Abstract
Purpose
The purpose of this study was to describe a case and to review the diameters, symptoms, locations, and treatment methods for vascular malformations (VMs) with phleboliths. Our case report is probably the first to mention this observation because of sizes and large number of phleboliths in buccal region.
Case presentation
A 26-year-old male patient was referred to Ordu University, Faculty of Dentistry, Department of Oral and Maxillofacial Surgery for the evaluation and management of a painless tender swelling in the left buccal region. Clinically, a bluish mucosal lesion of the posterior region of the left buccal mucosa and lip is apparent. Panoramic radiography and CT were obtained for radiographical examinations. Multiple giant phleboliths with the largest dimension of 32 mm were seen in this region. Left maxillary first molar teeth extraction was indicated. An aspiration was performed and revealed that there is a risk of severe hemorrhage. The patient did not want to take MRI and stated that he only wanted to have dental treatment. Therefore, it was decided that the maxillary first molar should be retained in the region and endodontic treatment should be done, if necessary.
Conclusions
The clinic of phlebolith patients is painless swelling and can reach up to 6 cm. The localization is not specific but is found in the masseter and parotid regions generally. Treatment may be invasive or non-invasive depending on, location, accessibility, depth of invasion, age, cosmetic issues, and risk of severe hemorrhage, as with the current case.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Vascular malformations (VMs) are benign lesions that are often present at birth and extend during growth [1,2,3]. According to ISSVA classification (2018) for vascular anomalies, VM’s are divided into four types as simple (capillary, lymphatic, venous, arteriovenous), combined, of major named vessels, and associated with other anomalies. They are present at birth, grow proportionally with the child, and does not undergo regression generally [4]. Although VMs are one of the most common soft tissue tumors of the head and neck, they are relatively rare in the oral cavity and are seldom encountered by clinicians [5].
VMs and phleboliths are rarely seen together [6,7,8,9]. They are seldom reported as solitary calcifications and/or not associated with a vascular lesion [10,11,12]. Changes in blood flow dynamics within the VM may result in a thrombus and phleboliths [10, 13, 14]. Trauma and regression of childhood VMs of the oral mucosa are considered causative for the occurrence of an isolated phlebolith [8, 9, 11, 12, 15, 16]. Phleboliths may be the only residual sign in the adult of a childhood VM, mainly because they are generally painless [3, 11]. In addition, bimanual palpation of the area has revealed a mass with hard nodules of various sizes, which may suggest that the VM is associated with phleboliths [11].
Radiographically, phleboliths present as oval structures with concentric radiolucent or radiopaque laminations [10]. They vary from 1 to 5 mm in diameter, but they may be 1 cm or larger [17]. In addition, particular radiographic techniques, such as computed tomography (CT), magnetic resonance imaging (MRI), angiography, and color-Doppler imaging may be needed in the diagnosis and treatment of phleboliths [4, 18].
Surgical excision, partial resection of masseter muscle [8], muscle trimmed or curetted [8], gland enuclation or excision [6, 9, 12,13,14, 19,20,21,22,23] are the surgical methods used in the treatment of AVMs. They usually yield successful results [8]. Non-invasive treatment methods such as endovascular embolization [23, 24] and sclerotherapy [25] may be preferred in cases of high risk of bleeding, esthetic problems after surgery, difficult access, and depth of the invasion [8].
In the literature, there have been 42 cases of reported phleboliths, which had diameters, symptoms, locations and treatment methods of the phleboliths presented here. The purpose of this study was to describe a new large sizes and multiple phleboliths case not seen yet in literature and to review the relevant literature.
Data sources
Literature was searched in the period between 1955 and 2017. Medline and Embase database were searched for the words “vascular malformations, multiple radiopaque laminations, phleboliths, vascular and venous malformations” Only articles written in English were included in present review. Twenty-six from 351 articles related phleboliths was chosen (6 case series, 20 case, and total 42 case). The 114 cases have VM but not phleboliths were excluded. Patient information (sex and age), clinical symptoms (pain, swelling, time) radiological findings (size and location) and treatment methods of phleboliths were collected.
