Abstract
Context
Based on the first-principles calculations, this paper investigates the structural, elastic, electronic, and optical properties of albite and kaolinite, respectively. It is determined that both of them show structural stability, mechanical stability, and brittleness by calculating formation enthalpy, phonon dispersion, elastic, and mechanically relevant properties. Both materials are insulators with an indirect bandgap. By calculating the TDOS and PDOS, the main characteristics of the electronic structure of NaAlSi3O8 come from O-2p and Si-3p states, O-2p, and Al-3p states hybridization, similar to Al4[Si4O10](OH)8. The covalence of Si–O bonds in NaAlSi3O8 is greater than Al–O bonds, and the covalent property sequence of Si–O bands in NaAlSi3O8 is Si2-O3 > Si1-O4 > Si2-O2 > Si1-O8 > Si1-O6 > Si3-O2 > Si3-O4. The optical anisotropy of NaAlSi3O8 and Al4[Si4O10](OH)8 is analyzed.
Methods
First-principles density functional theory (DFT) calculation was carried out by the CASTEP computer program. The GGA-PW91 exchange–correlation was used. The energy convergence tolerance, the maximum force, and the maximum displacement were set in the calculation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Both albite and kaolinite are aluminosilicate with good insulating properties [1]. Albite can be used to make ceramics [2,3,4,5,6], glass [7, 8], tiles [9], catalysts [10], etc. Kaolinite can be used as a raw material for ceramics, paper making, catalysts [11,12,13], adsorbents [14,15,16,17,18], nanotube raw materials [19, 20], refractory materials [21], etc.
The physical and chemical properties of albite are now widely investigated. Liu et al. [22,23,24] investigated the thermodynamic stability of albite at high temperatures and pressures by means of molecular simulations. Li et al. [25] calculated isotopic fractionation coefficients for albite using a reductive partitioning function. Factors responsible for the isotopic fractionation of silicon and oxygen in albite were investigated [26, 27]. Quantitative calculation of surface anisotropy of albite provided new ideas for selective grinding of aluminosilicate minerals based on crystal characteristics [28].
For kaolinite, the electronic structure of synthetic natural kaolinite was determined by combining X-ray absorption spectrum and first-principles calculation, and the band gap of synthetic kaolinite decreased with the formation of defects [29]. Based on DFT simulations to obtain the optical properties of kaolinite, the role of hydrogen bonding in the O–H bond has an impact on the photosynthesis of the crystal [30]. Weck et al. calculated the structural, mechanical, and thermodynamic properties of kaolinite using DFT-2. The calculated results are in good agreement with the experimental data [31]. The deformation and damage processes of kaolinite under tensile action were simulated by MD, with the tensile strength and Young’s modulus decreasing with increasing temperature [32]. Studies on the adsorption principles of kaolinite were also being carried out, and the microscopic adsorption mechanisms of Ca(II) and K(I) on the surface of kaolinite were calculated using DFT [33]. The plane wave pseudopotential calculation was used to study the adsorption of [PbCl] + by kaolinite on hydroxylated (001) substrate surface in aqueous system, and the mechanism of Pb–O (or Cl) bonding was discussed [34].
The computational simulations were accompanied by experimental measurements and calculations for both substances. By comparing the Raman spectra measured on naturally ordered and fully ordered albite with those calculated using WCILYD Hamiltonian quantities, it is demonstrated that the calculated vibrational wave numbers are in good agreement with those obtained from experimental measurements [35]. Cano et al. measured the optical absorption properties of albite by γ-rays, thermal resonance (EPR), and thermoluminescence (TL) and finally suggested the use of electron paramagnetism to understand the physical properties of albite [36]. The conductivity model of albite is proposed, which can clarify the conduction mechanism in a wide range of compositions and geological environments [37]. The elastic properties of albite are measured and compared with the simulation results [38, 39]. Liu et al. performed kinetic analysis of kaolinite decomposition at high temperature as well as the dehydroxylation reaction at 600 °C by TG-DSC [40]. Batch experiments were conducted to investigate the adsorption kinetics and equilibrium isotherm characteristics of vanadium (V) adsorption on natural kaolinite, and it was concluded that the adsorption kinetics on kaolinite was consistent with secondary kinetics, and the adsorption isotherm of vanadium (V) on kaolinite was more consistent with the Freundlich model [41]. However, a comprehensive study of the electronic, structural, elastic, and optical properties of albite and kaolinite has not been reported. In this paper, the electronic, structural, elastic, and optical properties of albite and kaolinite are analyzed computationally based on the first-principles calculations.
