Abstract
Background
The aim of this study was to analyze trabecular microarchitecture of augmented sinuses with hyaluronic matrix and xenograft by microcomputed tomography, and to investigate whether hyaluronic matrix has an effect on the newly formed bone quality.
Materials and methods
Thirteen patients undergoing maxillary sinus augmentation were included in this split-mouth study. Right and left sinus sites were randomly assigned to test and control group. In test group, the sinus was grafted with hyaluronic matrix and xenograft; in control group, only with xenograft. Four months after augmentation, bone samples were harvested during implant placement and analyzed for the following trabecular microarchitecture parameters using microcomputed tomography: bone volume (BV), total volume (TV), bone volume fraction (BV/TV), bone surface (BS), specific bone surface (BS/BV), bone surface density (BS/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), trabecular pattern factor (Tb.Pf), and fractal dimension (FD).
Results
There was statistically significant difference only for BS/TV parameter between two groups. BS/TV was higher in hyaluronic matrix group compared with control group.
Conclusions
Addition of hyaluronic matrix to xenograft may enhance bone quality in terms of bone surface density. However, more research investigating the microstructural variation of augmented sinuses is needed with a greater sample.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Maxillary sinus augmentation by lateral window approach allows implant placement in the resorbed posterior maxilla. It is a predictable procedure with high success rates [1]. Various graft materials have been applied for this procedure. Although autogenous graft is accepted as possessing ideal properties [2], it has been substituted by other graft materials because of its disadvantages such as postsurgical morbidity, increased surgical time, and higher resorption rates [3]. Allografts, xenografts, and alloplastic materials are frequently used for this purpose. Xenografts including anorganic bovine bone and collagenated heterologous bone graft (CHBG) have osteoconductive effects and can be used in combination with other biomaterials to promote osteoinductive efficacy [4, 5].
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan and used in various medical fields including ophthalmology, orthopedic surgery, and dermatology owing to its biochemical and biophysical properties [6]. Interactions of HA with extracellular matrix macromolecules and cell surface contribute to morphogenesis, tissue remodeling, and inflammation [7]. It participates in several biological procedures such as mediation of cellular signaling; regulation of cell adhesion, proliferation, and differentiation [6]. Sasaki and Watanabe [8] stated that high-molecular HA is capable of accelerating new bone formation through mesenchymal cell differentiation in bone wounds.
HA-based materials were applied with bone grafts in sinus augmentation studies [9, 10]. These studies supported that it has positive effects on bone formation by providing enough space between graft particles and allowing vascular, cellular invasion to the grafted area.
The success of sinus augmentation is based on the quality of newly formed bone. There are many factors that affect bone quality [11]. It depends not only on the bone mass but also on its distribution in three-dimensional (3D) space (i.e., microarchitecture) [12]. Bouxsein [12] described trabecular microarchitecture as the shape and orientation of basic structural elements. It is evaluated with the number of trabeculae, their average thickness, the average distance between adjacent trabeculae, and trabecular connectivity. Several methods are used for trabecular microarchitecture assessment [13]. Histomorphometric analysis is considered the gold standard for assessing bone, because only it gives the opportunity for direct analysis of cellular components [14]. However, different techniques allow more than one measurement in a nondestructive way compared with histomorphometric analysis. Microcomputed tomography (microCT), one of these techniques, was first introduced by Feldkamp et al. [15] and considered a promising method for 3D evaluation of maxillary sinuses after augmentation [16].
The aim of this study was to analyze trabecular microarchitecture of augmented sinuses with hyaluronic acid-based matrix and CHBG by microCT, and to investigate whether hyaluronic matrix has an effect on the newly formed bone quality in terms of microarchitecture.
Materials and methods
Thirteen individuals in good general health (five men and eight women, mean age 50 years, ranging from 33 to 69 years) participated in this case–control study. All patients were informed about the procedure, and written informed consent was obtained from the patients. The study protocol was prepared according to the Declaration of Helsinki [17, 18]. Approval was obtained from Clinical Researches Ethics Board of Hacettepe University. Patients with bilateral posterior maxillary edentulism (≤ 4 mm residual crest height) and requiring sinus augmentation before implant treatment were included in the study. Individuals taking medications known to affect bone metabolism (e.g., steroids, bisphosphonates), those with significant systemic disease, pregnant, nursing women, and smokers were excluded from the study.
