Abstract
This study demonstrates the potential of the electrochemical methods for the characterization of dissolved organic matter (DOM) in the drainage water of hydroameliorated agricultural areas. A study of drainage water could lead to a better understanding of the distribution and fate of terrestrial DOM in the freshwater systems. We have applied the voltammetric techniques which were developed by our group for the characterization of organic matter in the natural waters in general. Studied samples were collected in the experimental amelioration fields in the Sava river valley (45° 33′ 52″ N/16° 31′ 33″ E, 100 m above sea level), in the hydroameliorated agricultural areas in Croatia. The rough characterization of the type, nature and reactivity of DOM was done through the study of surface activity (SA) of dissolved organic carbon (DOC), copper complexing capacity (CuCC) and apperent stability constants, and measurements of organic and inorganic reduced sulfur species (RSS) fractions. The results confirm that the electrochemical approach gives a valuable and comprehensive insight into physicochemical characteristics of DOM in the drainage water and could be successfully applied to temporal studies in different terrestrial ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dissolved organic matter (DOM) is the most important form of organic carbon transport from terrestrial to aquatic ecosystems, with wide-ranging ecological impacts [1]. The composition of DOM is considered to be too complex to allow complete characterization. A number of techniques can be used for the characterization of DOM in the water samples. Electrochemical measurements along with chromatographic (HPLC) are the most used but challenging approaches in the speciation of DOM in natural water samples. These methods, especially voltammetry, have appropriate features to be used for monitoring (early warning tools) for assessment of water quality in aqueous systems in general [2]. Electrochemical techniques offer increasing degree of accuracy, decreasing detection limits and enabling prompt response. Their cost is dramatically lower compared to other more sophisticated techniques. Electrochemical methods—voltammetric techniques, simple, rapid and nondestructive, that were developed by our group, were used as tool for rough physicochemical characterization of DOM in different aquatic environments including seawater, freshwater and atmospheric water [2–15]. The biogeochemistry of different environmental compartments also needs to be considered in this kind of investigation. In the frame of the actual project The Sulphur and Carbon Dynamics in the Sea- and Fresh-water Environment (SPHERE), we are studying sulfur (S) and carbon (C) dynamics between different environmental compartments of the seawater and freshwater environment. In the International Year of Soils 2015, we were focused on the characterization of DOM in the drainage water of the experimental amelioration fields in the Sava river valley in Croatia [16].
Dissolved organic carbon (DOC) is the measurable elemental organic carbon (OC) content of DOM [17] and is generally defined as compounds that can pass through a 0.45 μm or 0.7 μm filter. Soil organic matter (SOM) includes non-humified fraction, that consists of compounds such as carbohydrates, amino acids, protein, and low molecular weight organic acids, and humified fraction (humic acids, HA, fulvic acids, FA, and humin), which is the complex of largely unidentifiable organic compounds [18, 19]. In the soil solutions DOC, the content of FA and HA are by far the most abundant. The fraction of carbon in low molecular weight (LMW) carboxylic acids (mainly aliphatic mono-, di-, and tricarboxylic acids and substituted benzoic and cinnamic acids) constitutes a maximum of 10 % of DOC in soil solutions [19]. In addition, microbial biomass itself provides an important pool of potential DOM [20]. The release of carbon to the potentially soluble organic carbon pool is controlled by the decomposition of plants by bacteria and fungi, leaching from the soil of the drainage basins by precipitation and formation of soluble substances [21]. The solubility of SOM is highly dependent on the structure of the organic compounds and the pH of the environment [22]. A large portion of sulfur (S) in soil is bonded to carbon (C). Sulfur in dissolved natural organic matter (DNOM) occurs as reduced (sulfide and thiol) or as oxidized species (sulfonate and sulfate). Organic sulfur is important in transport and leaching of sulfur (S) especially in soils that contain little inorganic S and receive little atmospheric inputs of SO4 2− [23]. Wang et al. [24] investigated dissolved organic sulfur (DOS) in the streams draining forested catchments in southern China and determined DOS in the concentration range 0–13.1 mg dm−3.
