Abstract
The eleven monohydrates of 1, 2, 3, 4-tetrahydroisoquinoline (THIQ) are analyzed through natural bond orbital (NBO) analysis and QTAIM methods employing M06-2X functional in DFT and MP2 methods. Here, the role of OH bonds as an acceptor and donor is critically analyzed. The role of lone pairs of O is critically monitored in two of the complexes, where N–H···O hydrogen bonds are present. The relative contributions of rehybridisation and hyperconjugation are compared in detail. Popelier criteria are satisfied in all the complexes barring a few exceptions involving weak hydrogen bonds. At the bond critical points (BCP), four monohydrates show higher values of electron density (ρC) and negative values of total electron energy density (HC), while Laplacian \({(\nabla }^{2}{\uprho }_{\mathrm{C}} )\) remains positive. These complexes satisfy the criteria of partial covalency. All these are O–H⋅⋅⋅N-type bonds. Remaining h-bonds are weaker in nature. These are also confirmed by the smaller values of ρC at the respective BCP. The variation of potential energy density (VC) among the complexes seems to be the most important factor in determining the nature of non-covalent interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Various kinds of non-covalent interactions play a major role in controlling a multitude of physical and chemical phenomena. The covalent interactions are conventionally represented by Lewis structures [1], which most of us experienced in our high school texts. Weaker non-covalent interactions on the other hand might arise from a variety of sources. Dispersion, dipole–dipole interactions, steric repulsions, and hydrogen bondings are a few of the prominent ones. The classic review by Kolman [2] discusses these parameters in great detail. Dispersive interactions play an important role from a wide range of microscopic to macroscopic phenomena. This weak attractive interaction is responsible for the “physiosorption” of gases in solids and is an important marker for characterizing bulk solids to nanomaterials [3]. This interaction is also responsible in explaining the adhesion of lizards on various surfaces [4] increasing its importance, although this is a weak interaction in nature. Dipole–dipole interactions are comparatively stronger than the dispersive ones, but among the non-covalent interactions, hydrogen bonding had stolen the limelight for the last few decades. Its variety and the range of applications from the microscopic world [5] to the extreme environments in interstellar media [6] continue to fascinate scientists and people in general.
On the per-atom basis, hydrogen bonding is the strongest among all the non-covalent interactions. The structure of proteins [7] and DNA [8] can only be explained by this non-covalent interaction. The hydrogen bonding is defined as X–H⋅⋅⋅Y, where the hydrogen atom (H) is placed in between the two electronegative atoms (X and Y). Y atom should have one or multiple lone pairs or appropriate electron distribution to form the H⋅⋅⋅Y bond. This bonding involves the physical transfer of electronic charge from the lone pair of Y atom (Lewis base) to the σ * orbital of the X–H bond (Lewis acid) changing the atomic characteristics of the individual atoms on the formation of the bond. One may also view this as a transfer of a proton from a hydrogen bond donor (X) to the acceptor (Y) site. Since the bonding characteristics of the H atom are different with these two atoms X and Y, even if X and Y are identical, we will observe some interesting phenomenon. The problem of understanding the non-covalent interactions becomes complicated if there are multiple sites of hydrogen bonding, mainly with the variation of Y. The strength of hydrogen bonding can vary over a large range with the variation of atomic properties of X and Y and also the neighborhoods of them. Numerous reviews [9,10,11,12,13,14] dealing with and comparing the strength of this h-bonding are available. The strength is also connected with the directionality of this bond mainly defined by the angle ∠XHY. The more linearity in orientation favors the strongest bonds. The covalency of the strongest of the hydrogen bonds arouses a lot of interest [9].
The nature of hydrogen bonds (HB) can be explored from various perspectives. Mere comparison of binding energies might not be the best idea to get hold of the problem. Natural bond orbital (NBO) analysis [15] deals with the wavefunctions of the optimized structures having HBs. It pictorially shows the different molecular orbitals involved in the intramolecular or intermolecular hydrogen bonding. This is mainly done in following nY → σXH* orbital overlap energy. The beauty of this analysis is one can follow all the bonds of the participating molecules individually to understand how this bonding affects even those regions of a molecule which are not directly involved in this non-covalent interaction. One can also follow the characteristics of the atoms taking part in this bonding in an elegant way. This paved the way for a facile observation of charge transfer and the formation and breaking of hydrogen bonds. This analysis also gives us the changes occurring in the hybridization characteristics in the hydrogen bond donor during the whole process. A number of problems were successfully solved by this analysis [16,17,18].