Case presentation
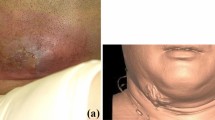
A 26-year-old man presented to Ordu University, Faculty of Dentistry, Department of Oral and Maxillofacial Surgery with swelling from the left buccal region (Fig. 1a, b). Clinically, a bluish mucosal lesion of the posterior region of the left buccal mucosa and lip is apparent and there was no pain (Fig. 1b). The patient stated that his swelling occurred at a very young age and had remained the same. In addition, the patient had referred to other hospitals for molar extractions, but no operations were performed because of the risk of bleeding.
CT were obtained for radiographical examinations (Fig. 2a). Panoramic radiography showed radiolucencies at the left mandible from the angulus to the corpus (Fig. 2b). Many phleboliths (< 33 mm wide) were also seen in this region. It was the large size and number of phleboliths that separated our patient from other reported VMs.
The patient was prepared for an incisional biopsy, but before beginning the biopsy, an aspiration was attempted. This was positive for a vascular process, so instead of an incisional biopsy, only an aspiration was performed. This revealed the lesion to be a vascular malformation.
We wanted MRI to create a pretreatment plan. However, the patient did not want to take MRI and stated that he only wanted to have dental treatment. Therefore, it was decided that the tooth should be retained in the region and endodontic treatment should be done, if necessary.
After radiographical and clinical examinations, a biopsy followed by histopathological examination, clinical history and immunohistochemistry can aid in arriving at a final diagnosis of the VMs and in this case, it was reached with clinical examinations, CT—panoramic radiography and an aspiration.
Discussion and conclusion
VMs and phleboliths are rarely seen together [6,7,8,9]. Phlebolith formation within a VM occurs in approximately 25–40% of cases [26, 27]. In the present case, giant buccal phleboliths were present and they are from one of the 42 cases discussed in the analysis of phleboliths (Table 1). In the literature, the cases reported to date suggest that phleboliths may occur across a wide age range (7–92 years), while the rates for genders are same (21 males and 21 females). Phleboliths can be anywhere in the head and neck region [1, 4, 6, 8,9,10,11,12,13, 16, 18,19,20,21,22,23,24,25, 27,28,29,30,31]. However, the phlebolith cases in this review have more commonly been reported in the masseter (14.2%) and parotid regions (19%) (Table 1).
Clinically, the most common sign of VM is a slow-growing palpable bluish purple mass that can fluctuate [6, 15, 32,33,34]. Swelling may be present in early childhood [8, 13]. In phlebolith cases, 32 of 40 patients showed swelling (80%). It can be seen at the salivary glands (parotid) [23], muscles [6, 8], soft tissues (lip, cheek etc.) [6, 12, 14, 18, 19, 24] (Table 1). A temporary increase in the size of the mass has been observed when the patients cried, laughed, performed handstands [8], experienced excitation, wore a tight collar [6], or when the patient clenched their teeth [11]. Palpation can reveal that the swelling is tender and the lesion is pulsatile [24]. Sometimes it can take a long time to notice the swelling [12, 19, 32]. In addition, some cases have stated that the swelling is formed at certain intervals [19, 22].
Pain is dependent on the speed of enlargement, pressure on adjacent anatomic structures, and thrombosis in VMs [15, 35]. In the present review, which evaluated phleboliths with/without VMs, only 11 of the 42 patients felt pain (26.1%). In painful cases, phleboliths have generally been located in the masseter muscle, parotid gland, lip, teeth, and in the orbit (Table 1) [1, 14, 19, 30, 31]. Phleboliths and pain can be in the different regions [1, 19]. There was no paresthesia or dysphasia [24]. However, some patients have discomfort resulting from facial asymmetry, restriction of mouth opening [12, 24, 27], headaches, coughing, blurred vision and feelings of heaviness in the eyelids [31].