Increasing DOC concentrations in surface waters have been observed for many areas in the Northern Hemisphere [25]. High DOC concentrations in surface waters have negative effects on the water quality and water habitats [26]. In the biogeochemical processes in aquatic environment, the physicochemical properties of organic matter are often more important than the amount present. The fraction of DOC which possesses surface active properties may be very important for natural freshwater. Organic surface active substances (SAS) are concentrated by adsorption processes at the interface of all natural water bodies, thus modifying interboundary layers and influencing mass and energy transfer through them. The investigation of the DOC-metal-biding properties can give information about the possible binding sites on the organic matter. The capacity of organic ligands to complex metal ions is of special interest for understanding the role of DOC components in the transport and reactivity of metals in natural waters. This study focused on copper-binding ligands. Characterization of dissolved sulfur fraction in the water samples was based on measurements of inorganic and organic reduced sulfur species (RSS) [8]. Reduced SS comprise a group of compounds that contain sulfur in nominally −2 and 0 oxidation states. Some of the most important members of this group in natural waters include sulfide, organic thiols, inorganic and organic di- and polysulfides or polysulfanes, and dissolved molecular S° [6].
In this study, qualitative and quantitative investigation of DOM in the drainage water was done by the determination the surface activity (SA) of DOC, copper complexing capacity (CuCC) and reduced sulfur species (RSS). The applied electrochemical methods are sensitive, rapid, and quantitative with appropriate calibration. These methods are combined with measurements of dissolved organic carbon (DOC) by high temperature catalytic oxidation (HTCO) [27].
The objective of this study was to demonstrate the potential of the electrochemical methods for the rough characterization of DOC composition and its reactivity in the drainage water samples of the hydroameliorated agricultural areas in Croatia after very intensive rainfall and during snowmelt (i.e., in the case of preferential flow).
Experimental
Sampling procedure
Drainage water samples were collected in the experimental amelioration fields in the Sava river valley (45° 33′ 52″ N/16○ 31′ 33″ E, 100 m above sea level), in the hydroameliorated agricultural areas in Croatia. Soil was drained in four different drainpipes that differ in water flows as described earlier [28]. Samples of drainage water were taken at a drainpipe outlet into a channel and into high density polyethylene (HDPE) bottles (0.5 dm−3), filled to the top and previously cleaned with nitric acid and rinsed with Mili-Q-water. Each set of samples were collected after precipitation events: December 2014; February 2015 (snowfall) and May 2015 when large precipitation events (up to 60 mm day−1) were recorded. The data for the amount of precipitation were obtained from the Meteorological Station Sisak, which is approximately 15 km far away from the experimental field (Fig 1). Upon collection, samples were transported to the laboratory and were immediately filtered (Whatman GF/F filters pore size 0.7 μm) and divided into several aliquots, according to the needs of each analytical technique that was later applied. For DOC measurement samples were preserved with HgCl2 solution (10−5 mol dm−3). Without pretreatment, samples for SAS, CuCC, RSS, and pH were analyzed within 48 h. Until then the samples were kept in the cold (4 °C) and in a dark place.
Methods and analyses
Electrochemical measurements were performed with μ-Autolab voltammeter that was PC controlled, using GPES 4.6 Windows software (Eco Chemie B.V., The Netherlands), in a three electrode cell using a hanging mercury drop electrode (HMDE), Ag|AgCl (3 mol dm−3 KCl) and a Pt wire as auxiliary electrode.
Adsorption effect of SAS from samples was measured at the HMDE using phase sensitive alternating current voltammetry (AC voltammetry) out-of-phase mode as described previously [12]. The measurement of the electrode double layer capacity changes offers the possibility of direct investigation of adsorption characteristics of a complex mixture of naturally present organics at the electrode-electrolyte interface. Solution of 0.5 mol dm−3 NaCl was used as a electrolyte in all electrochemical measurements. For the quantification of the SAS content in samples a calibration plot of the nonionic surfactant Triton-X-100 was used. Such calibration plot is in fact the apparent adsorption isotherm for the model surfactant at a selected accumulation time. The detection limit of SAS determination is 0.02 mg dm−3 eq. Triton-X-100. The relative standard deviation obtained for multiple analyses of the same solution containing SAS at the level of 0.1 mg per liter is less than 5 %. The method enables a rough characterization of SAS present in an aqueous medium, through comparison of the shape and intensity of the electrochemical responses obtained by AC voltammetry and those obtained with different model substances [2, 12].
Copper complexing capacity (CuCC) was determined by using differential pulse anodic stripping voltammetry (DPASV) [13].