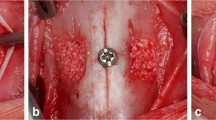
Bilateral maxillary sinus augmentation was performed with lateral window approach as described by Tatum [19]. In this split-mouth study, right and left sinus sites were randomly assigned to test and control group. The sinus was grafted with hyaluronic matrix (HyalossTM matrix, ANIKA Therapeutics, Italy) and CHBG (Apatos mix, OsteoBiol®, Italy) in test group and only with CHBG in control group. Hyaluronic matrix, in the form of fibers, consists of esterified bacterial origin HA. It immediately takes gel form in contact with sterile saline (Fig. 1).
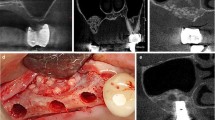
Four months after augmentation, during implant placement, 26 bone samples were taken from the grafted sinus areas with a 2-mm-diameter trephine bur. After removal, these samples were placed in 10% neutral buffered formalin solution.
MicroCT analysis
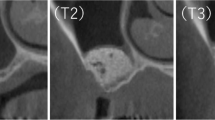
Bone samples were fixed to the scanner compartment of the microCT device (Skyscan 1174, Skyscan, Kontich, Belgium) via patafix. After the area setting of 800 µA, 50 kV, and 40.89 µm pixel size was arranged and saved, each sample was scanned with the same setting. The rotation step of microCT was set at 0.7° and the sample was determined to perform 180° rotation scan with 2300 ms exposure. Raw data were obtained during scanning and subsequent reconstructions of these data were carried out with the software NRecon (NRecon version 1.6.9.4, Skyscan, Kontich, Belgium), provided by the manufacturer. During reconstruction beam hardening, ring artifact reduction, smoothing, and frame averaging were individually adjusted to the optimum value for each sample. As a result of the reconstruction of the raw data, 8-bit gray value images were obtained. Reconstructed images were transferred to CTAn (version 1.13.5.1) software. Using CTAn scan, region of interest (ROI) was drawn within the sample to analyze the 3D microarchitecture of each sample.
From each ROI, the following trabecular microarchitecture parameters were analyzed according to the previously described variables in the literature [20, 21]: (1) bone volume (BV) (mm3), volume of the region segmented as bone; (2) total volume (TV) (mm3), volume of the entire ROI; (3) bone volume fraction (BV/TV) (%), ratio of the bone volume to the total volume of the ROI; (4) bone surface (BS) (mm2), surface of the region segmented as bone; (5) specific bone surface (BS/BV) (mm2/mm3), ratio of the segmented bone surface to the segmented bone volume; (6) bone surface density (BS/TV) (mm2/mm3), ratio of the segmented bone surface to the total volume of the ROI; (7) trabecular thickness (Tb.Th) (mm), mean thickness of the trabeculae in the ROI; (8) trabecular separation (Tb.Sp) (mm), mean distance between trabeculae; (9) trabecular pattern factor (Tb.Pf) (1/mm), which is an inverse connectivity index: the higher it is the trabeculae are less connected; (10) fractal dimension (FD), which indicates the complexity of the specimen surface.
Statistical analysis
The study sample was determined according to the previous study conducted by the same research group [9].
Statistical analysis was performed by the software IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA). All of the microCT parameters were summarized as median values and interquartile ranges [25th percentile (Q1)-75th percentile (Q3)]. The parameters between control and test group were compared with Wilcoxon’s rank-sum test. The correlations among the parameters for each group were calculated using Spearman’s rank correlation coefficient. Statistical significance was set at P < 0.05 and P < 0.01.
Results
Table 1 lists the median values of all measured parameters for test and control group. There was statistically significant difference only for BS/TV parameter between two groups. BS/TV was higher in hyaluronic matrix group compared with control group.
Spearman correlation analysis among microCT parameters revealed correlations at the 0.05 and 0.01 levels. Significant correlations were found in control group (Table 2). Tb.Sp was negatively correlated with BV/TV (r = − 0.758, P = 0.011), and BS/TV (r = − 0.661, P = 0.038). As an index showing inverse connectivity, Tb.Pf showed a positive correlation with BS/BV (r = 0.685, P = 0.029) in control group.
In hyaluronic matrix group, Tb.Sp was positively correlated with BS/BV (r = 0.721, P = 0.019) (Table 3). FD revealed strong positive correlation with Tb.Th both in the test (r = 0.673, P = 0.033) and control group (r = 0.806, P = 0.005).
Discussion
Dental implants are exposed to functional loading after prosthetic treatment. Therefore, it is important to learn the quality of newly formed bone, particularly in the posterior maxilla with a high rate of type 4 bone [22]. There are many factors that affect bone quality [11]. Microarchitecture is expressed as one of the determinants of bone quality. Orientation of trabeculae in 3D plane gives information about biomechanical properties of trabecular bone. Hence, as the implant is surrounded by trabecular bone, it is recommended to evaluate trabecular microarchitecture as part of bone assessment before implant surgery [23].
