Abstract
Purpose
The aim of the present study was to compare two therapeutic protocols of dexamethasone for the prevention of postoperative swelling, pain, and other complications after the extraction of impacted third molars, in a prospective, randomized, crossover, double-blinded clinical trial.
Methods
Fifty patients with symmetrical impaction of third molars were randomly assigned to two different protocols of dexamethasone for each side. Patients underwent two sessions performed at a 21-day interval. In group 1, patients took 8 mg of dexamethasone orally 1 h before the procedure, and in group 2, 4 mg dexamethasone orally 1 h before and 24 h after the procedure. Surgery duration, volume of local anesthetics, surgical technique, and rescue medication were standardized. Postoperative pain was evaluated using a visual analog scale (VAS) at predefined times: before operation; immediately after; 1, 2, 4, and 12 h; and 1, 2, 3, and 7 days after operation. The patients were also instructed to take notes of the number of rescue medication tablets taken. Edema and mouth opening were clinically evaluated before surgery and in the postoperative period (second and seventh postoperative days).
Results
There were no significant differences between groups for VAS scales (p = 0.5048), but the use of rescue medication was significantly lower in group 1 (p = 0.006). None statistically significant differences (p > 0.05) were observed between groups in any of the time points for all measurements of edema. However, the mouth opening limitation (DIINC) was bigger (p = 0.0069) for group 1 at 2 days.
Conclusion
Pre-emptive use of different dexamethasone regimens had a beneficial effect against pain, edema, and mouth opening limitation, especially when administered at an 8 mg concentration, which suggests that this protocol may also be efficient for more invasive surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The surgical extraction of impacted third molars usually causes moderate to intense postoperative discomfort because large amounts of inflammatory mediators are released during the manipulation of soft tissues, bone, and teeth. Therefore, adequate pharmacological control is required [1,2,3].
Efficient control of inflammation has an important role, as patients often see impacted tooth surgeries as a great trauma to their lives and try to avoid or delay treatment, which complicates the resolution of dental problems [4, 5]. To reduce postoperative complications, the prophylactic and preventive effects of several drugs have been investigated, and results have widely supported the use of corticosteroids [6, 7].
Dexamethasone is a steroidal anti-inflammatory drug used before and after oral surgeries to control pain, edema, and mouth opening limitations. It acts by inhibiting inflammatory mediators and reducing sensitivity to nociceptors and fluid extravasation to tissues. For several decades, numerous studies have reported on the efficacy of dexamethasone to control postoperative complications after the extraction of impacted third molars. However, the best indication and the most efficacious regimen have not been defined [2, 8, 9].
Even though the results of pre-emptive analgesia in extraction of impacted third molars has been shown to be significant when using 4 mg dexamethasone, recent studies have evaluated the dose of 8 mg dexamethasone for third molar surgeries. Some of these studies have demonstrated the efficacy of this dose for reduction of swelling and pain after third molar extraction [10, 11]. However, there are still few studies comparing different oral doses of dexamethasone in third molar extractions. Hence, this prospective study was designed to compare two therapeutic protocols of dexamethasone for the prevention of postoperative swelling, pain, and other complications after the extraction of impacted third molars.
Material and methods
Study design
This prospective randomized double-blind study was approved by the Ethics Committee of São Leopoldo Mandic Institute and Research Center (Campinas, São Paulo, Brazil—approval no. 811.667) and included 50 patients with an indication of bilateral removal of impacted third molars.
Sample selection
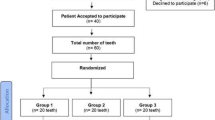
Volunteers were selected according to history and blood tests from a group of 118 patients initially evaluated. All study participants were in good health (ASA I); their mean age was 21 years, and sex distribution was similar. Inclusion criteria were panoramic radiograph and tooth impaction similar in right and left side of the mandible according to Winter (vertical and mesioangular) and Pell and Gregory (IIB or IIC class) classifications [3]. The CONSORT flow chart about sample selection is described in Fig. 1.
Patients with a history of pericoronitis, local infection, impairment of the immune system, hypersensitivity to the drug under test, recent use of any anti-inflammatory or antibiotic drug, prolonged use of any other drug, pregnancy, breastfeeding, premenstrual period, or smoking were excluded from the study, but treated by the surgical team as necessary.