Reduced sulfur species (RSS) were characterized and quantified by using cyclic voltammetry on the mercury electrode following the work of Ciglenečki and Ćosović [29] and Bura Nakić et al. [14]. The characterization of dissolved sulfur fraction in the water samples was based on measurements of inorganic and organic RSS that deposit HgS layer during an accumulation step at the deposition potential, followed by scanning potential from positive to more negative values at the HMDE [8, 30]. In solutions containing sulfide anions insoluble HgS layer at Hg electrode surface can be formed by the reversible process of two electron oxidation of Hg at potentials more positive than −0.5 V vs. Ag|AgCl. Organic sulfur compounds, (thiourea, thiols, oxines), thioanions, labile chalcogenide nanoparticles (CuS, PbS, HgS, FeS), polysulfides, and dissolved and colloidal S° are also found to deposit HgS layer by anodic oxidation of Hg at sufficiently positive potentials, around 0 V [6, 8, 11, 29, 30].
The DOC concentrations were determined by using a sensitive, high temperature catalytic oxidation (HTCO) technique. A Model TOC-5000 System (Shimadzu) with high sensitive Pt catalyst and non-dispersive infrared (NDIR) detector for CO2 measurements was used.
pH was measured by standard electrode methods with a digital pH-meter, either a Model Delta-OHM HD 86–02 or a Metrohm digital pH-meter.
Results and discussion
Concentrations of DOC, SAS, CuCC, and RSS in the drainage water samples of hydroameliorated agricultural areas in Croatia are presented in Table 1, together with pH values and dates of sampling.
Characterization of dominant SAS
Voltammetric curves for two filtered drainage water samples taken from the same drainpiper (denoted No. 2) during different precipitation events and in the different seasons (February and May 2015, Table 1) are presented in Fig. 2. All curves were recorded for the increase of deposition times. The decrease of the AC current (i) value at the initial deposition potential −0.6 V (vs. Ag|AgCl) with respect to the base line of the pure electrolyte (0.55 mol dm−3 NaCl) is the result of the SAS adsorption. It can be seen that the voltammetric curves for both investigated samples shows similar shape (characteristic hump, same position of desorption waves, without sharp and well-defined peaks) indicating no significant difference in properties of adsorbable organic substances between investigated samples. Qualitative characterization of SAS was done by comparing the obtained adsorption curves to those of different model substances. In our previous studies, a number of adsorbable organic substances (nonionic, anionic, and cationic surfactants, polyaromatic hydrocarbon, monocarboxylic, dicarboxylic acids, HA and FA, polysaccharides, proteins, and lipids), representing either naturally occurring substances in the aquatic systems or potential pollutants, have been studied under same condition as natural water samples [2, 3, 7, 9, 12]. In general, there are differences in adsorption behavior between strongly adsorbable, relatively small molecules and hydrophylic large polymer molecules. The high, sharp, and well-defined desorption peaks are characteristic for relatively low molecular mass substances while the low and extended desorption waves indicate the presence of macromolecular organic substances. In view of the above discussion, we have concluded that the DOC of drainage water samples consists mainly of high molecular substances. The low and extend wave around −1.4 to −1.7 V suggests a partial desorption of segments in the surfactant molecule. Similar effects were detected in the humic/fulvic acid isolated from lagoon sediments. In addition, in the case of HA, lower desorption wave have been observed [7]. Perhaps this is not surprising because in most soils, humic substances represent a predominant class of organic material. It is important to keep in mind that the surfactant activity is a collective parameter, i.e., all present adsorbable substances take a part in adsorption on the electrode in a competitive manner [3]. Therefore, the adsorption effect of other adsorbable compounds present in lower concentration in the samples could be overshadowed. The results of the SAS measurements from drainpipe No. 2 in May 2015 by dilution 1:2 showed stronger adsorption effect in diluted sample pointing to the presence of strongly adsorbable compounds. Adsorption effect of more predominant humic/fulvic material was probably diminished by dilution, and more strongly adsorbable compounds (more hydrophobic in its nature), present in lower concentration range were measured in the diluted sample. It seems that the high precipitation event can result in the increased concentration of strongly adsorbable/hydrophobic substances in the drainage water.