Computational details
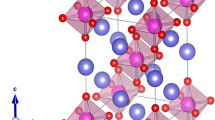
The calculations for albite (NaAlSi3O8) and kaolinite (Al4[Si4O10](OH)8) were based on first-principles density functional theory (DFT) and were carried out by the CASTEP computer program [42]. The space group of NaAlSi3O8 is \(P\overline{1 }\), and Al4[Si4O10](OH)8 is P1. And the crystal structure is triclinic, as shown in Fig. 1.
The calculated equilibrium lattice constants of NaAlSi3O8 are a = 8.12 Å, b = 12.76 Å, c = 7.16 Å; and of Al4[Si4O10](OH)8 are a = 5.21 Å, b = 9.05 Å, c = 7.50 Å. We used the GGA-PW91 functional to describe the exchange and correlation potential; the ultrasoft pseudopotential was used to describe electron-core interactions [43]. We set 5.0 10−6 eV atom−1 as the energy convergence tolerance, 0.01 eV Å−1 as the maximum force, 0.02 GPa as the maximum stress, and 5.0 10−4 Å as the maximum displacement. For NaAlSi3O8, we applied the Monkhorst–Pack grid to generate the 212 k-point grids [44]. We used the Na 2s22p63s1, O 2s22p4, Al 3s23p1, and Si 3s23p2 as valence electrons. For Al4[Si4O10](OH)8, we applied the Monkhorst–Pack grid to generate the 322 k-point grids. We took the H 1s1, O 2s22p4, Al 3s23p1, and Si 3s23p2 as valence electrons.
Results and discussion
Structural properties of NaAlSi 3 O 8 and Al 4 [Si 4 O 10 ](OH) 8
Based on the first-principles study, we carry out geometric optimization calculation for crystal structure in within the GGA-PW91 functional. The atom coordinates of NaAlSi3O8 and Al4[Si4O10](OH)8 are shown in Tables 1 and 2 [45, 46]. The obtained lattice constants are organized in Table 3. By comparing the calculated results, it can be seen that the lattice constants calculated by us are consistent with the previous calculations and experimental results [45, 46]. Therefore, the calculation method of geometric optimization is reasonable.
Structural stability of NaAlSi 3 O 8 and Al 4 [Si 4 O 10 ](OH) 8
It is necessary to predict the stability of structures before computing physical properties [47,48,49,50,51]. In order to predict the dynamic stability of materials, we calculated the phonon density of state and phonon dispersion of NaAlSi3O8 and Al4[Si4O10](OH)8.
The thermodynamic stability of the NaAlSi3O8 and Al4[Si4O10](OH)8 can be predicted by calculating the formation enthalpy (ΔH). It can be obtained by the following formula:
where \({E}_{\mathrm{total}}\left({\mathrm{NaAlSi}}_{3}{\mathrm{O}}_{8}\right),{ E}_{\mathrm{total}}\left({\mathrm{Al}}_{4}[{\mathrm{Si}}_{4}{\mathrm{O}}_{10}]{(\mathrm{OH})}_{8}\right), {E}_{\mathrm{Na}},{ E}_{\mathrm{Al}},{ E}_{\mathrm{Si}}, {\mathrm{E}}_{\mathrm{O}},\) and \({E}_{\mathrm{H}}\) are the calculated total energy of NaAlSi3O8, Al4[Si4O10](OH)8, Na, Al, Si, O, and H respectively.
