Abstract
In this work, the synthesised octadentate ligand immobilised multi-walled carbon nanotubes (MWCNTs) modified electrode as an electrochemical sensor of Hg2+ is reported. The octadentate/MWCNTs composites were coated on the polished surface of paraffin impregnated graphite electrode for fabricating the enhanced electrochemical sensing platform for Hg2+ determination. The octadentate ligand contains four N and four O donor atoms which coordinate with the metal ion in stripping medium have been investigated. Surface morphology of the fabricated modified electrode was studied using scanning electron microscope (SEM). The modified electrode was characterised by electrochemical impedance spectroscopy (EIS) and square wave anodic stripping voltammetry (SWASV). Further various factors such as preconcentration time, effects of pH and different electrolytes were optimised for the detection of Hg2+. Under the optimised condition, anodic stripping voltammetry of Hg2+ showed a response in a linear range from 2.4 - 180 nM and the limit of detection was 0.8 nM for Hg2+ (S/N = 3). Interference studies with Cd2+,As3+,Cu2+, Ag+,Ni2+,Fe3+,Zn2+,Sn2+ and Pb2+ showed an insignificant effect on the electrochemical response of Hg2+. The proposed modified electrode exhibited an excellent performance with good reproducibility, selectivity and stability. The practical application of the modified electrode was also evaluated by the detection of Hg2+ in well water and lake water samples with good recovery results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal ions (Hg2+, Pb2+, Cd2+ and As3+) are hazardous substances in the environmental, pharmaceutical and biomedical analysis [1, 2]. Among them, the accumulation of Hg2+ in the human body which damages the central nervous system, kidney, liver and some important cell functions is inactivated which leads to a wide variety of disease. Heavy metal ions are used in industries for manufacturing batteries, thermometers, cathodic tubes, mercury vapour lamps and other electronic productions [3, 4]. The waste from the above industries is source of metal contamination, and hence, it will be beneficial if selective method is available for determination of mercury in the environment.
Schiff bases are usually made by the reaction of an amine and aldehyde group, and sometimes it possesses the fluorescence properties with biological activity [5]. The Schiff base compounds are very reactive towards metal ions and are considered as excellent chelating agents. 1,2,4,5-tetrasalicylideneaminobenzene (TSAB) is a ligand prepared by condensation of salicylaldehyde and 3, 3′-diaminobenzidine. This ligand is a symmetrical molecule and has chiral properties.
The advantage of multi-walled carbon nanotubes (MWCNTs) has received a great attention due to its good stability on the electrode, chemical properties and wide potential range [6,7,8]. In electroanalytical methods, the presence of nanomaterials on the surface of the electrode has enhanced the sensitivity for determination of analytes in stripping analysis [9,10,11,12]. Numerous spectroscopy methods were used for trace analysis of mercury such as atomic absorption spectroscopy, inductively coupled plasma–atomic emission spectroscopy (ICP-AES) and inductively coupled plasma–mass spectrometry (ICP-MS) [13,14,15,16,17]. Unfortunately, these spectroscopy methods are more expensive, high operating cost, high labour and analytical cost [18, 19]. On the other hand, electroanalytical techniques are the cheapest and highly sensitive methods for determination of mercury. Therefore, electroanalytical techniques including stripping voltammetry are relatively widespread. The major advantages of anodic stripping voltammetry includes simplicity of the equipment, inexpensiveness, less electrical power consumption, more sensitivity and low detection limit. One of the disadvantages with the anodic stripping voltammetry for the metal ion determination with mercury electrode is the toxicity associated with mercury to the human system [20,21,22] and mercury-free electrodes are the need of the hour.
In this study, we have demonstrated the use of MWCNT/octadentate TSAB ligand to modify the graphite electrode for the electrochemical determination of Hg2+. The synthesised TSAB is an octadentate Schiff base ligand and is shown in Scheme 1. The complex formation reaction and voltammetry behaviour of Hg2+ at TSAB/MWCNT modified electrode are reported for the first time. The TSAB/MWCNT modified electrode has enhanced the sensitivity of determination of Hg2+ in seawater and lake water. The stripping voltammetric results of real samples were compared with atomic absorption spectrometry (AAS) and the details are discussed in this paper.