AC voltammetric curves of 0.55 mol dm−3 NaCl and filtered drainage water samples taken from drainpipe (No. 2) at different time: a February 2015 and b May 2015. All curves of water samples were recorded at different and increasing deposition times (t D = 15; 30; 60 and 120 s) at deposition potential, E D = −0.6 V vs. Ag|AgCl in 0.55 mol dm−3 NaCl solution. Experimental conditions: AC phase angle 90°, AC amplitude 10 mV, and frequency 75 Hz
The surface activity of all investigated samples was pH dependent [12]. Natural pH values of the drainage water samples ranged from 7.35–7.96, respectively. Upon acidifying, a stronger adsorption effect was observed (11–50 %). This can be explained by the presence of the organic ligands which due to protonation become more hydrophobic and more adsorbable on the Hg electrode [12]. These observations were in agreement with the fact that organic matter of the soil is a major source of negative charge. Tipping and Woof [31] reported that protonation of functional groups might reduce the solubility (or mobility) of DOM in the soil resulting from a low pH. Such behavior has been attributed to polyfunctional organic acids whose charge characteristics depend on the extent of dissociation of the acidic functional groups. At high pH these acids are negatively charged due to dissociation of carboxyl and phenolic hydroxyl groups whereas at pH < 3, these acids behave as uncharged polymers [32].
A quantification of adsorbable organic substances was performed by using a calibration plot of Triton-X-100.
The obtained SA value for samples from February (Fig 2a) and May (Fig 2b) was the same corresponding to 0.42 mg dm−3 eq. Triton-X-100 while DOC was different (DOC = 9.93 mg C dm−3 in February and 8.01 mg C dm−3 in May (Table 1). The relative concentrations of SAS values for all investigated drainage water samples (N = 10) were in the range from 0.11–0.45, with the average value of 0.36 ± 0.10 mg dm−3 eq. Triton-X-100. These results are very close to the ones obtained for SAS in the Sava River water samples (average value of 0.36 ± 0.14) [10]. In comparison, concentrations of SAS obtained for the continental rainwater samples in Croatia were in the range 0.06–0.45 mg dm−3 eq. Triton-X-100 (average concentration of 0.14 ± 0.06) [33]. The range of DOC values is extended from 1.07 to 9.93, with an average value of 5.16 ± 2.53 mg C dm−3. These DOC concentrations are higher than those measured in the surface freshwater systems of the Sava River, where DOC concentrations were reported to be in the range of 1.57–3.30, with an average value of 2.49 ± 0.66 mg C dm−3 [10]. Concentrations of DOC in natural freshwater range from ~1 to 60 mg C dm−3, but are commonly from 1 to 5 mg C dm−3 [34]. Dissolved OC concentrations in the soil solution are commonly in the range from 2 to 35 mg C dm−3 [35]. The DOC concentrations in the drainage water sampled from different drainpipes at the same time varied, which was probably caused by different amount of drainage water flowing through pipes [36]. The DOC concentration in the sample taken from the drainpipe No. 2. was lower by the factor 2–2.5 in December (3.95 mg C dm−3) than in the February (during snowmelt) and in May (after heavy rainfall), Table 1, indicating that large precipitation events/hydrological conditions may be an important factor in the controlling of DOC levels. Dissolved OC flux resulting from water transport, is partly controlled by sorption/desorption processes [37]. Several studies, summarized by Kalbitz [21], have shown that after heavy rainfall events and during snowmelt in the early stages, preferential flow through macropores in structure soils can affect DOC output by reducing contact time between the solid and solution phase. According to the laboratory experiments of Yu et al. [38], in the absence of water, organic compounds adsorb on the mineral surfaces in such a way that contact area is maximized. In the presence of a sufficient amount of water, some organic molecules can adsorb via a single functional group to mineral surfaces, while the bulk of the molecular structure is immersed in the aqueous phase. Many water molecules form a layer, excluding organic adsorbates from the mineral basal plane.
In the drainage water samples, there is no significant correlation between DOC and SAS concentrations (p > 0.05). This indicates that the total SA depends on the nature of the organic molecules in the samples. The graph in Fig. 3 is useful for rapid and rough DOM characterization in the studied samples based on comparison of its SAS/DOC content/ratio, with those of the model substances [2, 3, 12]. Model substances which were taken here are relevant for organic constituents in the soil: humic-like substances (HA from Aldrich and FA isolated from lagoon sediments in Perpignan, France), lipid-like material (oleic acid), protein-like material (albumin), polysaccharide-like material (xanhtan). The differences in the slopes of the curves in Fig. 3 are reflected in the ratios SAS/DOC, which are correlated to hydrophilic/hydrophobic substances. As can be seen, the data for drainage water samples are grouped between the lines for humic acid (SAS/DOC = 0.04) and fulvic acid (SAS/DOC = 0.17), pointing to the similar hydrophilic/hydrophobic nature of surface active material present. Furthermore, SAS/DOC values for different natural samples ranged 0.04–0.15 and do not preclude the presence some monocarboxylic acids [12]. This diagram also show that very small amounts of very strongly adsorbable compounds could increase surfactant activity of the complex mixture of organic mater in the drainage water samples.