The formation enthalpy, lattice parameters, and volume of the two materials are calculated as shown in Table 3. The results of the enthalpy of formation calculations show that both NaAlSi3O8 and Al4[Si4O10](OH)8 are thermodynamically stable, because the calculated formation enthalpy is less than zero.
The dynamical stability of materials is considered by the phonon dispersion and phonon density of state. No imaginary phonon frequency indicates that the material is dynamically stable. We calculated the phonon dispersion and phonon density of state of NaAlSi3O8 and Al4[Si4O10](OH)8 separately, and the results are shown in Fig. 2 The results of Fig. 2 show that both materials have no phonon imaginary frequency. This indicates that they are both dynamically stable.
Elastic and mechanical properties of NaAlSi 3 O 8 and Al 4 [Si 4 O 10 ](OH) 8
Mechanical stability is an important property when studying materials [52]. We use the elastic constant to describe the mechanical stability of a material and calculate it based on the linear stress–strain relationship equation \({\sigma }_{\mathrm{ij}}={C}_{\mathrm{ijkl}}{\varepsilon }_{\mathrm{kl}}\). The number of elastic constants is inversely proportional to the symmetry of the compound. For triclinic NaAlSi3O8 and Al4[Si4O10](OH)8 crystal structure, there are 21 twenty-one independent elastic coefficients, as shown in Table 4.
According to Born’s [53] stability criterion and Eq. (12) in the paper written by Gao [54] et al., we know the stability criterion of the triclinic system. Both NaAlSi3O8 and Al4[Si4O10](OH)8 are mechanically stable structures. Based on the Voigt–Reuss–Hill approximation [55], we can use the elastic constants to obtain mechanical properties. This research calculates the bulk modulus (B), shear modulus (G), B\G, Young’s modulus (E), and Poisson’s ratio (ν) of NaAlSi3O8 and Al4[Si4O10](OH)8 respectively. The calculation formulas of Young’s modulus and Poisson’s ratio are: \(E=\frac{9BG}{3B+G}\) and \(\nu =\frac{3B-2G}{6B+2G}\). The results of the calculations are shown in Table 5.
According to the data in Table 5, triclinic NaAlSi3O8 has greater resistance to volume deformation than Al4[Si4O10](OH)8, Brittleness/toughness is also the focus of material performance research. We can simply judge the brittleness/toughness of materials through B/G and Poisson’s ratio. If B/G is greater than 1.75 [56], the material shows toughness; otherwise, it shows brittleness. Both NaAlSi3O8 and Al4[Si4O10](OH)8 are brittle. The order of brittleness is: Al4[Si4O10](OH)8 > NaAlSi3O8.
Electronic properties of NaAlSi 3 O 8 and Al 4 [Si 4 O 10 ](OH) 8
As shown in the calculation results of energy band structure in Fig. 3, the black dashed line shows the Fermi energy level (\({E}_{F}\)). Both triclinic NaAlSi3O8 and Al4[Si4O10](OH)8 are insulators with an indirect bandgap of 5.22 eV and 5.03 eV, respectively.
The total density of states (TDOS) and partial density of states (PDOS) of NaAlSi3O8 and Al4[Si4O10](OH)8 are calculated based on DFT, as shown in Figs. 4 and 5.