The pathogenesis of phleboliths, which become organized and calcified, is thought to involve thrombi that are produced by the slowing of peripheral blood flow [6, 13, 14, 36]. Since phleboliths are calcified, they can be observed in X-rays. They vary from 1 to 5 mm in diameter, but they may also be 1 cm or larger [17]. From the phlebolith cases present in the literature, the diameters were between 1 mm and 60 mm. They exceeded 20 mm in only eight patients. In the present case, the diameter was 32 mm. The time since the onset of phlebolith formation can be related to the diameter. However, no study has examined this topic further.
In general, the diagnosis of an intramuscular VM of the head and neck region can be difficult [37, 38]. Only 8% of all cases of intramuscular VMs are diagnosed preoperatively [38]. Therefore, radiograms are useful in the confirmation of a clinical diagnosis. [39]. Imaging by MRI can be used to determine characterization, recognition, and the extent of the lesion [8, 35, 40]. In addition, dynamic contrast‑enhanced MRI has increased the specificity of vascular malformation diagnosis and can also be helpful in distinguishing between low-flow (capillary, venous, and lymphatic malformations) VM and high-flow (arteriovenous malformations) VM [27, 32]. However, detectability of phleboliths on CT images is superior to that on MRI [1]. In the present case, CT was used to examine the relationship between the tooth root and VMs (Fig. 3). In addition, feeding vessels of the VMs can be identified through angiography and angioxerography. Peripheral vascularity within the lesion for both arterial and venous nature of the flow can also be appreciated with color-Doppler as well as its velocity [1, 4, 18, 41]. In addition, ultrasound has been shown to be a reliable method for diagnosing alterations in the muscles [8]. Besides imaging exams, a biopsy followed by a microscopic examination can help in the final diagnosis [3, 10]. The patient in our case only an aspiration was performed, and this revealed that there is a risk of severe hemorrhage because of yielded venous blood or blood-tinged fluid. In addition, phleboliths may mimic sialoliths, calcified lymph nodes, carotid artery calcifications, miliary skin osteomas, cysticercosis, and traumatic myositis ossificans on various imaging modalities [2, 8, 9, 13, 16]. In such cases, a histopathological examination, combined clinical history, and immunohistochemistry can aid in arriving at a final diagnosis [9].
The treatment of VM is based on location, accessibility, depth of the invasion, age, and cosmetic considerations [8]. According to Rossiter et al. [42] the optimal treatment method for VMs is a complete surgical excision because of low recurrence rates. In the previous cases, surgical procedures included a partial resection of the masseter muscle [8], muscle trimmed or curetted [8] gland enucleation or excision [6, 9, 12,13,14, 19,20,21,22,23], intralesional injection of pingyangmycin hydrochloride (PH) used for sclerotherapy [25], and endovascular embolization [23, 24]. A sclerosing agent includes STS (sodium tetradecyl sulfate), polidocanol, and absolute alcohol [30]. Using up to 95% absolute alcohol prevent recurrence. [30] Recurrence following surgery has been reported in 18% of the cases [33]. The excision of large deforming lesions can be difficult because of the risk of severe hemorrhage. To solve this problem, corticosteroids, embolization, and an intralesional injection of fibrosing agents are sometimes advised [15]. In present case, the patient only wanted dental treatment. Therefore, the acceptable treatment method, embolization, was not performed.
In conclusion, asymptomatic, opaque, irregular radiopacities during routine radiography in the face and neck region with swelling, especially during childhood, increase the likelihood of phleboliths. The localization is not specific but is found in the masseter and parotid regions generally. The treatment method may be invasive or non-invasive depending on risk of severe hemorrhage, location, accessibility, depth of invasion, age, and cosmetic issues, but the optimal treatment method for VMs is a complete surgical excision because of low recurrence rates.
