical impedance spectroscopy (EIS) provides an important tool to study the interfaces between electrodes and electrolytes. EIS spectra of all Q-dot-sensitized solar cells were collected by Autolab potentiostat/galvanostat PGSTAT128 N with frequency response analyzer (Metrohm) in a frequency range between 0.01 Hz and 1 MHz under the simulated light of 100 mW cm−2 with AM 1.5 filter. Important electrochemical parameters such as charge transfer resistance, series resistance, recombination resistance, and electron lifetime were estimated using the fitted equivalent circuit.
Results and discussion
Morphology of the TiO2 triple-layer photoanode
The cross-section SEM image of the TiO2 triple-layer (nanoparticle/nanofiber/nanoparticle) composite structure is shown in Fig. 1. Total thickness of the TiO2 triple-layer is around 3.4 μm. The detailed configuration of the composite photoanode can be represented as follows: glass substrate with FTO layer/TiO2 compact layer/TiO2 P-90 nanoparticle layer (0.6 μm)/TiO2 P-25 nanoparticle layer (1.3 μm)/TiO2 nanofiber layer (0.8 μm)/TiO2 P-25 nanoparticle layer (1.3 μm).
Optical absorption of Au nanoparticles and photoanodes
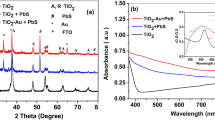
Figure 2 depicts the optical absorption spectrum of synthesized plasmonic colloidal Au nanoparticles showing a broad absorption in the visible region which peaks around 527 nm. From this absorption maximum, the average particle size of the Au nanoparticles has been estimated, which is in the range of 25–35 nm and the shape of the particles is spherical [33]. Stephan Link et al. [34] reported that, Au nanoparticles which have the diameter greater than 25 nm, the plasmon bandwidth increases with increasing size as the wavelength of the interacting light becomes comparable with the dimension of the nanoparticle. And also, the extinction coefficient depends on the size of the nanoparticle.
Figure 3 displays the optical absorption spectra of the TiO2 triple-layer and Au nanoparticle–incorporated TiO2 triple-layer with and without sensitization by PbS:Hg quantum dots. Bare TiO2 nanoparticle/nanofiber/nanoparticle nanostructure also shows increased optical absorption in the visible region (Fig. 3, curve (b)), quite likely due to the multiple light scattering events within the triple-layer. Au nanoparticle–incorporated TiO2 triple-layer photoanode shows a broad peak in the visible region between 500 and 550 nm (Fig. 3, curve (c)). This peak clearly confirms the presence of Au plasmonic nanoparticles in the TiO2 triple-layer nanostructure as described by Yin-Cheng Yen et al. [2]. The plasmonic absorption peak due to synthesized colloidal Au nanoparticles appears around 527 nm (Fig. 2). Correspondingly, the PbS:Hg Q-dot-sensitized, Au nanoparticle–incorporated TiO2 triple-layer photoanode shows a broad peak around 500–550 nm superimposed on an enhanced overall absorption curve (Fig. 3(d)). The cumulative effect due to the presence of TiO2 nanofibers (scattering enhanced), Au nanoparticles (plasmonic enhanced), and PbS:Hg Q-dots sensitized in the sandwich structure has resulted an overall increase in the optical absorption significantly. Also, this photoanode exhibits another very strong absorption peak at around 1050 nm in the near IR region evidently due to the optical absorption by PbS:Hg Q-dots [35]. The size of the PbS:Hg quantum dots corresponding to the absorption of 1050 nm can been estimated to be in the range of 3–4 nm [31, 35].
Figure 4 depicts the plots of (Ahν)2 against (hν) for the TiO2 triple-layer structure with and without Au plasmonic nanoparticles. Here, A is the absorption coefficient and ν is the frequency. According to these plots, the estimated value of the optical energy band gap for the TiO2 triple-layer electrode is 3.42 eV and for the Au nanoparticle–incorporated TiO2 triple-layer electrode is 3.04 eV. This result clearly shows that the energy band gap of the TiO2 semiconductor nanostructure has reduced by the defect-induced band gap narrowing caused by the presence of Au plasmonic nanoparticles. Similar observations have been made by other groups too [22, 36, 37].