Randomization
Dexamethasone regimen was defined randomly at the time of the first and the second operations considering patient sex, the mouth side, and the drug dose used [12].
Each study patient followed the two different drug regimens, one for each side of the mouth. The following pharmacological regimes were tested: group 1—two tablets of dexamethasone (4 mg Decadron®) 1 h before and one placebo tablet 24 h after surgery and group 2—one tablet of dexamethasone (4 mg Decadron®) and one placebo tablet 1 h before and one dexamethasone tablet (4 mg Decadron®) 24 h after surgery. The study was double-blinded: the dexamethasone and placebo tablets had the same appearance and were identified by codes [13]. Group identification was revealed only at the time for statistical analysis of data and preparation of results. These procedures followed the CONSORT Cochrane Collaboration checklist [14, 15].
Clinical procedures and drugs
The same experienced surgeon operated on the same patients twice using a standardized technique at an interval of 21 days between procedures [8, 10]. Before anesthesia, the study participants were asked to vigorously swish an aqueous solution of 0.12% digluconate chlorhexidine for 1 min [16]. Extraoral asepsis was achieved using an aqueous solution of 2% digluconate chlorhexidine. Local anesthesia consisted of standard inferior alveolar, lingual, and long buccal nerve block using 2% lidocaine hydrochloride with epinephrine 1:100,000 (Alphacaine®-DFL®, Brazil). A standard triangular mucoperiosteal flap was raised for surgical access. The bone around the tooth was removed using a round bur on a high-speed handpiece under continuous physiological irrigation. The crown and roots were separated when necessary. After complete extraction of the tooth and all its components, the dental alveolus was irrigated and carefully examined, after which the flap was closed using 4/0 nylon suture (Ethicon®). A small gauze pack was placed on the site, and the usual postoperative instructions were given to the patient. The duration of each surgery was recorded as minutes from incision to the last suture.
Postoperative analgesic rescue medication was 600 mg ibuprofen tablets (Spidufen®, Zambon, São Paulo, Brazil) administered every 8 h for both groups. The volunteers received six 600 mg ibuprofen tablets and were instructed to start rescue medication if there was pain and to continue the use according to the prescription, taking notes about the time and number of tablets taken [9, 12, 17].
All the patients received the aqueous solution of 0.12% digluconate chlorhexidine (Riohex®, Rio Química, São José do Rio Preto, Brazil) for the oral mouthwash twice a day beginning 24 h after the operation and continuing for five more days. No antibiotic medication was prescribed for the volunteers [15, 18].
Postoperative pain was evaluated using a visual analog scale (VAS) at predefined times: before operation; immediately after; 1, 2, 4, and 12 h; and 1, 2, 3, and 7 days after operation. The patients were also instructed to take notes of the number of rescue medication tablets taken [6, 10].
Edema and mouth opening limitations were measured before operation and on the second and seventh postoperative days by an independent examiner. Edema was evaluated according to linear measurements made from the mandibular angle to the following points: tragus (ANG-TRAG), ala of the nose (ANG-ALAN), lateral canthus (ANG-LCA), labial commissure (ANG-LCO), and mentum (ANG-SUBM). Mouth opening limitation was measured as the distance between the incisal edge of maxillary and mandibular central incisors (DIINC) in the right according to a scale that measures the amplitude of the movement of mouth opening [6, 10]. All volunteers received clinical follow-up of the surgical team at different time points: on the 2nd, 7th, and 30th postoperative days [8, 10].
Statistical analysis
The significance of differences between groups was calculated using the BioEstat 5.0 and the GraphPad Prism 6.0 programs. Means and analysis of variance (ANOVA) were used for descriptive analyses. Unpaired t tests with Welch correction, chi-squared and Wilcoxon tests, and two-way ANOVA were used as necessary to evaluate the significance between results. Different variables in the groups were compared using repeated measures ANOVA. Significance was set at p < 0.05.
Results
The study included 26 (52%) men aged (mean ± standard deviation) 21.3 (± 2.2) years and 24 (48%) women aged 21.7 (± 1.4) years. There were no statistically significant differences (p = 0.4873) in age between sexes, which confirmed that the sample was homogeneous. The most prevalent inclusion classification was mesioangular class IIB classification (p < 0.001), and there were no statistically significant (p = 0.2571) differences in surgery duration between groups.