Experimental
Chemical
MWCNT (size 110 nm and purity ≥ 90%) and graphite electrode (3 mm Dia) was purchased from Sigma-Aldrich. Salicylaldehyde and 1,2,4,5-tetraaminobenzene tetrahydrochloride, mercury acetate and sodium acetate were purchased from SRL, India. Other chemicals were used for analytical grade.
Apparatus
Scanning electron microscopy (SEM) was performed with Hitachi instrument (S-3400 N). Voltammetric determinations were carried out with a CHI 660 B electrochemical workstation (CHI instruments, USA). A conventional three-electrode system, consisting of a TSAB/MWCNT working electrode, a saturated calomel reference electrode (SCE) and a platinum wire counter electrode, was employed.
Synthesis of TSAB ligand
The ligand was prepared by the reported procedure [23] with a small reconstruction. Sodium methoxide was added to a stirred suspension of the 1,2,4,5-tetraaminobenzene tetrahydrochloride (0.93 g, 3.3 mmol) in 50 mL dry methanol under nitrogen atmosphere until reaction was complete, which resulted in a yellow solution. Salicylaldehyde (1.61 g, 13.2 mmol) was then immediately added dropwise to the warm solution. The mixture was refluxed overnight and the orange solid obtained was filtered, washed several times with methanol and ether and air-dried yield (90%, mp 270–290 °C).
Fabrication of modified electrode
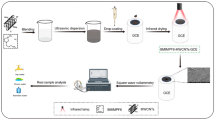
Paraffin wax-impregnated graphite electrodes (PIGE) have been used for electrode modification. The spectroscopic grade graphite rods have been impregnated with paraffin wax procedure developed by Scholz et al. [24]. Briefly, the graphite rods with the diameter of 3 mm and 4 cm length were put into the paraffin under a vacuum and kept until no more bubbles evolve from the rod. After that, the graphite rods were stripped and the excess paraffin from the surface of electrodes was cleaned. The one end of the electrode surface was polished with emery and smooth paper to get a mirror-like finish and rinsed with distilled water. The impregnation of graphite with paraffin helps to fill all the pores and in order to prevent the high background currents and spoiling of the electrode ingression of electrolyte or analyte solution [25]. Thus, we have chosen PIGE for the base electrode for modification. First, 0.1 mg MWCNT dispersion was prepared by dissolving 0.1 mg of MWCNT material in 1 mL of ethanol and sonicated for 15 min. Then, 10 μL of MWCNT suspension was coated on the bare electrode surface and allowed to dry for 10 min. Afterwards, the TSAB ligand was dissolved in 1 mL of acetone and 10 μL of the 1 mmol ligand was drop cast on the MWCNT electrode and dried in air for 5 min.
Voltammetric determination for Hg2+ ion
The voltammetric determination for Hg2+ procedure consisted of preconcentration, deposition potential, stripping and regeneration steps. In preconcentration steps, the modified electrode was dipped in a 0.1-M NaNO3 solution which contained 30 nM of Hg2+ stirred for 180 s such that the ligand on the electrode surface forms complexes. Afterwards, the TSAB/MWCNT electrode was then removed and transferred into a fresh background electrolyte of 0.1 M NaNO3. A negative potential of − 0.1 V was applied for 60 s so that the metal ion was reduced on the electrode surface. The metal ion on the electrode surface was stripped by scanning the potential from − 0.1 to 0.7 V. Then, the electrode was regenerated by immersing the electrode in 0.01 M EDTA solution (pH 4.5) as Hg2+ was removed from the electrode as water soluble Hg2+-EDTA complex and the electrode was washed with distilled water.
Results and discussion
Characterisation of MWCNT/TSAB ligand
The synthesised ligand was characterised by Fourier transform infrared (FT-IR) spectroscopy and nuclear magnetic resonance (NMR) spectroscopy. The modified electrode was characterised by SEM with energy dispersive x-ray analysis (EDAX) analysis, electrochemical impedance spectroscopy (EIS) spectroscopy and square wave anodic stripping voltammetry (SWASV). Fig. S1 shows the FT-IR spectrum of the ligand. The strong absorption band appears at 3315 cm−1 which could be assigned to be presence of OH groups. The presence of C=N and C–O groups were observed at 1611 and 1210 cm−1, respectively. H1-NMR spectra data of TSAB ligand are shown in Fig. S2. A sharp singlet peak is observed at 8.016 ppm were assigned to CH=N proton and OH proton of four phenolic group is observed at 4.82 ppm. The aromatic protons show peaks at 6.553-7.993 ppm. All the spectroscopic results are in good agreement with the reported values [23].