References
Altug HA, Buyuksoy V, Okcu KM, Dogan N (2007) Hemangiomas of the head and neck with phleboliths: clinical features, diagnostic imaging, and treatment of 3 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103:e60–e64
Lee JK, Lim SC (2005) Intramuscular hemangiomas of the mylohyoid and sternocleidomastoid muscle. Auris Nasus Larynx 32:323–327
Hassani A, Saadat S, Moshiri R, Shahmirzadi S (2014) Hemangioma of the buccal fat pad. Contemp Clin Dent 5:243–246
Mohan RP, Dhillon M, Gill N (2011) Intraoral venous malformation with phleboliths. Saudi Dent J 23:161–163
Sobhana CR, Beena VT, Soni A, Choudhary K, Sapru D (2012) Hemangiolymphangioama of buccal mucosa: report of a rare case and review of literature on treatment aspect. Natl J Maxillofac Surg 3:190–194
Sano K, Ogawa A, Inokuchi T, Takahashi H, Hisatsune K (1988) Buccal hemangioma with phleboliths. Report of two cases Oral Surg Oral Med Oral Pathol 65:151–156
Kanaya H, Saito Y, Gama N, Konno W, Hirabayashi H, Haruna S (2008) Intramuscular hemangioma of masseter muscle with prominent formation of phleboliths: a case report. Auris Nasus Larynx 35:587–591
Zengin AZ, Celenk P, Sumer AP (2013) Intramuscular hemangioma presenting with multiple phleboliths: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol 115:e32–e36
Nagaraja A, Kumar NG, Kumar BJ, Naik RM, Sangineedi YJ (2016) A solitary phlebolith in the buccal mucosa: report of a rare entity and clinicopathologic correlation. J Contemp Dent Pract 17:706–710
Gouvea Lima Gde M, Moraes RM, Cavalcante AS, Carvalho YR, Anbinder AL (2015) An isolated phlebolith on the lip: an unusual case and review of the literature. Case Rep Pathol 2015:507840
Zachariades N, Rallis G, Papademetriou J, Konsolaki E, Markaki S, Mezitis M (1991) Phleboliths. A report of three unusual cases. Br J Oral Maxillofac Surg 29:117–119
Kato H, Ota Y, Sasaki M, Arai T, Sekido Y, Tsukinoki K (2012) A phlebolith in the anterior portion of the masseter muscle. Tokai J Exp Clin Med 37:25–29
Cankaya H, Unal O, Ugras S, Yuca K, Kiris M (2003) Hemangioma with phleboliths in the sublingual gland: as a cause of submental opacity. Tohoku J Exp Med 199:187–191
Chuang CC, Lin HC, Huang CW (2005) Submandibular cavernous hemangiomas with multiple phleboliths masquerading as sialolithiasis. J Chin Med Assoc 68:441–443
Mandel L, Surattanont F (2004) Clinical and imaging diagnoses of intramuscular hemangiomas: the wattle sign and case reports. J Oral Maxillofac Surg 62:754–758
Gooi Z, Mydlarz WK, Tunkel DE, Eisele DW (2014) Submandibular venous malformation phleboliths mimicking sialolithiasis in children. Laryngoscope 124:2826–2828
Parker LA Jr, Frommer HH (1964) Phleboliths. Report of a Case Oral Surg Oral Med Oral Pathol 18:476–480
Ghosh S, Singh K, Garg A, Kumar P, Gupta S (2015) A rare case of low flow vascular malformation of head and neck region presenting with multiple phleboliths. J Clin Diagn Res 9:ZJ01–ZJ02
O'Riordan B (1974) Phleboliths and salivary calculi. Br J Oral Surg 12:119–131
Schwartz A, Salz N (1955) Cavernous hemangioma associated with phleboliths in the masseter muscle. Acta Radiol 43:233–234
Choi HJ, Lee JC, Kim JH, Lee YM, Lee HJ (2013) Cavernous hemangioma with large phlebolith of the parotid gland. J Craniofac Surg 24:e621–e623
Chen B-N (2017) Cavernous hemangioma with multiple phleboliths of the parotid gland in adult masquerading assialolithiasis. Int J Clin Exp Med 10:11097–11100