Photovoltaic characteristics of the solar cells
Current density (J) vs voltage (V) measurements have been performed to obtain the photovoltaic parameters of solar cells. Figure 5 displays the J-V plots for the PbS:Hg Q-dot-sensitized solar cells with TiO2 triple-layer photoanodes with and without Au nanoparticles under the simulated sunlight of 100 mW cm−2 with AM 1.5 spectral filter. Au nanoparticle–incorporated PbS:Hg Q-dot-sensitized solar cells show improved photovoltaic performance compared with the controlled device. The results are summarized in Table 1.
The overall efficiency of the cell is enhanced by 15.84% due to the Au plasmonic nanoparticles. As seen from Fig. 5, this is evidently due to the enhanced photocurrent in the Q-dot-sensitized solar cell with Au nanoparticles, compared with device without Au nanoparticles, under similar fabrication and light conditions caused by the localized surface plasmon resonance (LSPR) effect [19, 38]. Au nanoparticle–incorporated Q-dot-sensitized solar cell gives a significantly higher photocurrent density of 18.02 mA cm−2 while the controlled device shows a lower value of 12.98 mA cm−2. Clearly, the photocurrent density is enhanced by about 38.83% by the plasmonic effect due to Au nanoparticles.
Table 2 shows the variation of efficiency of the Q-dot-sensitized solar cell with the amount of colloidal Au nanoparticles. Optimum amount of colloidal Au nanoparticles added to the TiO2 P-25 nanoparticle paste is around 0.45 ml which gives a highest overall efficiency of 5.41% corresponding to the amount of 0.45 ml added to the TiO2 P-25 nanoparticle colloidal paste then decreases.
Electrochemical impedance spectra
In order to estimate and compare the charge transfer resistance, series resistance, and recombination resistance of the Q-dot-sensitized solar cells, electrochemical impedance spectra of the Au plasmonic Q-dot-sensitized solar cell and the controlled Q-dot-sensitized solar cell were analyzed using the most fitting equivalent circuit for the Q-dot-sensitized solar cell. Figure 6 exhibits the corresponding Nyquist plots and the equivalent circuit used for the analysis. In this equivalent circuit, Rs is the series resistance of the FTO/TiO2 interface and R1CT represents the resistance of the counter electrode/electrolyte interface. R2CT represents the resistance of the photoanode/electrolyte interface which is generally known as the “recombination resistance.” CPE1 and CPE2 represent the constant phase elements related to the interfaces. Zw refers to the finite Warburg impedance which originates from the difference of the diffusion coefficients of the positive and negative ions, and from the non-blocking character of the electrodes [39]. Figure 6 clearly shows that the series resistance of the Q-dot-sensitized solar cell is reduced due to the plasmonic effect.
Estimated electrochemical impedance parameters of the interfaces are given in Table 3. Au plasmonic nanoparticle–incorporated PbS:Hg Q-dot-sensitized solar cell shows low series and charge transfer resistances than the controlled Q-dot-sensitized solar cell. It is clear that the electron injection to the conduction band of the TiO2 and the charge transfer has been enhanced by the plasmonic Au nanoparticles. Plasmonic enhanced Q-dot-sensitized solar cell shows a high recombination resistance of 188.2 Ω compared with the controlled cell. Due to the increase in the recombination resistance, it is difficult for the photogenerated electrons to recombine with holes in the electrolyte. This results in the decrease of charge recombination and enhances the photocurrent [40].
Warburg impedance Zw gives the characteristics of the diffusing species. In this study, S2− and \( {S}_n^{2-} \)are the species in redox couple. Zw values of Au plasmonic nanoparticle–incorporated PbS:Hg Q-dot-sensitized solar cell and controlled Q-dot-sensitized solar cell are 9.92 Ω and 11.26 Ω respectively. Au plasmonic nanoparticle–incorporated PbS:Hg Q-dot-sensitized solar cell shows a lower value of Zw; this shows the better diffusion of electrolyte. Due to the better electrolyte diffusion, electron transport is enhanced and the performance of the Q-dot-sensitized solar cell is enhanced as described in Hee-Je Kim et al. [41].