For NaAlSi3O8, the partial density of states of different Al, Si, O atoms are shown in Fig. 4, but they have different peaks. The formation of covalent bonds between these different Al, Si, O atoms is revealed. The main features of electronic structure mainly come from O-2p (including: O1-2p, O2-2p, O3-2p, O4-2p, O5-2p, O6-2p, O7-2p, O8-2p) and Si-3p (including: Si1-3p, Si2-3p, Si3-3p) states, O-2p and Al-3p states hybridization. The lowest energy states are from Na-2 s states. States from − 22.5 to − 15.0 eV are mainly due to O1-2 s, O2-2 s, O3-2 s, O4-2 s, O5-2 s, O6-2 s, O7-2 s, O8-2 s, and Na-2p states along with a small amount of Al-3 s, Al-3p, Si1-3 s, Si1-3p, Si2-3 s, Si2-3p, Si3-3 s, and Si3-3p states. And then, the upper valence bands (VBs) are mainly derived from O-2p and Si-3p states, O-2p and Al-3p states hybridization. For Al4[Si4O10](OH)8, the partial density of states for different Al, Si, O atoms are shown in Fig. 5, indicating the formation of covalent bonds between different Al, Si, O atoms. The main features of electronic structure are mainly due to O-2p (including: O1-2p, O2-2p, O3-2p, O4-2p, O5-2p, O6-2p, O7-2p, O8-2p, O9-2p, O10-2p, O11-2p, O12-2p, O13-2p, O14-2p, O15-2p, O16-2p, O17-2p, O18-2p) and Si-3p (including: Si1-3p, Si2-3p, Si3-3p, Si4-3p) states, O-2p and Al-3p (including: Al1-3p, Al2-3p, Al3-3p, Al4-3p) states hybridization. Then, the upper valence bands (VBs) come from nonbonding O1-2p, O2-2p, O6-2p, O7-2p, O11-2p, and O15-2p states.
Tables 6 and 7 show the calculated Mulliken charges, bond lengths, and bond populations of triclinic NaAlSi3O8 and Al4[Si4O10](OH)8.
Positive or negative Mulliken charge means gaining or losing electrons. For NaAlSi3O8, Na, Al, and Si are all transferring charge while O is gaining charge. Si–O and Al–O bonds are mainly form covalent bonds and a small amount of ionic bonds, and Si is more capable of losing electrons than Al. Therefore, the covalence of Si–O bonds is greater than Al–O bonds. The ionic and covalent properties of atomic bonding can be directly characterized by the bond population. When the bond population value is positive/negative, it represents bonding/nonbonding. With the increase of the bond population, the covalent properties of bonding between atoms become stronger. As shown in Table 6, the Si–O bonds have the strongest covalency and are not easily broken. The order of covalency of Si–O bands is Si2-O3 > Si1-O4 > Si2-O2 > Si1-O8 > Si1-O6 > Si3-O2 > Si3-O4. Na–O bonds have the smallest bond population, which is mainly reflected in ionic property.
For Al4[Si4O10](OH)8, the individual atomic Mulliken charges are shown in Table 7. Si, Al, and H are all transfer charge, while O gains charge. The Si–O bonds and Al–O bonds are mainly covalent bonds. And O–H bonds form hydrogen bonds, which are much less powerful than chemical bonds. According to the calculation of bond populations and bond lengths, the bond populations of Si–O bonds are obviously larger than Al–O bonds, and bond lengths of Si–O bonds are smaller than Al–O bonds. Therefore, the covalence of Si–O bonds is greater than Al–O bonds. By comparing the different Si–O bonds, the Si1–O1 bond populations is the largest among different bonds. Compared with the Al–O bands, Al1-O18 bonds have the largest bond populations. In addition, both Al–O and Si–O bonds are covalent bonds. And the order of strength of the covalent bonding action is Si1-O1 > Si1-O4 > Si1-O3 > Si1-O10 > Al1-O18 > Al1-O13 > Al1-O16 > Al1-O1 > Al1-O15 > Al1-O2.
Charge density difference is also an important method to study the electronic structure, which can visualize the electron flow and bonding properties. We calculated the electron density difference for the NaAlSi3O8 along the (001) plane and Al4[Si4O10](OH)8 along the (010) plane as shown in Fig. 6.