Copper complexing capacity
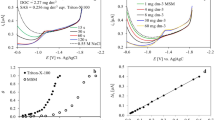
In Fig. 4 the copper complexing capacity (CuCC) determination in the filtered drainage water sample taken from drainpipe No. 4. in February 2015 (Table 1), is presented. In Fig. 4a the recorded voltammograms of the increasing copper additions to the sample at natural pH are presented (the additions were from 0 to 480 nmol dm−3 of copper ion). In Fig. 4b the results of direct electrochemical titration are presented as the dependence between the added copper ions concentrations and the corresponding current in the acidified sample (black circle; pH = 2.31) and in the sample of natural pH (red diamond; pH = 7.86). In Fig. 4c the results from Fig. 4b are presented after transformation (by dividing all the concentrations with the slope of the acidified solution); they are presented as copper ion added on the x-axis vs. copper ion found on the y-axis. In Fig. 4d, the data from Fig. 4c are linearized by the method of calculation by Ružić-van den Berg [39, 40], which enables the calculations of CuCC value from the slope of the line (slope = 1/CuCC) and the apparent stability constant (K app) from the intercept on the y-axis (intercept = 1/CuCC·K app). For the sample presented, the CuCC was 0.1 μmol Cu2+ dm−3 while log K app was 8.04. Copper complexing capacity of drainage water samples varied between 0.1 and 1.4 μmol Cu2+ dm−3. The highest CuCC value was measured during snowmelt (about three times higher than in December and twice higher than in May in the drainage water from drainpipe No. 1). Similar values of CuCC were obtained for the atmospheric bulk precipitations in the urban areas of Croatia (up to 1 μmol Cu2+ dm−3) [13, 33]. The values CuCC in the investigated samples were not correlated with concentrations of DOC or SAS. Additional information on the organic ligands characteristics was obtained by examining the complexing capacity values data normalized to DOC concentrations [41]. The values of CuCC/DOC indicate the partition of DOC copper ligands in the bulk organic matter. Determined values CuCC/DOC (μmol Cu2+ dm−3/mg C dm−3) were in the range from 0.01–0.47. The complexing capacity values data normalized to surfactant activity (CuCC/SAS) provide the information on the complexing capacity of the part of the organic matter, exhibiting surface active properties at the same time. The presence of organic ligands with surface active properties is very important as they influence the distribution between heavy metal species in the bulk water and their fractionation at phase boundaries. Obtained values were in the range of 0.38–3.25 μmol Cu2+ dm−3/mg dm−3 eq. Triton-X-100. The relative stability of the Cu complexes could be compared through the apparent stability constants (log K app) values. The calculated (log K app) values for all samples were in the range of 6.53–8.04. The stability constants determined for model humic, fulvic substances and melanoidins were in the same range of 6.5–8.4 [42]. Fulvic and humic acid are effective in cation complexation reactions due to the presence of carboxyl and phenolic hydroxyl groups as it was discussed above.