Electrochemical characterisation of TSAB/MWCNT
The surface bound feature of the bare electrode, MWCNT and TSAB/MWCNT ligand modified electrodes in 1 mM [Fe(CN)6]3−/4− solution was investigated by cyclic voltammetry (CV) and EIS as shown in Fig. 1.
Fig. 1(A) show the CV of bare electrode, MWCNT and TSAB/MWCNT modified electrodes in 1 mM of [Fe (CN) 6]3−/4−. The figure shows that the TSAB/MWCNT electrode exhibited higher anodic peak (Ip) and lower cathodic peak (Epc) compared to the bare electrode and MWCNT electrode. The electroactive surface area was estimated using Randles-Sevcik Eq. (1).
where A is the area of the electrode (cm2), D is the diffusion coefficient of the molecule in solution (cm2/s), C is the concentration of the molecule in the bulk solution (mol/cm3) and ν is the scan rate (V/S) for 1 mM of [Fe(CN)6]3−/4− in 0.1 M acetate buffer solution (ABS). The electroactive surface area of the bare electrode, MWCNT electrode and TSAB/MWCNT modified electrode demonstrated in the present work was calculated to be 0.012, 0.052 and 0.082 cm2, respectively.
In Fig. 1(B), its shows the Nyquist plots of the electrochemical impedance study for the bare (PIGE) electrode (curve a), MWCNT electrode (curve b) and TSAB/MWCNT modified electrode (curve c) in 1 mM [Fe(CN)6]3−/4− solution. The modified Randle’s equivalent circuit is given as an inset in Fig. 1(B). The resistance in solution (Rs) for the bare electrode was 52.36 Ω, for the MWCNT electrode was 38.16 Ω and for the TSAB/MWCNT electrode was 23.4 Ω. The charge transfer resistance (Rct) values observed were 7490 Ω for the bare electrode, 917.2 Ω for the MWCNT electrode and 94.83 Ω for the TSAB/MWCNT electrode.
The conductivity of the MWCNT electrode and TSAB/MWCNT electrode is given in Eq. (2)
where l is the length of the electrode, A is the surface area of the electrode and R is the conductance of the MWCNT electrode and MWCNT/TSAB ligand electrode. By applying Eq. (2), it shows that the electrode conductance of the bare electrode is 17.7 × 10−4 S cm−1, of the MWCNT electrode is 14.5 × 10−4 S cm−1 and of the TSAB/MWCNT ligand electrode is 140 × 10−4 S cm−1. From the equation, it was conformed that the TSAB/MWCNT electrode conductivity is higher than that of the MWCNT electrode.
The phase degree was calculated by using Eq. (3)
From the equation, the phase degree (ϕ) for the bare electrode was 44.4°, for the MWCNT electrode was 33.8° and for the TSAB/MWCNT was estimated to be 28.0°. According to Eq. (2), the phase angle (ϕ) is directly proportional to (Rct), respectively, so that the phase angles decrease at the TSAB/MWCNT ligand modified electrode and it has enhanced the electron transfer kinetics at the electrode interface. Therefore, this TSAB/MWCNT ligand modified electrode was suitable for stripping analysis.
SEM with EDAX image for different electrodes
The surface morphology of the bare electrode, MWCNT electrode, TSAB electrode, MWCNT/TSAB ligand modified electrodes and MWCNT/[TSAB-Hg2+] metal complex was studied by SEM with EDAX analysis, and the image obtained is shown in Fig. 2. The bare electrode has a smooth surface as shown in Fig. 2(A) and the EDAX image showed that the carbon element peak present in Fig. 2(B) and TSAB/MWCNT ligand is like the rod structure in Fig. 2(E), and the EDAX image showed the presence of carbon and oxygen peak in Fig. 2(F). The MWCNT modified electrode structure shows flexible surface (Fig. 2(C)) and the EDAX image for the MWCNT electrode showed an increase in carbon element peak (Fig. 2(D)) compared with the bare electrode. The preconcentration of Hg2+ on the modified electrode (metal complex) shows the needle-shape structure (Fig. 2(G)), and in the EDAX image, the results showed that the presence of Hg2+ peak (Fig. 2(H)) on the surface of MWCNT/TSAB ligand electrode is observed.