Figure 7 shows the Bode plate of the PbS:Hg Q-dot-sensitized solar cell. The frequency peak of the Au nanoparticle–incorporated Q-dot-sensitized solar cell shifted to lower frequency. Lifetime of the electrons in the TiO2 nanostructure can be calculated from Eq. (1). The electron lifetime is directly proportional to the recombination resistance [40].
where fmax is the maximum frequency of the middle frequency peak in the Bode plot
Table 4 shows the comparison of calculated electron lifetime and short circuit current density of each Q-dot-sensitized solar cell. Plasmon-enhanced PbS:Hg Q-dot-sensitized solar cell shows a high electron lifetime of 5.12 ms than the controlled cell. Lifetime of the electron is enhanced by 4.6 times. Therefore, electrons have a longer lifetime and are effectively transferred, substantially enhancing the photocurrent and the efficiency as discussed by Dinah Punnoose et al. [42], Yen et al. [43] and Jianjun Tian et al. [40].
Conclusion
PbS:Hg colloidal quantum dot–sensitized solar cells have been fabricated using TiO2 triple-layer photoanode nanostructure, nanoparticle/nanofiber/nanoparticle layers. Au plasmonic nanoparticles have been incorporated into the two nanoparticle layers. These Q-dot-sensitized, plasmonic solar cells show a significantly higher efficiency of 5.41% compared with the control device without Au nanoparticles. The enhancement is evidently due to the increased short circuit photocurrent by localized surface plasmon resonance effect and defect-induced energy band gap narrowing of TiO2 by Au nanoparticles.
References
Yuan M, Liu M, Sargent EH (2016) Colloidal quantum dot solids for solution-processed solar cellsColloidal quantum dot solids for solution-processed solar cells. J Nature Energy 1:16016
Carey GH, Abdelhady AL, Ning Z, Thon SM, Baker OM, Sargent EH (2015) Colloidal quantum dot solar cells. J Chem Rev 11:12732–12763
Kim MR, Ma D (2015) Quantum-dot-based solar cells: recent advances, strategies, and challenges. J Phys Chem Lett 6:85–99
Mc Donald SA, Konstantatos G, Zhang S, Cyr PW, Klem EJD, Levina L, Sargent EH (2005) Solution-processed PbS quantum dot infrared photodetectors and photovoltaics. Nature materials 4:138–142
Sargent EH (2008) Solution-processed infrared optoelectronics: photovoltaics, sensors, and sources. IEEE J Sel Top Quantum Electron 14:1223–1229
Tian J, Cao G (2013) Semiconductor quantum dot-sensitized solar cells. Nano Reviews 4:22578–22586
Chen G, Seo J, Yang C, Prasad PN (2013) Nanochemistry and nanomaterials for photovoltaics. J Chem Soc Rev 42:8304–8338
Li Y, Wei L, Chen X, Zhang R, Sui X, Chen Y, Jiao J, Mei L (2013) Efficient PbS/CdS co-sensitized solar cells based, on TiO2 nanorod arrays. Nanoscale Res Lett 8:67–74
Mali SS, Desai SK, Kalagi SS, Betty CA, Bhosale PN, Devan RS, Ma YR, Patil PS (2012) PbS quantum dot sensitized anatase TiO2 nanocorals for quantum dot-sensitized solar cell applications. Dalton Trans 41:6130–6136
Xu F, Benavides J, Ma X, Cloutier SG (2012) Interconnected TiO2 nanowire networks for PbS quantum dot solar cell applications. J Nanotechnology 9:709031–709037
Ratanatawanate C, Xiong C, Balkus KJ (2008) Fabrication of PbS quantum dot doped TiO2 nanotubes. ACS Nano 2:1682–1688
Lee SH, Jin H, Kim DY, Song K, Oh SH, Kim S, Schubert EF, Kim JK (2014) Enhanced power conversion efficiency of quantum dot sensitized solar cells with near single-crystalline TiO2 nanohelixes used as photoanodes. Optical Express 22:867–879