a Voltammograms of increased copper ion additions (0–480 nmol) to the filtered drainage water sample taken from drainpipe No. 4. February 2015: pH = 7.86; DPASV was applied under the following conditions: modulation time 0.04 s; interval time 0.31 s; modulation amplitude 25 mV, and step potential 5 mV. The deposition time, t D = 60 s at deposition potential, E D = −0.6 V vs. Ag|AgCl; b Direct titration curves of drainage water sample (black circle: pH = 2.31; red diamond: pH = 7.86) copper concentration added vs. current measured; c Titration curves from b presented as copper ion. concentration added vs. copper ion concentration found; d Calculated data from c (black diamond) accoding to Ružić-van den Berg method
Sulfur fraction
In Fig. 5 the cyclic voltammograms obtained for different deposition potentials (E D), in the filtered drainage water sample taken from drainpipe No. 2, on February (Table 1) are presented. The peak which appears at around −0.6 V is a measure for the presence of reduced sulfur species that deposit HgS layer at the Hg surface [8, 29]. Deposition at −0.2 V usually reveals HgS reduction peak from some organic (thio compounds) and inorganic sulfur species (sulfur, sulfide, polysulfide) while decrease of the same peak after the deposition at −0.4 V, indicates prevalence of organic sulfur compounds [8]. It is important to note that recorded peaks remained after purging with nitrogen in the acidic solution, which is characteristic of non volatile sulfur species [14]. For quantification of the mainly organic sulfur (organic, inorganic and non volatile) species in the samples, a calibration plot with sodium sulfide (Na2S) was used. The RSS were investigated in the samples from February and May, but RSS were detected only in the samples from the winter month. One possible reason for this could be anaerobic conditions in the soil during snowmelt. Another potential explanation is that the large precipitation in the spring diluted the sulfur fraction in the drainage water to concentration lower than our detection limit (1 nmol dm−3). The concentrations of RSS, including inorganic and organic fraction, were in the range between 0.24 and 0.36 μmol dm−3. The organic sulfur fraction in the total RSS was lower (23–36 %) than non volatile inorganic fraction (64–77 %). For comparison, measured values of RSS in anoxic freshwater systems (Visovac lake, Croatia) are up to 0.32 nmol dm−3 RSS (with mainly volatile inorganic sulfur fraction, i.e., sulfide species, from 80 to 95 %), while RSS concentrations (mainly sulfide) in seawater systems such as marine lake, Rogoznica Lake, Croatia, could be measured in range of mmol dm−3 [14].
Cyclic voltammetric curves for drainage water samples taken from drainpiper No. 2, February, 2015. The peak at around −0.6 V (deposition potential, E D = −0.2 V) is a measure for the presence of reduced sulfur species (group of compounds containing sulfur in oxidation state from −2 to 0). Decrease of the same peak after the deposition at the potential E D = −0.4 V, indicate presence of organic sulfur compounds. Experimental conditions: accumulation time with stirring, t D = 120 s at E D = −0.2 V and E D = −0.4 V (vs. Ag|AgCl), scan rate 100 mV s−1
Conclusion
We have shown that electroanalytical techniques are suitable for the investigation of physicochemical properties of DOM in the drainage water. The technique is sensitive, rapid, and quantitative. Additionally, such research generally deepens our knowledge and understanding of the electrochemistry, particularly voltammetry in the investigation of organic matter in natural waters.
In the drainage water samples taken after different precipitation events, SAS, CuCC, RSS, and DOC concentrations were measured. Although seasonal variations of the DOC characteristics in the drainage water samples need to be further studied, some differences in the properties of DOC in the case of different precipitations regime were observed.
-
Some organic constituents of drainage waters DOC have surface active properties which can be studied by AC voltammetry (out of phase), on the basis of the adsorption effects at the Hg electrode. Relative SAS concentrations (mg dm−3 eq. Triton-X-100), were very similar to those obtained in the surface waters of River Sava and atmospheric water. The SA of all investigated samples was pH dependent which indicates the presence of polyfunctional organic acids.
-
Large precipitation events affect the DOC fluxes by increasing DOC concentrations. There is no significant correlation between DOC and SAS concentrations, implying that the SAS concentration depends on the nature of the organic molecules. On the basis of the AC voltammetric curves and the surfactant activity normalized to the organic carbon content, it was concluded that the SAS in the drainage water samples corresponds to mainly humic/fulvic type of the material. However, higher precipitation events can influence the appearance of the different organic material with more adsorbable/hydrophobic characteristics.
-
Copper complexing capacity of drainage water samples varied between 0.1 and 1.4 μmol Cu2+ dm−3. The highest CuCC value was measured during snowmelt. The organic ligands responsible for complexing copper were not proportional to DOC and SAS concentrations. The calculated apparent stability constants (log K app) for all samples were in the range from 6.53–8.04. The obtained log K app were similar to the ones determined for model humic/fulvic substances and melanoidins.
-
The non volatile organic sulfur fraction in the samples from February was lower (23–36 %) than the inorganic fraction (64–77 %). The presence of the measurable concentration of RSS only in the winter samples was speculated to be a consequence of anaerobic conditions that probably prevailed in the soil during snowmelt.