It can be seen from Fig. 6(a) that Si and Al atoms in NaAlSi3O8 form covalent bonds with O atoms respectively, where both Si and Al atoms lose electrons and O atoms get electrons. It can be clearly seen that the ability of Si atom to lose electrons is greater than that of Al atom, and the covalence of Si–O bonds is greater than that of Al–O bonds. Similarly, it can be seen from Fig. 6(b) that both Si and Al atoms in Al4[Si4O10](OH)8 lose electrons, and O atoms gain electrons, and the covalence of Si–O bonds is greater than that of Al–O bonds.
Optical properties of NaAlSi 3 O 8 and Al 4 [Si 4 O 10 ](OH) 8
The optical properties resulting from the electronic transition can be calculated by the first-principles. First, dispersion of optical functions can be expressed as [57]:\(\upvarepsilon \left(\upomega \right)={\upvarepsilon }_{1}\left(\upomega \right)\)+\(\mathrm{i}{\upvarepsilon }_{2}\left(\upomega \right)\). The imaginary part \({\upvarepsilon }_{2}\left(\upomega \right)\) is related to electronic transition, and the expression formula is shown below:
(\(\mathrm{Eq}. M-\mathrm{dipole matrix}\); \(i-\mathrm{initial states };j-\mathrm{ final states};\) \({f}_{i}-\mathrm{the Fermi}\)\(\mathrm{distribution function for the }i\mathrm{th state};\) \({E}_{i}-\mathrm{energy of electron in the ith state}\))
According to the Kramers–Kronig dispersion relation system [58], the real part \({\varepsilon }_{1}\left(\omega \right)\) can be expressed as [59, 60]:
From the above formula, it can be deduced that the material’s refractive index \(n\left(\omega \right)\), extinction coefficient \(k\left(\omega \right)\), reflectivity \(R\left(\omega \right)\), absorption coefficient \(I\left(\omega \right)\), loss function \(L\left(\omega \right),\) and optical conductivity \(\sigma \left(\omega \right)\).The specific relationship is as follows:
The calculated dielectric functions are shown in Fig. 7.
It can be seen that NaAlSi3O8 and Al4[Si4O10](OH)8 have different optical anisotropy. For NaAlSi3O8, imaginary part \({\varepsilon }_{2}\left(\omega \right)\) depends on the transition of valence band electrons and conduction band electrons. We can see that the imaginary part \({\varepsilon }_{2}\left(\omega \right)\) shows two peaks which mainly come from the electron transition of Si1-3p, Si2-3p, Si3-3p, and Al-3p states. The first peak position appears near 10 eV, similar to real part \({\varepsilon }_{1}\left(\omega \right)\). With regard to Al4[Si4O10](OH)8, there are three peaks present in the \({\varepsilon }_{2}\left(\omega \right)\), it mainly comes from the electron transition of O-2p states and the hybridization between O-2p and Si-3p states, O-2p, and Al-3p states. Specially, the peak of the dielectric imaginary part in the z direction moves towards the low-energy region and increases in peak value.
The calculated values of absorption coefficient, reflectivity, optical conductivity, and loss function of NaAlSi3O8 and Al4[Si4O10](OH)8 are shown in Figs. 8 and 9.