MicroCT allows us to evaluate bone samples in 3D plane and to learn about trabecular microarchitecture of the samples. It is a non-destructive method and gives high-resolution images of bone structure [15]. There are a limited number of studies examining augmented sinus region by microCT in terms of trabecular microarchitecture [16, 24,25,26,27,28,29,30,31,32,33]. In these sinus augmentation studies, heterogeneity exists with respect to the grafting material, healing time, and measured outcomes. Moreover, a detailed discussion of microarchitecture parameters is unavailable. This may be attributed to the evaluation of trabecular microarchitecture being a relatively new area of research for implant surgery. However, the majority of these studies agreed that microCT is effective for evaluating 3D bone structure. Huang et al. [27] evaluated bone microarchitecture by microCT after sinus augmentation with autogenous bone graft and stated that it is important to understand the trabecular remodeling of autogenous bone graft and hereby to determine the implant prognosis in grafted maxillary sinus region. Kühl et al. [16] performed sinus augmentation using different graft materials and evaluated whether microCT is suitable for examining the morphometric structure of healing grafts. Consequently, it was stated that this method is promising.
Although microCT is a successful method in this regard, there is a need for a clinically applicable method as microCT can only be applied to ex vivo bone samples. Apart from histomorphometry and microCT, there are some microarchitecture evaluation methods such as high-resolution magnetic resonance imaging (HR-MRI) and high-resolution peripheral quantitative computed tomography (HR-pQCT). HR-MRI and HR-pQCT are capable of 3D imaging, but they cannot be applied in vivo in the craniofacial region either.
To the authors’ knowledge, this is the first study that microCT technique has been used to perform a microarchitecture evaluation of bone samples, retrieved from hyaluronic matrix applied maxillary sinuses. Microstructural properties displayed statistically significant difference only for BS/TV parameter between two groups. As a parameter showing bone surface density, BS/TV was higher in hyaluronic matrix group compared with control group. This is a notable result that addition of hyaluronic matrix to xenograft may be an alternative treatment for implant placement in poor bone density areas such as type 4 bone. This result should be confirmed by implant stability and torque analyses at the time of implant placement.
HA (also termed hyaluronan) has several biological properties. The cellular effects of HA are mostly explained by its unique hydrodynamic properties and its interactions with structural hyaluronan-binding proteins of extracellular matrices. It acts as a template for assembly of a multi-component pericellular matrix [7]. In this matrix, interactions with cell surface hyaluronan receptors (e.g., CD44, RHAMM) and direct transmembrane attachment to hyaluronan synthase affect cell behavior in terms of cell proliferation, motility, and invasion. Although it is not clear whether HA has direct or indirect effect on osteogenic cells, it was shown to increase bone formation in vitro by mesenchymal cell migration and differentiation [34]. Huang et al. [35] concluded that HA increases the proliferation and differentiation of osteoprogenitor cells to osteoblasts in the rat calvarial-derived cell culture and also found increased alkaline phosphatase activity and osteocalcin gene expression with HA administration. As a result, it was suggested that HA may enhance osteogenic and osteoinductive properties of bone grafts due to its stimulatory effects on osteoblasts. Stiller et al. [36] reported higher osteogenic marker expression with HA containing graft application in a sinus augmentation study. Another possible explanation of increased bone formation is that low molecular weight degradation products of HA promotes neovascularization by increasing endothelial cell proliferation and blood vessel invasion [8, 37]. This possibility has been noted in sinus augmentation studies using HA containing grafts and finding abundant vascular spaces [9, 10].
Some microstructure parameters (BV, Tb.Pf, Tb.Sp) were more favorable in hyaluronic matrix group; however, there was no statistically significant difference (Table 1). Moreover, BV/TV, BS, and Tb.Th were statistically insignificant higher in the control group. Given the biological properties of HA, these discrepancies may be attributed to the small sample of this study. In addition, a longer healing period may be required to show a statistically significant difference for all these parameters.
Conclusions
Considering the relationship between bone quality and implant success [38], it is important to evaluate the quality of the newly formed bone after augmentation. While HA was determined to have favorable effect on bone quality in terms of bone surface density, further studies are required with a greater sample and implant survival results. In addition, more microarchitecture analyses of augmented sinuses are essential to be able to compare microarchitecture parameters between studies.