Dissanayake MAKL, Jaseetharan T, Seenadeera GKR, Thotawatthage AC (2018) A novel, PbS:Hg quantum dot-sensitized, highly efficient solar cell structure with triple layered TiO2 photoanode. J Electrochimica Acta 269:172–179
Dissanayake MAKL, Sarangika HNM, Seenadeera GKR, Divarathna HKDWMNR, Ekanayake EMPC (2017) Application of a nanostructured, tri-layer TiO2 photoanode for efficiency enhancement in quasi-solid electrolyte-based dye- sensitized solar cells. J Appl Electrochem 47:1239–1249
Dissanayake MAKL, Divarathna HKDWMNR, Dissanayake CB, Seenadeera GKR, Ekanayake PMPC, Thotawattage CA (2016) An innovative TiO2 nanoparticle/ nanofibre/ nanoparticle, three layer, composite photoanode for efficiency enhancement in dye-sensitized solar cells. J Photochem and Photobio A: Chemistry 323:110–118
Ye W, Long R, Huang H, Xiong Y (2017) Plasmonic nanostructures in solar energy conversion. J Mater Chem 5:1008–1021
Erwin WR, Zarick HF, Talbert EM, Bardhan R (2016) Light trapping in mesoporous solar cells with plasmonic nanostructures. J Energy Env Sci 9:1577–1601
Mathpal MC, Kumar P, Tripathi AK, Balasubramaniyan R, Kumar Singh M, Chung JS, Agarwal A (2015) Facile deposition and plasmonic resonance of Ag–Au nanoparticles in titania thin film. New J Chem 39:6522–6530
Smith JG, Faucheaux JA, Jain PK (2015) Plasmon resonances for solar energy harvesting: A mechanistic outlook. Nano Today 10:67–80
Luan X, Wang Y (2014) Plasmon-enhanced performance of dye-sensitized solar cells based on electrodeposited Ag nanoparticles. J Mater Sci Tech 30:1–7
Standridge SD, Schatz GC, Hupp JT (2009) Distance dependence of plasmon - enhanced photocurrent in dye-sensitized, solar cells. J Am Chem Soc 131:8407–8409
Dissanayake MAKL, Kumari JMKW, Senadeera GKR, Thotawatthage CA (2016) Efficiency enhancement in plasmonic dye-sensitized solar cells, with TiO2 photoanodes incorporating gold and silver, nanoparticles. J Appl Electrochem 46:47–58
Muduli S, Game O, Dhas V, Vijayamohanan K, Bogle KA, Valanoor N, Ogale SB (2012) TiO2–Au plasmonic nanocomposite for enhanced dye-sensitized solar cell (DSSC) performance. J Solar Energy 86:1428–1434
Chou CH, Yang RY, Yeh CK, Lin YJ (2009) Preparation of TiO2/nano-metal composite particles and their applications in dye-sensitized solar cells. J Powder Technology 194:95–105
Kawawaki T, Wang H, Kubo T, Saito K, Nakazaki J, Segawa H, Tatsuma T (2015) Efficiency enhancement of PbS, quantum dot/ZnO nanowire bulk-heterojunction solar cells by plasmonic silver nanocubes. ACS Nano 9:4165–1472
Wang Y, Zhang Q, Huang F, Li Z, Zheng YZ, Tao X, Cao G (2018) In situ assembly of well-defined Au nanoparticles in TiO2 films for Plasmon - enhanced quantum dot sensitized solar cells. J Nano energy 44:135–143
Naresh Kumar P, Deepa M, Srivastava AK (2015) Ag plasmonic nanostructures and a novel gel electrolyte in a high efficiency TiO2/CdS solar cell. J Phy Chem Chem Phy 17:10040–10052
Kawawaki T, Tatsuma T (2013) Enhancement of PbS quantum dot-sensitized photocurrents using plasmonic gold nanoparticles. J Phy Chem Chem Phy 15:20247–20251
Lee JW, Son DY, Ahn AK, Shi HW, Kim IY, Hwang SJ, Ko MJ, Su S, Han H, Park NG (2013) Quantum dot-sensitized solar cell with unprecedentedly high photocurrent. Sci Rep 3:1050