The calculated (a) refractive index \(n\left(\omega \right)\); (b) refractive index \(k(\omega )\); (c)optical conductivity \({\sigma }_{1}\left(\omega \right)\); (d) optical conductivity \({\sigma }_{2}\left(\omega \right)\); (e) reflectivity \(R\left(\omega \right)\); (f)loss function \(L\left(\omega \right)\); and (g)absorption coefficient \(I\left(\omega \right)\) of NaAlSi3O8
The calculated (a) refractive index \(n\left(\omega \right)\); (b) refractive index \(k(\omega )\); (c)optical conductivity \({\sigma }_{1}\left(\omega \right)\); (d) optical conductivity \({\sigma }_{2}\left(\omega \right)\); (e) reflectivity \(R\left(\omega \right)\); (f)loss function \(L\left(\omega \right)\); and (g)absorption coefficient \(I\left(\omega \right)\) of Al4[Si4O10](OH)8
It can be seen from Fig. 8 that the tendency of the refractive index \(n\left(\omega \right)\) of NaAlSi3O8 corresponds to the real part \({\varepsilon }_{1}\left(\omega \right)\) of the dielectric function. The refractive index fluctuates widely in the energy range from 10 to 20 eV. It rises slowly from 20 to 30 eV. The extinction coefficient \(k\left(\omega \right)\) achieves a maximum value at the energy of 16.3 eV. And the absorption coefficient is the proportion of energy attenuation of a light wave per unit distance through a semiconductor medium. NaAlSi3O8 has a light absorption coefficient of 0 near the visible wavelength band. The absorption peak appears in the energy range of 15 eV, which is 2.9 × 105 \({\mathrm{cm}}^{-1}\). The main reason for the absorption peak is the electron transitions of the valence band electrons to the conduction band. The reflection coefficient is the proportion of the photon energy reflected from the surface to the photon energy striking the surface. When the energy is 20 eV, the reflectivity reaches the maximum value. The reflectivity in x, y, and z directions are 32.0%, 33.0%, and 35.2%, respectively. The loss function represents the energy loss of photoelectrons in homogeneous media. The diagram shows a large number of dissipation peaks, which are highly relevant to plasma oscillations. Among them, the plasma frequencies \({L}_{n}\)(\(n=xx,yy,zz\)) in three directions are 22.67, 22.63, and 22.78 eV, respectively.
As illustrated in Fig. 9, the refractive index of Al4[Si4O10](OH)8 reaches a maximum value of 1.96 in the energy range of 10 eV. The trend of the extinction coefficient in the x, y direction is the same. In particular, the second peak of the extinction coefficient in the z direction is much larger than other two directions, when the light utilization is improved. The absorption coefficient has two peaks, which achieve the maximum value of 3.2 × 105 \({\mathrm{cm}}^{-1}\) at the energy of 17 eV. When the energy is 21.6 eV, the reflectivity reaches the maximum value, and the reflectivity in the three directions of x, y, and z is 40.0%, 42.0%, and 45.8%, respectively. The calculated value of the plasma oscillation \({L}_{n}\)(\(n=xx,yy,zz\)) are 21.13, 20.99, and 21.06 eV, respectively.
Conclusions
Overall, we calculated the structural, elastic, electronic, and optical properties of albite and kaolinite on the basis of first-principles calculations. The calculated lattice constants are consistent with the experimental data. The mechanical properties of NaAlSi3O8 and Al4[Si4O10](OH)8 are studied. It shows that they have mechanical stability and brittleness. The energy band structure indicates that both of them are insulators with an indirect bandgap. By calculating the total density of states and the partial density of states, it can be seen that the main characteristics of the electronic structure of NaAlSi3O8 come from O-2p and Si-3p states, O-2p and Al-3p states hybridization, similar to Al4[Si4O10](OH)8. For the purpose of investigating the bonding behavior of the materials, Mulliken charges, bond lengths, and bond populations were calculated for triclinic NaAlSi3O8 and Al4[Si4O10](OH)8. The covalent property sequence of Si–O bands in NaAlSi3O8 is Si2-O3 > Si1-O4 > Si2-O2 > Si1-O8 > Si1-O6 > Si3-O2 > Si3-O4. The order of strength of the covalent bonding action in Al4[Si4O10](OH)8 is Si1-O1 > Si1-O4 > Si1-O3 > Si1-O10 > Al1-O18 > Al1-O13 > Al1-O16 > Al1-O1 > Al1-O15 > Al1-O2. Optical anisotropy of triclinic NaAlSi3O8 and Al4[Si4O10](OH)8 is observed.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request and available within the article.