Voltammetric behaviour of TSAB/MWCNT modified electrode
Electrochemical detection of Hg2+ that is based on the TSAB/MWCNT ligand modified electrode using CV and square wave anodic stripping voltammetry was studied as shown in Fig. 3. In CV, the TSAB/MWCNT ligand modified electrode is immersed in 0.1 M NaNO3 containing 0.5 μM of Hg2+ ion for 180 s (Fig. 3(A)). After preconcentration, the modified electrode is washed with double distilled water and then transferred into freshly NaNO3 solution (pH 5.0). From the CV of the modified electrode (curve b), a well-defined anodic peak (0.34 mV) and cathodic peak (0.38 mV) were observed. In the absence of Hg2+ ion (curve a), no peak shows. The CV result implies that the metal ion was preconcentrated at the TSAB/MWCNT electrode surface. Therefore, the modified electrode is used for the determination of Hg2+ ion using anodic stripping voltammetry.
(A) Cyclic voltammograms of modified electrode in 0.1 M NaNO3 solution containing 0.5 μM Hg2+ without (a) and with (b) 180 s preconcentration at the scan rate of 50 mV s−1. Reduction potential − 1.2 V for 60 s. (B) Stripping current of 30 nM for Hg2+ ion at PIGE (a), MWCNT (b) and MWCNT/TSAB modified electrode (c) in 0.1 M NaNO3. Preconcentration time 180 s, deposition potential − 0.1 V, amplitude 50 mV
In SWASV, preconcentration was performed by immersing the electrode in 0.1 M NaNO3 solution containing 30 nM of Hg2+ ion under stirred conditions. The electrode was transferred into fresh electrolyte and reduced to − 0.1 V for 60 s. The anodic stripping voltammetry was carried out from − 0.1 to 0.7 V. The result obtained with the stripping analysis is shown in Fig. 3(B). From the figure, it can be observed that the modified electrode shows a high current enhancement compared to the bare electrode (MWCNT without ligand). The nature of ligand containing imine and hydroxyl groups is in coordinate with Hg2+ in solution. Therefore, the TSAB/MWCNT ligand modified electrode has high sensitivity current for determination of Hg2+ using anodic stripping voltammetry (ASV). The stripping mechanism of Hg2+ using the TSAB/MWCNT ligand modified electrode is explained by the following steps:
-
1)
Preconcentration step for surface MWCNT electrode (0.1 M NaNO3, for 180 s pH − 5.0)
-
2)
Reduction step (0.1 M NaNO3, pH − 5.0, − 0.1 V)
-
3)
Stripping step (0.1 M NaNO3, pH − 5.0, − 0.1 to 0.7 V)
Optimisation parameters
Figure 4 shows the electrochemical detection of Hg2+ ion using anodic stripping voltammetry. Using the TSAB/MWCNT ligand modified electrode, various parameters such as preconcentration time, effect of pH, different supporting electrolyte and stripping media were investigated.
In electrochemical determination, the choice suitable for different supporting electrolytes of a stripping medium on the current response was investigated using KNO3, NH4NO3, acetate buffer and NaNO3. Figure 4(A) shows the stripping voltammogram for Hg2+ ion which was preconcentrated from these different media. The maximum current response was found in 0.1-M NaNO3 solution as the most suitable medium for preconcentration of Hg2+ ion. Hence, in subsequent experiments, the 0.1-M NaNO3 solution was used for preconcentration of Hg2+ ion.
The effect of pH that was evaluated for Hg2+ in the pH range of 3.0–6.0 in the 0.1-M NaNO3 medium was studied and the results obtained are given in Fig. 4(B). The preconcentration of Hg2+ increases from pH 3.0–5.0 and then decreases from 5.0 to 6.0, showing a maximum pH 5.0 in 0.1 M NaNO3 solution. The protonation of the TSAB ligand occurs at low pH which has resulted in low preconcentration amount as complexation does not occur with protonated TSAB. On the other hand, metal hydroxide forms at higher pH, reducing the amount of Hg2+ present in the solution leading to lower amount of Hg2+ preconcentration. A pH 5.0 is most suitable for the preconcentration of Hg2+ of the electrode surface. Hence, subsequent experiments have been carried out at pH 5.0 in 0.1 M of NaNO3 medium.
