Abstract
Pilomatrixoma is considered a rare benign tumor arising from the hair follicle, most common in the head and neck region, but it is rarely diagnosed on a clinical basis. This report describes a new case of giant pilomatrixoma in a 36-year-old female patient. The nodule was localized in the preauricular area on the right side, appearing as a slow-growing, fixed, painless, with a hardened consistency, unusual giant (4.5 cm). A cone-beam computed tomography (CBCT) examination showed a slightly hyperdense lesion, and fine-needle aspiration cytology (FNAC) revealed peripheral blood and mononucleated inflammatory cells. After enucleation of lesion, a diagnosis of pilomatrixoma was confirmed. The differential diagnosis of pilomatrixoma is broad, because its characteristics also can be found in other lesions common to the head and neck. Thus, a lesion in the head and neck, adherent to the skin, and well demarcated, mainly in the young and in females, should be suspected as pilomatrixoma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pilomatrixoma, also known as pilomatricoma, is a rare benign tumor, arising from the hair follicle, with an estimated incidence of 1 in 1000 skin biopsies [1]. The lesion usually occurs in the upper extremities, as in the head and neck region, and is rarely diagnosed clinically [2].
Typically, the pilomatrixoma is described as a firm, painless, well-defined solitary nodule, which may have a bluish-red coloration [3]. The size normally ranges from 0.5 to 4.5 cm in diameter, and the highest incidence is found in children and females [3].
Its histopathological aspects include irregular epithelial cells agglomerated in islands, presence of the shadow cells (named “ghost” cells), and areas of calcification [1]. Although it is well known by dermatologists and pathologists, it can sometimes be confused by clinicians with a malignant neoplasm, resulting in unnecessary extensive surgery [1, 4]. When present over the preauricular area, it can be confused with lesions of the parotid gland [4]. We are reporting a case of pilomatrixoma presenting as a subcutaneous nodule of the preauricular region.
Case report
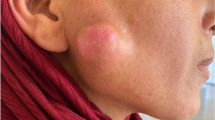
A 36-year-old female visited our university complaining of slow-growing, painless nodule in the preauricular area and mandibular angle on the right side, which the patient had noticed for about 14 months (Fig. 1). No other relevant medical information was identified in the patient’s clinical history.
Upon physical examination, a 4.5-cm fixed, painless nodule with a hardened consistency was observed near the tail of the right parotid gland (Fig. 1). Milking the gland showed clear, unchanged salivary flow without pain.
At cone-beam computed tomography (CBCT) examination, it was possible to see, with intermediate electrodensity, the lesion and small focal areas hyperdense. The lesion was measured with software Ozirix v.5.0 32-bit and showed an approximate area of 1.4 cm2 and approximately 4.5-cm length as the largest (Fig. 2a–d).
Fine-needle aspiration cytology (FNAC) revealed only peripheral blood and mononuclear inflammatory cells. It was decided to perform the surgical resection under general anesthesia, with a retromandibular incision (Hinds’ access), dissection, and enucleation of lesion.
The lesion was localized in the subcutaneous region above the platysma, limited by delicate hair tissue. The dissection preserved the mandibular branch of the right facial nerve and, after excision, planes were sutured (Fig. 3a).
The anatomic sample obtained showed well-defined, encapsulated, yellow-white tumor, measuring approximately 4.5 cm in greatest diameter (Fig. 3b). Histological sections revealed a tumor mass with the central area rich in stratified squamous epithelium forming multiple cystic areas. The central zones contained sheets of amorphous eosinophilic cells with ghosted clear nuclei (“shadow cells”). Focal areas of dystrophic calcification also were present. The periphery of the tumor was surrounded by a thin capsule of the organized conjunctive tissue (Fig. 4). A diagnosis of pilomatrixoma was made.
The patient recovered uneventfully, and no recurrence has been noted 12 months later.
Discussion
Pilomatrixoma was first described in 1880 [5] as a benign neoplasm of sebaceous gland or epithelioma calcifying of the sebaceous glands, because these who was thought to be its origin [4, 6]. Since then, it was named calcifying epithelioma of Malherbe, referring to the author who described the tumor [5]. In 1949, it was suggested that the tumor originated from cells present in the hair follicle [7], and, in 1961, the term pilomatrixoma was proposed to emphasize the lesion origin [8], avoiding the term “epithelioma,” which has a connotation with malignancy [3, 9].