References
Li B, Knowles KM (2013) Modell Simul Mater Sci Eng 21:055012
Chen JH, Liu PS, Cheng W (2019) Multidiscip Model Mater Struct 15:659
Sánchez-Soto PJ, Garzón E, Pérez-Villarejo L, Angelopoulos GN, Eliche-Quesada D (2021) Minerals 11:232
Rouabhia F, Nemamcha A, Moumeni H (2018) Cerâmica 64:126
Lerdprom W, Chinnam RK, Jayaseelan DD, Lee WE (2016) J Eur Ceram Soc 36:4319
Pali M, Das S, Das SK (2015) Bull Mater Sci 38:551
Gaudio SJ, Lesher CE, Maekawa H, Sen S (2015) Geochim Cosmochim Acta 157:28
Siqueira RL, Peitl O, Zanotto ED (2016) J Sol-Gel Sci Technol 80:619
Frizzo RG, Zaccaron A, Nandi VDS, Bernardin AM (2020) Journal of Building Engineering 31:101432
Wang JY, Wang ZY, Yang LM, Yang GX, Miao CL, Lv PM (2017) Energy 141:1650
Cao Z, Wang QZ, Cheng HF (2021) Chin Chem Lett 32:2617
Alaba PA, Sani YM, Daud WMAW (2015) RSC Adv 5:11127
Barbosa LV, Marcal L, Nassar EJ, Calefifi PS, Vicente MA, Trujillano R, Rives V, Gil A, Korili SA, Ciuffifi KJ, de Faria EH (2014) Catalysis Today 246:133
Zhao J, Gao W, Qin XZ, He MC (2020) Journal of the International Adsorption Society 26:443
Chen GB, Zhao HZ, Li X, Xia SW (2021) Appl Clay Sci 210:106120
Sarma GK, Gupta SS, Bhattacharyya KG (2019) SN Applied Sciences 1:211
Zhang B, Kang JT, Kang TH (2018) Appl Surf Sci 439:792
Underwood T, Erastova V, Greenwell HC (2016) J Phys Chem 120:11433
Xu HL, Jin XZ, Chen P, Shao G, Wang HL, Chen DL, Lu HX, Zhang R (2015) Ceram Int 41:6463
Detellier C (2018) Chem Rec 18:868
Kambel RD, Aliyu BA, Barminas JT, Akinterinwa A (2017) International Journal of Materials and Chemistry 7:14
Liu TQ, Hu MY, Lu WT, Zhan JX, Cui XY, Zhan XH, Yu J (2019) Physica B 567:55
Blasco M, Gimeno MJ, Auqué LF (2017) Procedia Earth and Planetary Science 17:120
Sims M, Rucks M, Lobanov S, Young J, Daly JA, Pakhomova A, Konopkova Z, Liermann HP, Hrubiak R, Whitaker ML, Glotch TD, Ehm L (2019) Lunar and Planetary Science Conference 50:2132
Li YH, Wang WZ, Huang SC, Wang K, Wu ZQ (2019) Geochim Cosmochim Acta 245:374
Qin T, Wu F, Wu ZQ, Huang F (2016) Contrib Mineral Petrol 171:1
Zhu C, Zhang YL, Rimstidt JD, Gong L, Burkhart JAC, Chen KY, Yuan HL (2021) Geochim Cosmochim Acta 303:15
Xu LH, Peng TF, Tian J, Lu ZY, Hu YH, Sun W (2017) Appl Surf Sci 426:1005
Pietzsch A, Nisar J, Jämstorp E, Gråsjö J, Arhammar C, Ahuja R, Rubensson JE (2015) J Electron Spectrosc Relat Phenom 202:11
Shafei L, Adhikari P, Ching WY (2021) Curr Comput-Aided Drug Des 11:618
Weck PF, Kimb E, Jové-Colóna CF (2015) Dalton Trans 44:12550
Yang H, He MC, Lu CS, Gong WL (2019) Science China Physics. Mechanics & Astronomy 62:1
Zhang ZJ, Zhou Q, Zhuang L, Zhao ZF (2021) Mol Phys 119:1896047
Wang J, Xia SW, Yu LM (2015) Appl Surf Sci 330:411
Aliatis I, Lambruschi E, Mantovani L, Bersani D, Andò S, Gatta GD, Gentile P, Salvioli-Mariani E, Prencipe M, Tribaudinoa M, Lottici PP (2015) J Raman Spectrosc 46:501
Cano NF, Santos LHED, Chubaci JFD, Watanabe S (2015) Spectrochim Acta Part A Mol Biomol Spectrosc 137:471
Amulele GM, Lanati AW, Clark SM (2022) Am Miner 107:614