The influence of preconcentration on the stripping peak current at different time intervals from 60 to 420 s was studied in Fig. 4(C). The amount of Hg2+ preconcentrated at the TSAB/MWCNT ligand modified electrode surface was found to increase with the increase in the preconcentration time. Therefore, the stripping peak current of Hg2+ also increased. Thus, preconcentration time of 180 s was fixed in order to shorten the analysis time.
Analytical performance
Calibration curve
In SWASV, the TSAB/MWCNT ligand modified electrode was the response between the stripping peak (Ip) and concentration (C) of Hg2+ that were investigated. In Fig. 5, after the accumulation of Hg2+ for 180 s, the Ip that is proportional to the C ranging from 2.4 to 180 nM was observed; the regression equation is expressed as Ip (μA) = (0.019 ± 0.0003) x + (0.027 ± 0.03) [Hg2+] with a correlation coefficient of R2 = 0.996, and the lowest detectable concentration of Hg2+ is achieved at 0.8 nM after 180 s accumulation. A calibration graph has been drawn by plotting the Ip vs. C of Hg2+ taken and is shown as an inset in Fig. 5. Further, linear range and the detection limits obtained with the TSAB/MWCNT ligand modified electrode have shown better sensitivity and linear range with previously reported modified electrodes (Table 1). From the table, the present modified electrode showed a better linear working range and low detectable Hg2+.
Reproducibility and stability
To examine the reproducibility, stability of the TSAB/MWCNT electrode for electrochemical sensors of Hg2+ ion was investigated. The reproducibility of electrodes was performed by four electrodes prepared with the determination of standard solution of 50 nM Hg2+ ion. The RSD for the response between electrodes was 3.0% for Hg2+, respectively. The stability of stripping voltammetry for the same modified electrode was used for 3 weeks, and the maximum deviation obtained was 3.5%, respectively. The stability of the modified electrode is shown in Fig. 6. The results indicate that the multi-walled carbon nanotube/TSAB ligand electrode has good sensitivity, reproducibility and long-time stability.
Anti-interference studies
Under optimal conditions, the interference studies were carried out by the addition of some metal ions which contain the 50 nM of Hg2+ ion. The results that show in the presence of 50 nM concentration of Pb2+, Cd2+, Zn2+, Sn2+, Ni2+,Co2+, Ag+ and 30 nM of Cu2+ and As3+ have shown insignificant effect on the stripping peak current for 50 nM Hg2+ as shown in Fig. 7. The prepared electrode shows excellent reproducibility and stability and the result of the interference was also investigated.
Effects of various metal ions on the analytical signal of 50 nM Hg2+ in the presence of 50 nM concentration of Pb2+, Cd2+, Zn2+, Sn2+, Ni2+, Co2+, Ag+, Cu2+ and As3+. Other experimental conditions are the same as in Fig. 4
Real sample analysis
The use of the MWCNT/TSAB ligand modified electrode for the determination of Hg2+ in well water and lake water. The lake water sample was collected from Kodaikanal lake and well water from poonamallee from Tamil nadu, India. The results are shown in Table 2, and the recovery test had been carried out by spiking in different concentrations of Hg2+ to the water samples which was analysis of the prepared electrode. The result of well water (Fig. 8a) and lake water (Fig. 8b) are shown in Fig. 8. From Table 2, it was confirmed that the determination of Hg2+ at TSAB/MWCNT ligand modified electrode was not affected by sample matrix, and the results of well water and lake water corroborated with those obtained by atomic absorption spectrometry (AAS), with the R.S.D values lower than 2.3 and 3.0% for Hg2+ which showed a good recovery in the presence of Hg2+ for analysis. In all the samples, good recoveries (between 100.4 and 104%) had been obtained (Table 2). Therefore, the prepared electrode was extremely showed good recovery for determination of Hg2+ in different water samples.
Conclusions
Fabrication of MWCNT and synthesised TSAB ligand modified electrode was prepared. Compared with a MWCNT electrode, the TSAB/MWCNT ligand shows an extremely improves in stripping current peak for the Hg2+. The developed method is highly selective and shows good sensitivity and wide linearity ranges for Hg2+. The electrode modified with the MWCNT/TSAB ligand exhibited high sensitivity and selectivity for the determination of Hg2+ and the LOD was 0.8 nM at S/N = 3 was achieved. Due to the combination of the good stability of MWCNT and the strong chelating ability of Hydroxyl groups to Hg2+. The performances of the prepared method were favourably utilised for determining Hg2+ in well water and lake water with good recovery ranging from 100.4% to 104%. Additionally, the sensor showed remarkable stability and repeatability.