VAS values for both groups indicated that pain was mild. There were no statistically significant differences (p = 0.5048) between groups at any time point. Increasing pain was recorded in the first 4 h after operation, and, after that, VAS values decreased continually and reached the initial levels at 48 h (Fig. 2).
Table 1 shows the correlation between the number of rescue medication tablets used and recorded VAS values at each time point.
It was not possible to calculate values “before” and “after 7 days” because all VAS values were zero for these intervals. A direct and significant (p < 0.05) association between the number of rescue medication tablets taken and the VAS values was found for each time point in the two groups. Total amount of rescue medication was significantly greater (p = 0.006) in group 2. However, there were no statistically significant differences (p = 0.1610) between the time point at which the volunteers initiated the use of rescue medication after each surgery.
None statistically significant differences (p > 0.05) were observed between groups in any of the time points for all measurements of edema. However, the mouth opening limitation (DIINC) was bigger (p = 0.0069) for group 1 at 2 days. The comparison among time points showed increased (p < 0.05) values at 2 days for all the distances measured (except DIINC) for both groups. Besides, at 7 days, all the distances (including DIINC) showed no significant differences (p > 0.05) with the baseline (time 0) (Figs.3, 4, 5, 6, 7 and 8).
Discussion
Perioperative use of corticosteroids is a frequent pharmacological approach to control postoperative complications of oral surgery [7, 19, 20]. The choice of corticosteroid should take into consideration a low adverse effect, its potency, and therapeutic efficiency [19]. Dexamethasone meets these requisites, as it has a lower mineralocorticoid activity, 36–72-h half-life, and potency 25 times greater than hydrocortisone in the reduction of inflammatory mediators that have a minor effect on leukocyte chemotaxis [21,22,23]. Several controlled clinical studies that used the divided mouth model confirmed the effect of dexamethasone in the reduction of pain, edema, and mouth opening limitation after surgery to extract impacted third molars, but its preventive effect in the different oral protocols has not been thoroughly discussed, which was a motivation to conduct our study [2, 7,8,9,10, 12].
The selection of participants according to history, blood tests, and imaging studies ensured sample homogeneity of health status, age, sex, and tooth impaction classification. The control of surgery duration, the standardization of operative technique, and the randomized double-blind crossover design of the study are great advantages for clinical pharmacology studies, as patients receive high-quality follow-up and the results are reliable [6, 18,19,20,21,22,23,24].
Adequate pain control consists of the handling of pain mechanisms at different time points, beginning before the surgical trauma, to decrease sensitivity to nociceptors, and continuing during and after operation to decrease the transmission of nociceptive information from the site of trauma to the central nervous system and to provide more comfort to patients [20, 25, 26]. Despite the differences in dexamethasone dosing between groups, the two protocols under study had a good analgesic result according to the recorded VAS values [8,9,10]. Several authors classify rescue medication as an important limitation to the measurement of prophylactic effects of corticosteroids, because when the patient starts feeling pain and uses rescue medication, the comparisons between groups may become not representative and difficult to measure. Therefore, the choice of rescue medication should take into consideration the least interference in what the study aims to test [10, 25, 26].
In this context, the same rescue medication (600 mg ibuprofen tablet) was used in both groups, and the choice of this drug was based on its pharmacokinetic properties according to similar controlled studies [9, 12, 17]. The patients were instructed to begin taking the rescue medication only in case they felt pain and to continue it if pain persisted. They should follow the prescription and record the time and number of tablets taken [1, 8,9,10].
The participants used rescue medication between 3 and 4 h after operation in both treatment groups, a time interval classified as the one of greatest pain by several studies that measured it as amount of pain medication taken [6, 8,9,10]. The group treated with 8 mg dexamethasone had lower VAS pain values, as well as a lower use of rescue medication, which demonstrated a better analgesic effect of this treatment protocol [9,10,11].
Inflammatory edema and mouth opening limitation after oral surgeries result from the action of inflammatory mediators released because of the surgical trauma [7]. These mediators signal a dynamic reaction in the local microcirculation in the first minutes, with contraction of the cytoskeleton of endothelial cells and plasma exudation into the tissues. This explains the effect of prophylactic administration of drugs in edema control [8, 17, 18].
The differences in concentration and time of dexamethasone administration between treatments revealed that the results for edema control and mouth opening limitation were better in group 1. The administration of 8 mg dexamethasone did not double the effectiveness of the administration of 4 mg dexamethasone because of drug absorption and distribution patterns. However, the protocol used in group 1 was significantly more effective [10].