It is an unusual benign neoplasm, slow-growing, present for months or years before diagnosis. It manifests as cutaneous, firm lesions of the upper extremities [6], where the head and neck are the most common sites [10]. Although most masses measure less than 1.5 cm in diameter, lesions with 13.5 cm were reported in literature [4]. The nodule presented in our case report had approximately 4.5-cm diameter as the largest, having higher volume than presented in most of the cases in literature, and classified as giant [11].
It typically appeared as a solitary mass, as is the case of our patient. In cases of the multiple lesions [10], it is suspected of related Gardner, Turner, Rubinstein–Taybi, or Churg–Strauss syndromes, besides xeroderma pigmentosum or sarcoidosis [3, 10]. The presence of calcification gives firm characteristic to lesion, with irregular angulated shapes when stretched, called “tent sign” [2].
The variant morphology of pilomatrixoma, sometimes similar to more common lesions, induces to difficulty to establish a clinical diagnosis [6]. The bluish-red discoloration overlying skins, due to dilated blood vessels [3], may confuse with hemangiomas [2]. Presence of tenderness in a firm subcutaneous swelling may lead to make a clinical diagnosis as neural tumor-like neurofibromas [2]. Other differential diagnosis previously reported includes lymphadenitis [12]; sebaceous, dermoid, and epidermoid cysts [1, 6, 10]; parotid tumor [1]; atheroma [13]; keratoacanthoma; fibroxanthoma; atypical infections by mycobacteria; sinuses; ossifying hematoma; tumor of giant cell; or chondroma [6, 10], besides malignant lesions such as squamous cell carcinoma [6], carcinoma matricial, carcinoma basocelular, and malignant melanoma [14].
Adenoid cystic carcinomas, also of slow growth, are most common in the minor salivary glands and appear as masses involved in glands, submucosal and smooth, overlying ulcerations [15], which could invade bone or a perineural spread, leading to pain [15]. FNAC shows round to ovoid basaloid cells with hyperchromatic nuclei, besides globules of mucus, and the treatment is local resection, and radiation therapy or chemotherapeutic for advanced disease statuses [15].
Among the benign neoplasms of the salivary gland, the pleomorphic adenoma in the parotid gland is the most common [16, 17] and is considered in the differential diagnosis of the pilomatrixoma [16]: sessile nodule, slow and asymptomatic growth, well delimited, and firm palpation. May be by reduction of salivary flow. It affects females more than males but is commonly seen in the third to sixth decades of life [18].
FNAC has been utilized. However, it is argued that the presence of ghost cells in the aspirate is difficult, which impairs the correct diagnosis [2, 10]. That is because the yield may contain numerous keratinized squamous cells and few basaloid and shadow cells, or the aspirate is made in an early lesion, giving basaloid cells and absence of other components [2]. In our case, FNAC revealed only peripheral blood and eventual leukocytes mononucleated.
The use of radiographs can help identify calcifications but is considered of low diagnostic value by superficial location of pilomatrixoma [10]. There have also been reports of the use of computed tomography (CT) or magnetic resonance imaging (MRI) [1, 10, 19].
CT demonstrates sharply demarcated mass of soft tissue density containing micro-calcifications. However, these characteristics also can be found in other lesions, as in the sebaceous cysts, foreign body reaction, and metastatic bone formations [6]. Thus, imaging CT has been used mainly for differentiating preauricular tumors from parotid tumors or large and aggressive tumors [1]. The MRI revealed that the lesion presented as a homogenous mass for pilomatrixoma [10], and no demonstrate specific features, like this has not been used to definitive diagnostic [6]. We utilized CBCT imaging and proved with histopathological evaluation. The ultrasound also has demonstrated good accuracy rates for diagnosis of the pilomatricoma, because it shows characteristic results, as heterogeneous echotexture, the posterior shadowing, ovoid complex mass, and focus of subcutaneous fat [6].
Table 1 presents the diagnostic methods and preoperative diagnosis of the 77 case reports, with 42 in the preauricular region. In these 77 cases, only 20 included pilomatrixoma in the preoperative diagnosis. The diagnostic methods included CT imaging, FNAC, fluorodeoxyglucose-positron emission tomography (FDG-PET/CT) scan, MRI, ultrasonography, test for paraphenylenediamine (PPD), radiograph exams, and histologic examination.