Faber RG, Ibrahim SZ, Drew DS, Hobsley M (1978) Vascular malformations of the parotid region. Br J Surg 65:171–175
Orhan K, Icen M, Aksoy S, Avsever H, Akcicek G (2012) Large arteriovenous malformation of the oromaxillofacial region with multiple phleboliths. Oral Surg Oral Med Oral Pathol Oral Radiol 114:e147–e158
Su YX, Liao GQ, Wang L, Liang YJ, Chu M, Zheng GS (2009) Sialoliths or phleboliths? Laryngoscope 119:1344–1347
Morris SJ, Adams H (1995) Case report: paediatric intramuscular haemangiomata--don't overlook the phlebolith! Br J Radiol 68:208–211
Scolozzi P, Laurent F, Lombardi T, Richter M (2003) Intraoral venous malformation presenting with multiple phleboliths. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 96:197–200
Tosios K, Koutlas IG, Kapranos N, Papanicolaou SI (1995) Spindle-cell hemangioendothelioma of the oral cavity. A case report. J Oral Pathol Med 24:379–382
Aynali G, Unal F, Yariktas M, Yasan H, Ciris M, Yilmaz O (2014) Submandibular hemangioma with multiple phleboliths mimicking sialolithiasis: the first pediatric case. Kulak Burun Bogaz Ihtis Derg 24:168–171
Saurabh Prakash, V.N., Jawahar Dhanavel (2017) Multiple low flow vascular malformation with phleboliths: a case report. J Young Pharm 9
Lloyd GA (1965) Phleboliths in the orbit. Clin Radiol 16:339–346
Chava VR, Shankar AN, Vemanna NS, Cholleti SK (2013) Multiple venous malformations with phleboliths: radiological-pathological correlation. J Clin Imaging Sci 3:13
Terezhalmy GT, Riley CK, Moore WS (2000) Intramuscular hemangiomas. Quintessence Int 31:142–143
Addante RR, Donovan MG (1994) Right facial mass. J Oral Maxillofac Surg 52:1061–1065
Itoh K, Nishimura K, Togashi K, Fujisawa I, Nakano Y, Itoh H, Torizuka K (1986) MR imaging of cavernous hemangioma of the face and neck. J Comput Assist Tomogr 10:831–835
H., R (1917) Die Phlebolithen. Virchows Arch 223:339–350
Elahi MM, Parnes L, Fox A (1992) Hemangioma of the masseter muscle. J Otolaryngol 21:177–179
Avci G, Yim S, Misirliogolu A, Akoz T, Kartal LK (2002) Intramasseteric hemangioma. Plast Reconstr Surg 109:1748–1750
Zou ZJ, Wu YT, Sun GX, Zhu XP, Meng XZ, He ZQ (1983) Clinical application of angiography of oral and maxillofacial hemangiomas. Clinical analysis of seventy cases. Oral Surg Oral Med Oral Pathol 55:437–447
Yonetsu K, Nakayama E, Miwa K, Tanaka T, Araki K, Kanda S, Ohishi M, Takenoshita Y, Yoshida K, Katsuki T (1993) Magnetic resonance imaging of oral and maxillofacial angiomas. Oral Surg Oral Med Oral Pathol 76:783–789
Gianfranco G, Eloisa F, Vito C, Raffaele G, Gianluca T, Umberto R (2014) Color-Doppler ultrasound in the diagnosis of oral vascular anomalies. N Am J Med Sci 6:1–5
Rossiter JL, Hendrix RA, Tom LW, Potsic WP (1993) Intramuscular hemangioma of the head and neck. Otolaryngol Head Neck Surg 108:18–26
Author information
Authors and Affiliations
Contributions
SEC and CB summarized the case and wrote the manuscript. OMM is the principal surgeon and provided the overall supervision in the writing of this article. AF and ZE performed the radiological examination of the lesion. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sivrikaya, E.C., Cezairli, B., Ayranci, F. et al. Buccal vascular malformation with multiple giant phleboliths: a rare case presentation and review of the literature. Oral Maxillofac Surg 23, 375–380 (2019). https://doi.org/10.1007/s10006-019-00767-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10006-019-00767-0