Systematic reviews have shown that the effectiveness of pharmacological interventions does not depend solely on the potency of the drugs used, but also on their effective presence in the peripheral sites of action [19, 22, 23]. All measures showed less pain, edema, and mouth opening limitation in group 1, which demonstrates that the 8 mg oral dexamethasone regimen initiated before the operation had a better anti-inflammatory effect against these complications, with a lower impact on mastication after the extraction of impacted third molars, a finding that corroborates those reported in previous studies [6, 9, 10].
The anti-inflammatory power of 8 mg dexamethasone administered orally as prophylaxis controls inflammatory complications better, and this regimen seems to be a good strategy to provide greater postoperative comfort to patients that undergo the extraction of impacted third molars, particularly in the case of more invasive surgeries [10, 11].
The rational use of pharmacological regimens to control postoperative complications should consider not only drug concentration and administration mode but also drug potency and absorption and distribution patterns. Therefore, further similar controlled clinical studies should be conducted to define treatment choices.
Conclusion
Pre-emptive use of different dexamethasone regimens had a beneficial effect against pain, edema, and mouth opening limitation, especially when administered at an 8 mg concentration, which suggests that this protocol may also be efficient for more invasive surgery.
References
Antunes AA, Avelar RL, Martins Neto EC, Frota R, Dias E (2011) Effect of two routes of administration of dexamethasone on pain, edema, and trismus in impacted lower third molar surgery. Oral Maxillofac Surg 15:217–223. https://doi.org/10.1007/s10006-011-0290-9
Falci SGM, Lima TC, Martins CC, Santos CRRD, Pinheiro MLP (2017) Preemptive effect of dexamethasone in third-molar surgery: a meta-analysis. Anesth Prog 64:136–143. https://doi.org/10.2344/anpr-64-05-08
Bello SA, Adeyemo WL, Bamgbose BO et al (2011) Effect of age, impaction types and operative time on inflammatory tissue reactions following lower third molar surgery. Head Face Med 28:7–8. https://doi.org/10.1186/1746-160X-7-8
Ibikunle AA, Adeyemo WL (2016) Oral health-related quality of life following third molar surgery with or without application of ice pack therapy. Oral Maxillofac Surg. 20:239–247. https://doi.org/10.1007/s10006-016-0558-1
Avellaneda-Gimeno V, Figueiredo R, Valmaseda-Castellón E (2017) Quality of life after upper third molar removal: a prospective longitudinal study. Med Oral Patol Oral Cir Bucal 22:e759–e766. https://doi.org/10.4317/medoral.21781
Mehra P, Reebye U, Nadershah M, Cottrell D (2013) Efficacy of anti-inflammatory drugs in third molar surgery: a randomized clinical trial. Int J Oral Maxillofac Surg 42:835–842. https://doi.org/10.1016/j.ijom.2013.02.017
Sotto-Maior BS, Senna PM, de Souza Picorelli Assis NM (2011) Corticosteroids or cyclooxygenase 2-selective inhibitor medication for the management of pain and swelling after third-molar surgery. J Craniofac Surg 22:758–762. https://doi.org/10.1097/SCS.0b013e318207f3fe
Paiva-Oliveira JG, Bastos PR, Cury Pontes ER et al (2016) Comparison of the anti-inflammatory effect of dexamethasone and ketorolac in the extractions of third molars. Oral Maxillofac Surg 20:123–133. https://doi.org/10.1007/s10006-015-0533-2
Bauer HC, Duarte FL, Horliana AC et al (2013) Assessment of preemptive analgesia with ibuprofen coadministered or not with dexamethasone in third molar surgery: a randomized double-blind controlled clinical trial. Oral Maxillofac Surg. 17:165–171. https://doi.org/10.1007/s10006-012-0360-7
Chaudhary PD, Rastogi S, Gupta P, Niranjanaprasad Indra B, Thomas R, Choudhury R (2015) Pre-emptive effect of dexamethasone injection and consumption on post-operative swelling, pain, and trismus after third molar surgery. A prospective, double blind and randomized study. J Oral Biol Craniofac Res 5:21–27. https://doi.org/10.1016/j.jobcr.2015.02.001