References
Afkhamia A, Bagherib H, Khoshsafara H, Tehranic MS, Masoumeh Tabatabaeed M, Shirzadmehra A (2012) Simultaneous trace-levels determination of Hg(II) and Pb(II) ions in various samples using a modified carbon paste electrode based on multi-walled carbonnanotubes and a new synthesized Schiff base. Anal Chim Acta 746:98–106
Sanchez J, Castillo E, Corredor P, Agreda J (2011) Determination of mercury by anodic stripping voltammetry in aqua regia extracts. Port Electrochim Acta 29(3):197–210
Yin Z, Wu J, Yang Z (2010) A sensitive mercury (II) sensor based on CuO nanoshuttles/poly(thionine) modified glassy carbon electrode. Microchim Act 170(3-4):307–312
Abollino O, Giacomino A, Piscionieri G, Mentasti E (2008) Determination of mercury by anodic stripping voltammetry with a gold nanoparticle-modified glassy carbon electrode. Electroanalysis 20(1):75–83
Jayaseelan P, Akila E, Usha Rani M, Rajavel R (2016) Synthesis, spectral characterization, electrochemical, anti-microbial, DNA binding and cleavage studies of new binuclear Schiff base metal(II) complexes derived from o-hydroxyacetophenone. J Saudi Chem Soc 20(6):625–634
Tu Y, Lin Y, Yantasee W, Reng Z (2005) Carbon nanotubes based nanoelectrode arrays: fabrication evaluation and application in voltammetric analysis. Electroanalysis 17(1):79–84
Bankim J, Ashiwini S, Srivastava (2011) Biometic sensor for certain catecholamines employing copper (II) complex and silver nanoparticle modified glassy carbon paste electrode. Electrochim Acta 56:4188–4196
Golikand AM, Asgari M, Maraghah MG, Lohrasbi E (2009) Carbon nanotube-modified glassy carbon electrode for anodic stripping voltammetric detection of Uranyle. J Appl Electrochemi 39(1):65–70
Gadapudi RP, Lu C, Su F (2007) Sorption of divalent metal ions from aqueous solution by carbon nanotubes: a review. Sep Purif Technol 58:224–231
Ulloa PJ, Rosales PC, Nunez-Vergara LJ, Squella JA (2011) Adsorptive stripping voltammetric determination of nitroimidazole derivative on multi-walled carbon nanotube modified electrodes: influence of size and functionalization of nanotubes. J Braz Chemi Soc 22(7):1271–1278
Chicharro M, Bermejo E, Mareno M, Sanchez A, Zapardiel A, Rivas G (2005) Adsorptive stripping voltammetric of amitrole at a multi-walled carbon nanotubes paste electrode. Electroanalysis 17:476–482
Mohadesi A, Parvaresh S, Eshaghi Z, Karimi MA (2016) 4-Aminohippuric acid-functionalized carbon nanotubes for stripping voltammetric determination of copper (II) ions. Electrochemistry 84(3):138–142
Bulska E, Kandler W, Pashawski P, Hulanickin A (1995) Atomic absorption spectrometric determination of mercury in soil standard reference material following microwave sample pretreatment. Microchim Acta 119(1-2):137–146
Jamshid L, Manzoori JL, Mohammad H, Sorouraddin MH, Haji Shabani AM (1998) Determination of mercury by cold vapour atomic absorption spectrometry after preconcentration with dithizone immobilized on surfactant-coated alumina. J Anal At Spectrom 13:305–308
Fengxiang X, Dean Patterson HW, Xi Y, Maruthi Sridhar BB, Su Y (2006) Rapid determination of mercury in plant and soil samples using inductively coupled plasma atomic emission spectroscopy, a comparative study. Water Air Soil Pollut 170:161–171
Passariello B, Barbaro M, Quaresima S, Casciello A, Marabini A (1996) Determination of mercury by inductively coupled plasma-mass spectrometry. Microchemical 54(4):348–354
Allibone J, Fatemian E, J Walker JP (1999) Determination of mercury in potable water by ICP-MS using gold as a stabilising agent. J Anal At Spectrom 14(2):235–239
Stozhko NY, Malakhova NA, Fyodorov MV, Brainina KZ (2008) Modified carbon-containing electrode in stripping voltammetry of metals. J Solid State Electrochem 12(10):1185–1204