References
Karlsson J, Byström P, Ask J, Ask P, Persson L, Jansson M (2009) Light limitation of nutrient-poor lake ecosystems. Nature 460:506–509
Ćosović B, Kozarac Z, Frka S, Vojvodić V (2010) Electrochemical adsorption study of natural organic matter in marine and freshwater systems. A plea for use of mercury for scientific purposes. Electroanalysis 22:1994–2000
Ćosović B, Vojvodić V (1998) Voltammetric analysis of surface active substances in natural seawater. Electroanalysis 10:429–434
Kozarac Z, Ćosović B, Vojvodić V (1986) Effects of natural and synthetic surface active substances on the electrochemical reduction of cadmium in natural waters. Water Res 20:295–300
Batina N, Ciglenečki I, Ćosović B (1992) Determinatin of elemental sulfur, sulfide, and their mixtures in electrolyte solutions by ac voltammetry. Anal Chim Acta 267:157–164
Ciglenečki I, Ćosović B (1996) Electrochemical study of sulfur species in seawater and marine phytoplankton cultures. Mar Chem 52:87–97
Gašparović, B (1995) Characterisation of dissolved organic matter in the seawater using o-nitrophenol as a probe, PhD Thesis, University of Zagreb, Croatia
Krznarić D, Ciglenečki I, Ćosović B (2001) Voltammetric investigations of 2-dimethylarsinylethanol sulfide in NaCl and seawater. Anal Chim Acta 431:269–278
Orlović-Leko, P (2001) Physico-chemical interactions of surface active substances with lead in the aquatic systems, PhD thesis, University of Zagreb, Croatia
Orlović-Leko P, Kozarac Z, Ćosović B (2004) Surface active substances (SAS) and dissolved organic matter (DOC) in atmospheric precipitation of urban area of Croatia (Zagreb). Water Air Soil Pollut 158:295–310
Ciglenečki I, Krznarić D, Helz GR (2005) Voltammetry of copper sulfide particles and nanoparticles; investigation of the cluster hypothesis. Environ Sci Technol 39:7492–7498
Ćosović B, Orlović-Leko P, Kozarac Z (2007) Rain water dissolved organic carbon: characterization of surface active substances by electrochemical method. Electroanalysis 19:2077–2084
Plavšić M, Orlović-Leko P, Kozarac Z, Bura-Nakić E, Strmečki S, Ćosović B (2008) Complexation of copper ions in atmospheric precipitation in Croatia. Atmos Res 87(1):80–87
Bura-Nakić E, Helz GR, Ciglenečki I, Ćosović B (2009) Reduced sulfur species in a stratified seawater lake (Rogonica Lake, Croatia); seasonal variations and argument for organic carriers of reactive sulfur. Geochim Cosmochim Acta 73:3738–3751
Orlović-Leko P, Kozarac Z, Ćosović B, Strmečki S, Plavšić M (2011) Characterization of atmospheric surfactants in the bulk precipitation by electrochemical tools. J Atmos Chem 66(11–26)
Orlović-Leko P, Vidović K, Plavšić M, Ciglenečki I, Šimunić I, Minkina, T Characterization of Dissolved Organic Matter (DOM) in the Drainage Water of Hydroameliorated Agricultural Areas in Croatia. The Proceeding of the International Soil science Congress on Soil Science in International Year of Soils. Article book, Shein E (ed), 19–23 October, 2015. Sochi, Russia
Filella M (2009) Freshwaters: which NOM matters? Environ Chem Lett 7:21–35
Milne E (2008) Soil organic carbon. In: Cleveland CJ (ed) Encyclopedia of Earth. Environmental Information Coalition, National Council for Science and the Environment, Washington, DC
Strobel BW (2001) Influence of vegetation on low-molecular-weight carboxylic acids in soil solution—a review. Geoderma 99:169–198
Yavitt JB, Fahey TJ (1984) An experimental analysis of solution chemistry in a lodgepole pine forest floor. Oikos 43:222–234
Kalbitz K, Solinger S, Park JH, Michalzik B, Matzner E (2000) Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci 165:277–304
Kleber M, Johnson MG (2010) Chapter three—advances in understanding the molecular structure of soil organic matter: implications for interactions in the environment. Adv Agron 106:77–142
Kaiser K, Guggenberger G (2005) Dissolved organic sulphur in soil water under Pinus sylvestris L. and Fagus sylvatica L. Stands in northeastern Bavaria, Germany-variations with seasons and soil depth. Biogeochemistry 72:337–364