Abbreviations
- CHBG:
-
Collagenated heterologous bone graft
- HA:
-
Hyaluronic acid
- 3D:
-
Three dimensional
- MicroCT:
-
Microcomputed tomography
- ROI:
-
Region of interest
- BV:
-
Bone volume
- TV:
-
Total volume
- BV/TV:
-
Bone volume fraction
- BS:
-
Bone surface
- BS/BV:
-
Specific bone surface
- BS/TV:
-
Bone surface density
- Tb.Th:
-
Trabecular thickness
- Tb.Sp:
-
Trabecular separation
- Tb.Pf:
-
Trabecular pattern factor
- FD:
-
Fractal dimension
- HR-MRI:
-
High-resolution magnetic resonance imaging
- HR-pQCT:
-
High-resolution peripheral quantitative computed tomography
References
Wallace SS, Tarnow DP, Froum SJ, Cho S-C, Zadeh HH, Stoupel J et al (2012) Maxillary sinus elevation by lateral window approach: evolution of technology and technique. J Evid Based Dent Pract 12:161–171
Moy PK, Lundgren S, Holmes RE (1993) Maxillary sinus augmentation: histomorphometric analysis of graft materials for maxillary sinus floor augmentation. J Oral Maxillofac Surg 51:857–862
Scarano A, Degidi M, Iezzi G, Pecora G, Piattelli M, Orsini G et al (2006) Maxillary sinus augmentation with different biomaterials: a comparative histologic and histomorphometric study in man. Implant Dent 15:197–207
Miron RJ, Zhang Q, Sculean A, Buser D, Pippenger BE, Dard M et al (2016) Osteoinductive potential of 4 commonly employed bone grafts. Clin Oral Investig 20:2259–2265
Froum SJ, Wallace S, Cho S-C, Rosenberg E, Froum S, Schoor R et al (2013) A histomorphometric comparison of Bio-Oss alone versus Bio-Oss and platelet-derived growth factor for sinus augmentation: a postsurgical assessment. Int J Periodontics Restorative Dent 33:268–279
Zhao N, Wang X, Qin L, Zhai M, Yuan J, Chen J et al (2016) Effect of hyaluronic acid in bone formation and its applications in dentistry. J Biomed Mater Res A 104:1560–1569
Toole BP (2001) Hyaluronan in morphogenesis. Semin Cell Dev Biol 12:79–87
Sasaki T, Watanabe C (1995) Stimulation of osteoinduction in bone wound healing by high-molecular hyaluronic acid. Bone 16:9–15
Dogan E, Dursun E, Tosun E, Bilgic E, Akman AC, Orhan K et al (2017) Evaluation of hyaluronic matrix efficacy in sinus augmentation: a randomized-controlled histomorphometric and micro-computed tomography analysis. Int J Oral Maxillofac Surg 46:931–937
Emam H, Beheiri G, Elsalanty M, Sharawy M (2011) Microcomputed tomographic and histologic analysis of anorganic bone matrix coupled with cell-binding peptide suspended in sodium hyaluronate carrier after sinus augmentation: a clinical study. Int J Oral Maxillofac Implants 26:561–570
Compston J (2006) Bone quality: what is it and how is it measured? Arq Bras Endocrinol Metabol 50:579–585
Bouxsein ML (2005) Determinants of skeletal fragility. Best Pract Res Clin Rheumatol 19:897–911
Müller R (2003) Bone microarchitecture assessment: current and future trends. Osteoporos Int 14:89–99
Iwaniec UT, Wronski TJ, Turner RT (2008) Histological analysis of bone. Methods Mol Biol 447:325–341
Feldkamp LA, Goldstein SA, Parfitt MA, Jesion G, Kleerekoper M (1989) The direct examination of three-dimensional bone architecture in vitro by computed tomography. J Bone Miner Res 4:3–11
Kühl S, Götz H, Hansen T, Kreisler M, Behneke A, Heil U et al (2010) Three-dimensional analysis of bone formation after maxillary sinus augmentation by means of microcomputed tomography: a pilot study. Int J Oral Maxillofac Implants 25:930–938
Rickham PP (1964) Human Experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki. Br Med J. 2:177
Palacios R (2013) Post-trial access and the new version of the Declaration of Helsinki. Colomb Med (Cali) 44:206–207
Tatum H Jr (1986) Maxillary and sinus implant reconstructions. Dent Clin North Am 30:207–229
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ et al (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units: report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R (2010) Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J Bone Miner Res 25:1468–1486
Ulm C, Kneissel M, Schedle A, Solar P, Matejka M, Schneider B et al (1999) Characteristic features of trabecular bone in edentulous maxillae. Clin Oral Implants Res 10:459–467
Ibrahim N, Parsa A, Hassan B, van der Stelt P, Aartman IH, Wismeijer D (2014) Accuracy of trabecular bone microstructural measurement at planned dental implant sites using cone-beam CT datasets. Clin Oral Implants Res 25:941–945