Raghu GK, Sampath S, Pandurangappa M (2012) Chemically functionalized glassy carbon spheres: a new covalent bulk modified composite electrode for the simultaneous determination of lead and cadmium. J Solid State Electrochem 16(5):1953–1963
Afkhami A, Madrakian T, Ghaedi H, Khanmohammadi H (2012) Construction of a chemically modified electrode for the selective determination of nitrile and nitrate ions based on a new nanocomposite. Int J Electrochem Sci 66:255–264
Gholivand MB, Azadbakht A, Pashabadi A (2011) Simultaneous determination of trace zinc and cadmium by anodic stripping voltammetry using a polymeric film nanoparticle self-assembled electrode. Electroanalysis 23(2):364–370
Salmanipour A, Taher MA (2011) An electrochemical sensor for stripping analysis of Pb(II) based on multiwalled carbon nanotube functionalized with 5-Br-PADAP. J Solid State Electrochem 15(11-12):2695–2702
Mishra I, Bindu K, Bhattacharya S (2004) Synthesis spectroscopic studies, molecular structure optimization, and superoxide dismutase activity of copper(II) and zinc(lI) bipyridyl assisted supramolecular motifs containing octadentate Schiff base. Ind J Chem 43:315–319
Scholz F, Lange B (1992) Abrasive stripping voltammetry an electrochemical solid state spectroscopy of wide applicability. Trends Anal Chem 11(10):359–367
Scholz F, Schröder U, Gulaboski R (2015) Electrochemistry of immobilized particles and droplets, Springer 2015
Weia J, Yanga D, Chena H, Gaoa Y, Li H (2014) Stripping voltammetric determination of mercury(II) based on SWCNT-PhSH modified gold electrode. Sensors Actuators B 190:968–974
Yantasee W, Lin Y, Zemanian TS, Fryxell GE (2003) Voltammetric detection of lead(II) and mercury(II) using a carbon paste electrode modified with thiol self-assembled monolayer on mesoporous silica (SAMMS). Analyst 128(5):467–472
Cesarino I, Marino G, Matos JR, Cavalheiro EC (2008) Evaluation of a carbon paste electrode modified with organofunctionalised SBA-15 nanostructured silica in the simultaneous determination of divalent lead, copper and mercury ions. Talanta 75(1):15–21
Rajabi HR, Roushani M, Shamsipur M (2013) Development of a highly selective voltammetric sensor for nanomolar detection of mercury ions using glassy carbon electrode modified with a novel ion imprinted polymeric nanobeads and multi-wall carbon nanotubes. J Electroanal Chem 693:16–22
Selvan SK, Narayanan SS (2018) Synthesis and characterization of carbon nanotubes/asymmetric novel tetradentate ligand forming complexes on PIGE modified electrode for simultaneous determination of Pb(II) and Hg(II) in well water, lake water and well water using anodic stripping voltammetry. J Electroanal Chem 810:176–184
Wu Z, Jiang L, Zhu Y, Xu C, Ye Y, Wang X (2012) Synthesis of mesoporous NiO nanosheet and its application on mercury (II) sensor. J Solid State Electrochem 16(10):3171–3177
Shahar T, Tal N, Mandler D (2013) The synthesis and characterization of thiol-based aryl diazonium modified glassy carbon electrode for the voltammetric determination of low levels of Hg(II). J Solid State Electrochem 17(6):1543–1552
Acknowledgements
One of the authors gratefully acknowledges the financial assistance as JRF received from the Department of Science and Technology for DST-PURSE Program in support of this research work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 120 kb)
Rights and permissions
About this article
Cite this article
Gayathri, J., Selvan, K.S. & Narayanan, S.S. A novel sensor for the determination of Hg2+ in waters based on octadentate ligand immobilized multi-walled carbon nanotube attached to paraffin wax impregnated graphite electrodes (PIGE). J Solid State Electrochem 22, 2879–2888 (2018). https://doi.org/10.1007/s10008-018-3984-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-018-3984-1