The more reliable method of diagnosis is histopathological evaluation. Often, pilomatrixoma is located in the lower dermis [1], and the histological pattern seen is of a well-circumscribed nodule-cystic tumor [6, 10]. This nodule usually is surrounded by a connective tissue, with irregular islands of epithelial cells; in the center are ghost cells and, in the periphery, the basaloid cells [6, 10].
As the tumor matures, these basaloid cells of the periphery of the tumor that have attempted to produce hair keratinize and degrade centrally forming the anucleated ghost, called ghost cells [6, 10]. The age of the tumor could be stipulated by proportion of shadow cells to basophilic cells, where younger lesions have greater numbers of proliferating basophilic cells [1]. Recently, the expression of interleukin-8 (IL-8), IL-8 receptor alpha (CXCR1), and IL-8 receptor beta (CXCR2) in pilomatrixomas has been found, which may help in the definition of future diagnoses [32].
Presence of the keratinized debris can produce foreign body reactions. Also, areas of calcification within the shadow cell regions can be found. However, only shadow cells are not sufficient for the diagnosis [6], and presence of basaloid cells or ghost cells need not be present in all the cases of pilomatrixoma, making the diagnosis difficult [2].
The transition of the pilomatrixoma to his malignant version is exceedingly rare. According Zloto and colleagues [3], to date, his publication of 101 cases of pilomatrix carcinoma has been reported. Pilomatrix carcinoma shows male predominance and is common in the elderly [2, 3, 6].
As is not observed the spontaneous regression of the pilomatrixoma tumors, surgical excision is sufficient as treatment, with good prognosis [6] and recurrence uncommon [3, 6, 27]. Yoshimura and colleagues [13], analyzing 37 pilomatrixoma tumors, observed that the treatment was by enucleation in 29, and seven included the removal of the overlying skin. There was no recurrence during a follow-up period of 43 months.
In summary, we observe that the case reports of pilomatrixomas increased in the last years, but yet have been considered in the differential diagnosis of these lesions. Thus, pilomatrixomas are frequently misdiagnosed. The clinical difficulty distinguishing pilomatrixomas from more common skin lesions eventually led a treatment more aggressive. In lesions of the head and neck, adherent to the skin that are well demarcated, mainly in the young and females, oral surgeons should suspect pilomatrixoma tumors.
References
Lee KH, Kim HJ, Suh CH (2000) Pilomatricoma in the head and neck: CT findings in three patients. J Comput Assist Tomogr 24:332–335
Sinhasan SP, Jadhav CR, Bhat RV, Amaranathan A (2013) Pilomatrixoma - presented as hypopigmented tender nodule: diagnosed by FNAC: a case report with review of literature. Indian J Dermatol 58:405
Zloto O, Fabian ID, Vishnevskia Dai V, Ben Simon GJ, Rosner M (2015) Periocular pilomatrixoma: a retrospective analysis of 16 cases. Ophthal Plast Reconstr Surg 31:19–22
Makek M, Franklin DJ, Fisch U (1989) Preauricular pilomatrixoma: a diagnostic pitfall. Oral Surg Oral Med Oral Pathol 68:451–454
Malherbe A, Chenantais J (1880) Note surl’epitheliome calcifit: desglandes sebaces. Progr Med 8:826–828
De Rosa DC, Lin-Hurtubise K (2012) Pilomatricoma: an un usual dermatologic neoplasm. Hawaii J Med Public Health 71:282–286
Lever WF, Griesemer RD (1949) Calcifying epithelioma of Malherbe; report of 15 cases, with comments on its differentiation from calcified epidermal cyst and on its histogenesis. Arch Dermatol Syphilol 59:506–518
Forbis R Jr, Helwig EB (1961) Pilomatrixoma (calcifying epithelioma). Arch Dermatol 83:606–618
Souto MP, Matsushita MM, Matsushita GM, Souto LR (2013) An unusual presentation of giant pilomatrixoma in an adult patient. J Dermatol Case Rep 7:56–59