Azpiroz JM, Ugalde JM, Etgar L, Infante I, De Angelis F (2013) The Effect of TiO2 morphology on the electron injection efficiency in PbS quantum dot solar cells: a first-principles study. J Phy Chem Chem Phy 17:6076–6086
Tang J, Sargent EH (2011) Infrared colloidal quantum dots for photovoltaics: Fundamentals and recent progress. J Adv Mater 23:12–29
Huang H, Yang X (2003) Chitosan mediated assembly of gold nanoparticles multilayer. Colloids and Surfaces A Physicochem Eng Aspects 226:77–86
Link S, El-Sayed MA (1999) Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J Phy Chem B 103:4212–4217
Link S, El – Sayed MA (1999) Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J Phy Chem B 103:8410–8426
Hyun BR, Zhong YW, Bartnik AC, Sun L, Abrun HD, Wise FW, Goodreau JD, Matthews JM, Leslie TM, Borrelli NF (2008) Plasmon - enhanced photocurrent using gold nanoparticles on a three-dimensional TiO2 nanowire - web electrode. Acs Nano 2:2206–2212
Ansari SA, Khan MM, Ansari MO, Cho MH (2015) Gold nanoparticles-sensitized wide and narrow band gap TiO2 for visible light applications: A comparative study. New J Chem 39:4708–4815
Zolanvari A, Sadeghi H, Norouzi R, Ranjgar A (2013) Surface plasmons and optical properties of TiO2/X (X=Au and Ag) nanostructure thin films. Chin Phy Lett 30:096201
Gonfa BA, Kim MR, Zheng P, Cushing S, Qiao Q, Nianqiang W, Khakania MAE, Ma D (2016) Investigation of plasmonic effect in air-processed PbS/CdS core-shell quantum dot based solar cells. J Mater Chem A 4:13071–13080
Barbero G (2017) Theoretical interpretation of Warburg’s impedance in unsupported electrolytic cells. J Phy Chem Chem Phy 19:32575–32579
Tian J, Lv L, Fei C, Wang Y, Liu X, Cao G (2014) A highly efficient (>6%) Cd1-xMnxSe quantum dot sensitized solar cell. J Mater Chem A 2:19653–19659
Kim HJ, Kim DJ, Rao SS, Savariraj AD, Soo-Kyoung K, Son MK, Gopi CVVM, Prabakar K (2014) Highly efficient solution processed nanorice structured NiS counter electrode for quantum dot sensitized solar cells. Electrochimica Acta 127:427–432
Punnoose D, Rao SS, Kim SK, Kim HJ (2015) Exploring the effect of manganese in lead sulfide quantum dot sensitized solar cell to enhance the photovoltaic performance. RSC Adv 5:33136–33145
Yen YC, Chen JA, Ou U, Chen YS, Lin KJ (2017) Plasmon - enhanced photocurrent using gold nanoparticles on a three-dimensional TiO2 nanowire - web electrode. Sci Rep 7:42524
Acknowledgments
The authors would like to thank the Department of Physics at University of Jaffna, Sri Lanka, for providing facilities for optical absorption measurements.
Funding
This research was financially supported by the South Eastern University of Sri Lanka and Postgraduate Research Scholarship Award (NSF/SCH/2018/4) by the National Science Foundation, Sri Lanka.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Gold nanoparticle–incorporated, Hg-PbS quantum dot–sensitized photoanode was made.
• DSSCs fabricated with above photoanode showed an efficiency of 5.41%.
• Efficiency enhancement of 38.8% was achieved by a plasmonic resonance effect.
Rights and permissions
About this article
Cite this article
Dissanayake, M., Jaseetharan, T., Senadeera, G. et al. Highly efficient, PbS:Hg quantum dot–sensitized, plasmonic solar cells with TiO2 triple-layer photoanode. J Solid State Electrochem 23, 1787–1794 (2019). https://doi.org/10.1007/s10008-019-04280-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-019-04280-y