Wang Z, Zhang X, Wang Z, Zhang Y, Li B, Vogt R (2012) Dissolved organic sulfur in streams draining forested catchments in southern China. J Environ Sci (China) 24(4):704–710
Evans CD, Monteith DT, Cooper DM (2005) Long-term increases in surface water dissolved organic carbon: observations, possible causes and environmental impacts. Environ Pollut 137:55–71
Delpla I, Jung AV, Baures E, Clement M, Thomas O (2009) Impacts of climate change on surface water quality in relation to drinking water production. Environ Int 35(8):1225–1233
Dafner EV, Wangersky PJ (2002) A brief overview of modern directions in marine DOC studies part II—recent progress in marine DOC studies. J Environ Monit 4:55–69
Šimunić I, Mesić M, Sraka M, Likso T, Čoga L (2011) Influence of drainpipe spacing on nitrate leaching and maize yield. Cereal Res Commun 39(2):274–283
Ciglenečki I, Ćosović B (1997) Electrochemical determination of thiosulfate in seawater in the presence of elemental sulfur and sulfide. Electroanalysis 9:1–7
Milanović I, Krznarić D, Bura-Nakić E, Ciglenečki I (2014) Deposition and dissolution of metal sulfide layers at a Hg electrode surface in seawater electrolyte conditions. Environ Chem 11(2):167–172
Tipping E, Woof C (1990) Humic substances in acid organic soils: modeling their release to the soil solution in terms of humic charge. J Soil Sci 41:573–586
Tate KR, Theng, BKG (1980) Organic matter and its interactions with inorganic soil constituents In: Theng BKG (ed) Soils with Variable Charge, N.Z. Soc. Soil Sci., Lower Hutt, New Zealand 225–249
Orlović-Leko P, Plavšić M, Bura-Nakić E, Kozarac Z, Ćosović B (2009) Organic matter in the bulk precipitations in Zagreb and Šibenik, Croatia. Atmos Environ 43(4):805–811
McDonald S, Bishop AG, Prenzler PD, Robards K (2004) Analytical chemistry of freshwater humic substances. Anal Chim Acta 527(2):105–124
Michalzik B, Kalbitz K, Park JH, Solinger S, Matzner E (2001) Fluxes and concentrations of dissolved organic carbon and nitrogen—a synthesis for temperate forests. Biogeochemistry 52(2):173–205
Šimunić I, Tomić F, Ostojić Z (2002) Concentration and leaching of atrazine into drainage water in Gleyic Podzoluvisol. Rostlinná Výroba 48(4):167–174
Guggenberger G, Kaiser K (2003) Dissolved organic matter in soil: challenging the paradigm of sorptive preservation. Geoderma 113:293–310
Yu CH, Newton SQ, Norman MA, Schäfer L, Miller DM (2003) Molecular dynamics simulations of adsorption of organic compounds at the clay mineral/aqueous solution Interface. Struct Chem 14(2):175–185
Ružić I (1982) Theoretical aspects of the direct titration of natural waters and its information yield for trace metal speciation. Anal Chim Acta 140:99–113
van den Berg CMG (1982) Determination of copper complexation with natural organic ligands in seawater by equilibration with manganese dioxide: I Theory. Mar Chem 11:307–322
Scoullos M, Plavšić M, Karavoltsos S (2004) Copper speciation in the Gulf of Elefsis, the role of macroalgae Ulva rigida. Mar Chem 86:51–63
Plavšić M, Lee C, Ćosović B (2006) Copper complexing properties of melanoidins and marine humic material. Sci Total Environ 366:310–319
Acknowledgments
This work is fully supported by the project “The Sulphur and Carbon Dynamics in the Sea and Fresh-Water Environment” (IP-11-2013-1205 SPHERE) and partly by the project AMBIOMERES (IP-11-2013-8607) from the Croatian Science Foundation. The authors thank Zdeslav Zovko for DOC measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orlović-Leko, P., Vidović, K., Plavšić, M. et al. Voltammetry as a tool for rough and rapid characterization of dissolved organic matter in the drainage water of hydroameliorated agricultural areas in Croatia. J Solid State Electrochem 20, 3097–3105 (2016). https://doi.org/10.1007/s10008-016-3245-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-016-3245-0