Whittemore KR, Cohen M (2012) Imaging and review of a large pre-auricular pilomatrixoma in a child. World J Radiol 4:228–230
Cappellesso R, Bellan A, Saraggi D, Salmaso R, Ventura L, Fassina A (2015) YAP immunoreactivity is directly related to pilomatrixoma size and proliferation rate. Arch Dermatol Res 307:379–383
Rachakonda T, Kacker A, Koizumi J (2010) Pediatric pilomatrixoma of the preauricular region. Acta Cytol 54:724–726
Yoshimura Y, Obara S, Mikami T, Matsuda S (1997) Calcifying epithelioma (pilomatrixoma) of the head and neck: analysis of 37 cases. Br J Oral Maxillofac Surg 35:429–432
Williams CM, Bozner P, Oliveri CV, Horenstein MG (2003) Melanocytic matricoma: case confirmation of a recently described entity. J Cutan Pathol 30:275–278
Vidyalakshmi S, Aravindhan R (2014) Adenoid cystic carcinoma of the buccal mucosa: a case report with review of literature. J Clin Diagn Res 8:266–268
Aydin S, Bilmez ZE, Erdogdu S, Altintoprak N, Kayipmaz S (2016) Complicate giant pilomatrixoma of the parotid region. J Maxillofac Oral Surg 15:111–115
Sciandra D, Dispenza F, Porcasi R, Kulamarva G, Saraniti C (2008) Pleomorphic adenoma of the lateral nasal wall: case report. Acta Otorhinolaryngol Ital 28:150–153
Vicente OP, Marqués NA, Aytés LB, Gay Escoda C (2008) Minor salivary gland tumors: a clinicopathological study of 18 cases. Med Oral Patol Oral Cir Bucal 13:E582–E588
Just T, Hingst V, Kreutzer HJ, Pau HW (2005) Hard, expanding preauricular swelling in a 6-year-old child. HNO 53:1074–1076
Bernier FE, Schreiber A, Coulombe J, Hatami A, Marcoux D (2017) Pilomatricoma associated with kabuki syndrome. Pediatr Dermatol 34:e26–e27
Bajpai M, Arora M, Chandolia B (2016) A rare case of pilomatrixoma (calcifying epithelioma of Malherbe) of parotid space masquerading as salivary gland tumor. Iran J Pathol 11:418–420
Dutta M, Chatterjee I (2016) A lesson learnt: retrospection in a case of pilomatricoma mimicking as parotid neoplasm. Einstein (Sao Paulo) 14:104–105
Bellafiore S, Tagliavini E, Carlinfante G, Piana S (2016) Pilomatrixoma is a diagnostic trap in fine-needle aspiration cytology of the parotid region. Diagn Cytopathol 44:516–518
Bhatt MK, Sommerville R, Ravi Kumar AS (2012) FDG PET/CT appearance of benign pilomatricoma. Clin Nucl Med 37:684–686
Hwang JY, Lee SW, Lee SM (2005) The common ultrasonographic features of pilomatricoma. J Ultrasound Med 24:1397–1402
Yuca K, Kutluhan A, Cankaya H, Akman E (2004) Giant pilomatrixoma arising in the preauricular region: a case report. Kulak Burun Bogaz Ihtis Derg 12:147–149
Silva TA, Moraes EF Jr, Consolaro A, Lara VS (2003) Pilomatricoma of the auricular region: case report. Braz Dent J 14:223–226
Sari A, Yavuzer R, Isik I, Latifoglu O, Ataoglu O (2002) Atypical presentation of pilomatricoma: a case report. Dermatol Surg 28:603–605
Phyu KK, Bradley PJ (2001) Pilomatrixoma in the parotid region. Laryngol Otol 115:1026–1028
Ooi LL, Sim CS, Soo KC (1992) Calcifying epithelioma of Malherbe--a case report of a large preauricular tumour. Ann Acad Med Singap 21:379–381
Yoshimura Y, Oka M (1990) Pilomatrixoma of the preauricular region. Br J Oral Maxillofac Surg 28:416–418
Cianga CM, Cianga P, Dumitrescu GF, Sava A (2016) IL-8, IL-8RA (CXCR1) and IL-8RB (CXCR2) expression in pilomatricoma. Romanian J Morphol Embryol 57:59–64
Acknowledgements
Special thanks to Professor Brad Neville for the English stile review and advise during the diagnostic process.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
The consent of patient was obtained for publication of this case report and images.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Pinheiro, T.N., Fayad, F.T., Arantes, P. et al. A new case of the pilomatrixoma rare in the preauricular region and review of series of cases. Oral Maxillofac Surg 22, 483–488 (2018). https://doi.org/10.1007/s10006-018-0724-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10006-018-0724-8