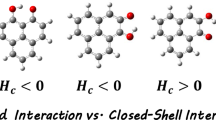
Quantum Theory of Atoms in Molecules [QTAIM] [9, 18,19,20,21] is another approach which provides us with geometrical, topological, and energetic characteristics of various kinds of hydrogen bonds [22, 23]. According to this theory [18, 19], nuclei are considered as attractors defined as the gradient vector field of charge distributions. These are denoted as (3, − 3) critical points having maxima of charge density. An atom is looked upon as a union of an attractor (nucleus) and its associated basin (electron density distribution). Here, the electron density (ρ) is plotted in the region occupied by the atoms in the molecules; consequently one can visualize the bond path connecting the atoms. At the bond critical point (BCP) Hessian matrix of the charge density has two negative and one positive eigenvalues. These are denoted as (3, − 1). At these points, ∇ρ vanishes. The respective value of ρ at these points is termed \({\uprho }_{\mathrm{C}}\). Similarly, for a ring, we get ring critical points (RCP) denoted as (3, + 1). The nature of the bonds is characterized by the values of ρ and its second derivative or Laplacian (∇2ρ). The corresponding eigenvalues of the Hessian matrix and the sign of Laplacian indicate the nature of the non-covalent interactions [9]. For non-covalent interactions between two atoms, if the electron density reduces in the interatomic region, Laplacian will be positive which indicates dipole–dipole, van der Waals, and conventional hydrogen bonds in general. But, if Laplacian becomes negative, it hints at an accumulation of charges and consequent electron density in that region. It implies sharing of electron charges and points to a covalent nature of the corresponding non-covalent interaction [9]. It needs to be mentioned that the characteristics of energy at the BCP are connected with the Laplacian through the following relations:
where HC, GC, and VC are the total electron energy density, the kinetic electron energy density, and the potential electron energy density, respectively. Here, GC is always positive and VC is negative, making the sign and value of HC as an indicator of the nature of bonding. Strong hydrogen bonds are characterized by positive values of \({\nabla }^{2}{\uprho }_{\mathrm{C}}\) and negative values of HC. When both of them appear with positive values then it is a moderate to weak h-bonding [9]. On the other hand, extreme negative values of both \({\nabla }^{2}{\uprho }_{\mathrm{C}}\) and HC indicate very strong hydrogen bonding. This can also be looked at as h-bonding with covalent characteristics [9]. According to the AIM approach, the topological criteria for the existence of hydrogen bonding were put forward by Popelier [24]. These criteria include (i) the correct topological pattern for bond path and BCP, (ii) a relatively high value ( 0.002–0.034 a.u.) of ρH…Y ( at the H atom of the H···Y hydrogen bond) at the BCP, (iii) ∇2ρH…Y should be within the range 0.024–0.139 a.u. at BCP, (iv) the formation of hydrogen bonding will be revealed by the mutual penetration of H atom and acceptor atoms, (v) evidence of loss of charge on H atom on hydrogen bond formation, (vi) destabilization in the energy of H atom on bonding, (vii) decrease in dipolar polarization of H atom on the formation of cluster, and (viii) decrease in the volume of hydrogen atom due to intermolecular interaction.