Brown JM, Angel RJ, Ross NL (2016) Journal of Geophysical Research: Solid Earth 121:663
Lacivita V, D’Arco P, Mustapha S, Bernardes DF, Dovesi R, Erba A, Rérat M (2020) Physics and Chemistry of Minerals 47:45
Liu XX, Liu XW, Hu YH (2015) Clay Miner 50:199
Zhu HW, Xiao XY, Guo ZH, Han XQ, Liang YQ, Zhang Y, Zhou C (2018) Appl Clay Sci 161:310
Clark SJ, Segall MD, Pickard CJ, Hasnip PJ, Probert MIJ, Refson K, Payne MC (2005) Z Kristallogr 220:567
Perdew JP, Zunger A (1981) Phys Rev B 23:5048
Monkhorst HJ, Pack JD (1976) Phys Rev B 13:5188
Winter JK, Ghose S, Okamura FP (1977) Am Miner 62:921
Young RA, Hewat AW (1988) Clays Clay Miner 36:225
Yu ED, Pan Y (2022) Journal of Materials Chemistry A 46:24866
Yu ED, Pan Y (2022) Electrochim Acta 435:141391
Chen S, Pan Y (2022) Appl Surf Sci 599:154041
Pan Y, Yu ED (2022) Int J Hydrogen Energy 47:27608
Pan Y (2023) J Phys Chem Solids 174:111152
Liu WH, Liu QJ, Zhong M, Gan YD, Liu FS, Li XH, Tang B (2022) Acta Mater 236:118137
Born M (1940) Math Proc Cambridge Philos Soc 36:160
Gao J, Liu QJ, Jiang CL, Fan DH, Zhang M, Liu FS, Tang B (2022) Chinese Journal of High Pressure Physics 36:051101
Chung DH, Buessem WR (1967) J Appl Phys 38:2010
Pugh SF (1954) The London, Edinburgh, and Dublin Philosophical Magazine and Journal of. Science 45:823
Gao J, Zeng W, Tang B, Zhong M, Liu QJ (2021) Mater Sci Semicond Process 121:105447
Horsley SAR, Artoni M, Rocca GCL (2015) Nat Photonics 9:436
Zhong M, Zeng W, Liu FS, Fan DH, Tang B, Liu QJ (2022) Mater Today Phys 22:100583
Ambrosch-Draxl C, Sofo JO (2006) Comput Phys Commun 175:1
Funding
This work was supported by the Sichuan Science and Technology Development Project (Grant No. 2021ZYD0027) and the Original Scientific Research Instrument and Equipment Development Project of Southwest Jiaotong University (Grant No. XJ2021KJZK055).
Author information
Authors and Affiliations
Contributions
Ya-Le Tao: data curation, formal analysis, investigation, methodology, writing—original draft; Juan Gao: formal analysis, investigation, writing—review and editing; Qi-Jun Liu: conceptualization, investigation, methodology, project administration, supervision, writing—review and editing; Zheng-Tang Liu: methodology, software; all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable for both human and/or animal studies, we allow the journal to review all the data, and we confirm the validity of results. There is none of the financial relationships. This work was not published previously and it is not submitted to more than one journal. It is also not split up into several parts to submit. No data have been fabricated or manipulated.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tao, YL., Gao, J., Liu, QJ. et al. Structural, elastic, electronic, and optical properties of NaAlSi3O8 and Al4[Si4O10](OH)8 from first-principles calculations. J Mol Model 29, 111 (2023). https://doi.org/10.1007/s00894-023-05508-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05508-5