Pereira R, Menezes J, Bonardi J, Griza G, Okamoto R, Hochuli-Vieira E (2018) Comparative study of volumetric changes and trabecular microarchitecture in human maxillary sinus bone augmentation with bioactive glass and autogenous bone graft: a prospective and randomized assessment. Int J Oral Maxillofac Surg 47:665–671
Monje A, Monje F, González-García R, Suarez F, Galindo-Moreno P, García-Nogales A et al (2015) Influence of atrophic posterior maxilla ridge height on bone density and microarchitecture. Clin Implant Dent Relat Res 17:111–119
Dursun E, Dursun CK, Eratalay K, Orhan K, Celik HH, Tözüm TF (2015) Do porous titanium granule grafts affect bone microarchitecture at augmented maxillary sinus sites? a pilot split-mouth human study. Implant Dent 24:427–433
Huang HL, Chen MY, Hsu JT, Li YF, Chang CH, Chen KT (2012) Three-dimensional bone structure and bone mineral density evaluations of autogenous bone graft after sinus augmentation: a microcomputed tomography analysis. Clin Oral Implants Res 23:1098–1103
Márton K, Tamás SB, Orsolya N, Béla C, Ferenc D, Péter N et al (2018) Microarchitecture of the augmented bone following sinus elevation with an albumin impregnated demineralized freeze-dried bone allograft (BoneAlbumin) versus anorganic bovine bone mineral: a randomized prospective clinical, histomorphometric, and micro-computed tomography study. Materials 11:202
Chackartchi T, Iezzi G, Goldstein M, Klinger A, Soskolne A, Piattelli A et al (2011) Sinus floor augmentation using large (1–2 mm) or small (0.25–1 mm) bovine bone mineral particles: a prospective, intra‐individual controlled clinical, micro‐computerized tomography and histomorphometric study. Clin Oral Implants Res. 22:473–480
Caubet J, Ramis JM, Ramos-Murguialday M, Morey MÁ, Monjo M (2015) Gene expression and morphometric parameters of human bone biopsies after maxillary sinus floor elevation with autologous bone combined with Bio-Oss® or BoneCeramic®. Clin Oral Implants Res 26:727–735
Chopra PM, Johnson M, Nagy TR, Lemons JE (2009) Micro-computed tomographic analysis of bone healing subsequent to graft placement. J Biomed Mater Res B Appl Biomater 88:611–618
Rebaudi A, Maltoni AA, Pretto M, Benedicenti S (2010) Sinus grafting with magnesium-enriched bioceramic granules and autogenous bone: a microcomputed tomographic evaluation of 11 patients. Int J Periodontics Restorative Dent 30:53–61
Wang F, Zhou W, Monje A, Huang W, Wang Y, Wu Y (2017) Influence of healing period upon bone turn over on maxillary sinus floor augmentation grafted solely with deproteinized bovine bone mineral: a prospective human histological and clinical trial. Clin Implant Dent Relat Res 19:341–350
Pilloni A, Bernard GW (1992) Low molecular weight hyaluronic acid increases osteogenesis in vitro. J Dent Res. 71:574(IADR abstract #471}
Huang L, Cheng Y, Koo P, Lee K, Qin L, Cheng J et al (2003) The effect of hyaluronan on osteoblast proliferation and differentiation in rat calvarial-derived cell cultures. J Biomed Mater Res A 66:880–884
Stiller M, Kluk E, Bohner M, Lopez-Heredia MA, Müller-Mai C, Knabe C (2014) Performance of β-tricalcium phosphate granules and putty, bone grafting materials after bilateral sinus floor augmentation in humans. Biomaterials 35:3154–3163
Raines AL, Sunwoo M, Gertzman AA, Thacker K, Guldberg RE, Schwartz Z et al (2011) Hyaluronic acid stimulates neovascularization during the regeneration of bone marrow after ablation. J Biomed Mater Res A 96:575–583
Jaffin RA, Berman CL (1991) The excessive loss of Branemark fixtures in type IV bone: a 5-year analysis. J Periodontol 62:2–4
Funding
This work was supported by Hacettepe University, Scientific Research Projects Coordination Unit [grant number THD-2015–5190].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Approval was obtained from Clinical Researches Ethics Board of Hacettepe University (2014/08—16 (KA-14030)). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gurbuz, E., Dursun, E., Vatansever, A. et al. Microcomputed tomographic analysis of bone microarchitecture after sinus augmentation with hyaluronic matrix: a case–control study. Oral Maxillofac Surg 26, 431–437 (2022). https://doi.org/10.1007/s10006-021-01002-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10006-021-01002-5