In this paper, we will find out the non-covalent intermolecular interactions present in the monohydrated complexes of 1, 2, 3, 4-tetrahydroisoquinoline (THIQ). TA, TE, and BA are the conformers of bare THIQ molecule [25], where T stands for “twisted” and B for “bent” conformations of the saturated ring. “Axial” and “Equatorial” orientations of the NH bonds in the above conformations are indicated by A and E, respectively. Recently, we explored the different conformations of the THIQ:(H2O) [26] and THIQ: (NH3) [27] complexes in S0. We also succeeded in reassigning the IR-UV and UV-UV double resonance spectra [28] of the monohydrated complex [25]. Bare THIQ poses different conformations in S0. TA and TE are the two close-lying structures [25, 29] in S0. There is some ambiguity [25] in the determination of the global minimum in S0. But, when conformer selective water complexes were studied [28], it is unambiguously observed and corroborated experimentally that the monohydrates with the twisted equatorial (TE) configuration of THIQ are the global minimum. The other close-lying monohydrated complex with the twisted axial (TA) conformer lies about 200 cm−1 higher than the corresponding complex in the TE form. In the former, both the OH bonds of water participate in the h-bonding as O–H⋅⋅⋅N and O–H⋅⋅⋅π type,while in the latter O–H⋅⋅⋅N type is only present. Although the H bond length is not shorter in the TE form, the overall contribution from the two HBs energetically separates the conformer selective water complexes and (THIQ) TA: (H2O) becomes the global minimum in the potential energy surface (PES) [26] of the cluster study. Apart from TE and TA forms, the bare THIQ [25] also shows a bent conformation of the saturated ring in the axial configuration of the N atom, termed as bent axial (BA) form. This is located about 700 cm−1 higher in energy. The other bent form bent equatorial (BE) is interestingly a transition state (TS) in the bare molecule [25]. In monohydrates of THIQ, the BE conformer forms the complex with the largest binding energy [26], even larger than the TE and TA forms. This raises a lot of questions and we feel it necessary to distinguish the nature of hydrogen bonding on the basis of QTAIM theory to enquire about the possibility of covalency [9] in any of the conformer selective monohydrates of THIQ. We also observed that the behavior of the two OH bonds in the complexes was different. Out of the two OH bonds of water, the one not participating in the H bonding behaves quite differently from the participating one in the TE conformer of THIQ. As, we focused mainly on the identification of different conformers and reassigning the vibrational spectrum in our earlier work, here we will try to explore these monohydrated complexes in greater detail.
The questions this paper will try to address are:
-
1.
Is there any of the monohydrates of THIQ that show covalency character as classified by QTAIM computations and aptly described by Grabowski et al. [22]?
-
2.
Do all the monohydrates follow the criteria proposed by Popelier [24]?
-
3.
In our earlier article [26] we observed a difference in the behavior of two OH bonds of water on hydrogen bonding. Will the behavior of free (not participating in hydrogen bonding) OH bonds remains identical irrespective of the behavior of the O atom as an acceptor or donor of H bonds?
-
4.
Does the hydrogen bonding behavior change identically as that observed [26] in TA and TE forms of THIQ when the role of donor and acceptors are interchanged?
-
5.
How far can we distinguish different kinds of hydrogen bonding interactions on the basis of different properties at BCP?
Computational methods
We will use both Density Functional theory (DFT) [30] as well as 2nd order Moller–Plesset perturbation theory, MP2 [31] methods. Within the DFT framework, M06-2X functional [32] will be utilized here for describing these non-covalent interactions. The suitability of this functional over other functionals in these types of cases was well documented and also observed in our earlier works [26]. The basis set 6-311G + + (2d, 3p) [33] is used in both these methods. All the different structures of these complexes are computed in Gaussian 09 [34] suits of the program. The wavefunctions of different optimized complexes were used in natural bond orbital (NBO) analyses. This is achieved through NBO 3.1 associated with Gaussian09. AIM analysis has been done with the help of Multiwfn [35].
Results and discussions
In our previous work, [26] we observed 11 distinct monohydrated complexes of THIQ. There are multiple centers of h-bondings in THIQ. We had classified [26] these different HBs according to the primary HB centers. There are three different types of HBs where the primary HB centers are—(a) O–H···N, (b) N–H···O, and (c) O–H···π. Apart from these primary centers, we observed secondary HBs in some of the complexes. The nomenclature of the different complexes will remain identical as earlier [26]. Respective monohydrates for TE conformation of THIQ were coined as TEW1a, TEW1b, TEW1c, and respectively so for TA, BA, and BE conformers of bare THIQ.
NBO analysis
The different intra- and intermolecular interactions within a pair of covalent bonds (A-H···X-B) related through hydrogen bonding were conveniently probed by NBO analysis [18]. Here the X usually has one or multiple lone pairs. This is an important analytical tool for understanding non-covalent interactions. This analysis assesses the relative contribution of two different factors involved in hydrogen bonding–- (i) hyperconjugation following the electronic charge transfer (QCT) from the filled lone pair orbitals (nX) in X to the empty anti-bonding orbital (σAH*) of the HB donor (A-H) and (ii) rehybridization depicting the change in hybridization characteristics in the donor. In our previous publication on this complex [26], we analyzed the NBOs in the two lowest energy complexes, TEW1a and TAW1a only. It is to be noted that both the studied complexes are of “a” type, where O–H···N is the primary HB. Hyperconjugation was the predominant mechanism. The behavior of individual OH bonds of water was thoroughly probed. If any of the two OH bonds of water remains “free” (non-participation in h-bonding), it behaved differently from the other one. The former OH bond was found to shorten on HB. It was observed only in the TAW1a complex. We also looked at bond-specific non-covalent interactions in those complexes. We observed that energy associated with NBO (ENBO) accounts for this quite well.