Lima TC, Bagordakis E, Falci SGM, dos Santos CRR, Pinheiro MLP (2018) Pre-emptive effect of dexamethasone and diclofenac sodium associated with codeine on pain, swelling, and trismus after third molar surgery: a split-mouth, randomized, triple-blind, controlled clinical trial. J Oral Maxillofac Surg 76:60–66. https://doi.org/10.1016/j.joms.2017.06.012
Aznar-Arasa L, Harutunian K, Figueiredo R, Valmaseda-Castellón E, Gay-Escoda C (2012) Effect of preoperative ibuprofen on pain and swelling after lower third molar removal: a randomized controlled trial. Int J Oral Maxillofac Surg 41:1005–1009. https://doi.org/10.1016/j.ijom.2011.12.028
Lima CAA, Favarini VT, Torres AM, da Silva RA, Sato FRL (2017) Oral dexamethasone decreases postoperative pain, swelling, and trismus more than diclofenac following third molar removal: a randomized controlled clinical trial. Oral Maxillofac Surg 21:321–326. https://doi.org/10.1007/s10006-017-0635-0
Moher D, Schulz KF, Altman D (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Lancet 357:1191–1194
Milani BA, Bauer HC, Sampaio-Filho H, Horliana ACRT, Perez FEG, Tortamano IP, Jorge WA (2015) Antibiotic therapy in fully impacted lower third molar surgery: randomized three-arm, double-blind, controlled trial. Oral Maxillofac Surg. 19:341–346. https://doi.org/10.1007/s10006-015-0521-6
Hedström L, Sjögren P (2007) Effect estimates and methodological quality of randomized controlled trials about prevention of alveolar osteitis following tooth extraction: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103:8–15
Bailey E, Worthington HV, van Wijk A, Yates JM, Coulthard P, Afzal Z, Cochrane Oral Health Group (2013) Ibuprofen and/or paracetamol (acetaminophen) for pain relief after surgical removal of lower wisdom teeth. Cochrane Database Syst Rev 12:CD004624. https://doi.org/10.1002/14651858.CD004624.pub2
Xue P, Wang J, Wu B, Ma Y, Wu F, Hou R (2015) Efficacy of antibiotic prophylaxis on postoperative inflammatory complications in Chinese patients having impacted mandibular third molars removed: a split-mouth, double-blind, self-controlled, clinical trial. Br J Oral Maxillofac Surg 53:416–420. https://doi.org/10.1016/j.bjoms.2015.02.001
Beirne OR (2013) Corticosteroids decrease pain, swelling and trismus. Evid Based Dent 14:111. https://doi.org/10.1038/sj.ebd.6400968
Piecuch JF (2012) What strategies are helpful in the operative management of third molars? J Oral Maxillofac Surg 70:S25–S32. https://doi.org/10.1016/j.joms.2012.04.027
Alexander RE, Throndson RR (2000) A review of perioperative corticosteroid use in dentoalveolar surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 90:406–415
Markiewicz MR, Brady MF, Ding EL, Dodson TB (2008) Corticosteroids reduce postoperative morbidity after third molar surgery: a systematic review and meta-analysis. J Oral Maxillofac Surg 66:1881–1894. https://doi.org/10.1016/j.joms.2008.04.022
Herrera-Briones FJ, Prados Sánchez E, Reyes Botella C, Vallecillo Capilla M (2013) Update on the use of corticosteroids in third molar surgery: systematic review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol 116:e342–e351. https://doi.org/10.1016/j.oooo.2012.02.027
Meechan JG, Seymour RA (1993) The use of third molar surgery in clinical pharmacology. Br J Oral Maxillofac Surg 31:360–365
Ong CK, Lirk P, Seymour RA, Jenkins BJ (2005) The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesth Analg 100:757–773 table of contents
Penprase B, Brunetto E, Dahmani E, Forthoffer JJ, Kapoor S (2015) The efficacy of preemptive analgesia for postoperative pain control: a systematic review of the literature. AORN J 101:94–105.e8. https://doi.org/10.1016/j.aorn.2014.01.030
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Vicentini, C.B., Ramacciato, J.C., Groppo, F.C. et al. Clinical evaluation of two dexamethasone regimens in the extractions of impacted third molars—a randomized clinical trial. Oral Maxillofac Surg 22, 177–183 (2018). https://doi.org/10.1007/s10006-018-0687-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10006-018-0687-9