Apart from the above-mentioned two complexes, we found that TAW1b, TAW1c, TEW1b, BAW1a, and BAW1b complexes have at least one “free” OH bond in water. We will study these in greater detail and try to verify and conclude on the behavior of “free” OH bonds in the monohydrates of THIQ. The bonded H atom in water is termed Ha and the “free” one Hb. Some common features observed earlier in TAW1a and TEW1a complexes were ––(i) The O-Hb bond length (BL) decreases when Hb is free, (ii) the O-Ha BL increases in all cases including the case when Hb is not “free”, and (iii) charges on Ha (when free) increases and Hb decreases on complexation. The changes were more pronounced in TAW1a than TEW1a, (iv) Charge on O atom decreases and the change was more in TAW1a. In both these complexes, the OH bonds behave as HB donor while N lone pair as acceptor. But within the above-mentioned complexes, TAW1b, BAW1b, and TEW1b complexes show N–H···O type h-bond signifying the reversal of the role of N and O atoms in this HB. Then, NH bond will behave as a donor and lone pair of O atom as HB acceptor. The C-H···O bonds are weakest among all the h-bonds here and we left out those complexes where they appear as a primary or secondary HBs. Since BAW1a and BAW1a* bear the same signature of H bonding as TAW1a, we will leave them out of this discussion here. Similarly, TEW1a has its identical counterparts as BEW1a, TEW1c, and BAW1c and we will not explore the behavior of OH bonds in these complexes. Considering all these aspects we will thoroughly investigate the HBs in TAW1b and BAW1b only shown in Fig. 1 computed at M06-2X/aug-cc-pVDZ level. Corresponding results with M06-2X/6–311 + + G (2d, 3p) level and respective coordinates are given in figures. F1 and F2 respectively in the supplementary file. Consistency in the results is noteworthy.
In both cases, the primary HB is N–H···O type [26] as clearly evident from the values of equilibrium H-bond lengths. In both TAW1b and BAW1b, there is an additional O–H···π bond. We follow both the O–H bonds in these two complexes as we vary the N–H···O bond lengths. The H⋅⋅⋅O hydrogen bond lengths are varied from a value smaller than the equilibrium bond lengths and extended up to the point where H-bond disappears. We also closely watch the lone pairs on the O atom, whether they are forming any new H bonds or not. The change in the s-character of the O atom on h-bonding is also calculated to ascertain which interaction among rehybridization and hyperconjugation dominates here. These plots are shown in Fig. 2.
Correlations of charge transfer, change in O–H bond length, and the change of s-character (%) of O–H bond at O due to nO/πC-C → σ*(O–H) interactions with O⋅⋅⋅H-N H-bond length of two complexes a TAW1b and b BAW1b. The vertical line indicates the H-bond length at the equilibrium geometry and horizontal lines indicate % of s-character and O–H bond length of bare H2O. Computations are performed with M06-2X/aug-cc-pVDZ level
Figure 2 shows the variation of O⋅⋅⋅H-N hydrogen bond lengths in TAW1b and BAW1b complexes over a range of 1.50 Å to 3.50 Å. The corresponding equilibrium bond lengths are around 2.30 Å. Due to hyperconjugative interaction electronic charge is transferred to the N–H bond from O. On the other hand, weak O–H···π bond signifies a transfer of charge from π electrons to an O–H bond. So, some competitive processes are going on here as we change the O⋅⋅⋅H-N bond lengths. In TAW1a and TEW1a, NH bonds do not form any h-bonds with lone pairs of O atom. So, charge transfer from O was not feasible [26].
For TAW1b (Fig. 1a), as the N–H···O bond length is increased the charge transfer to the N–H bond also decreases, consequently, the charge on O decreases and charge on N increases from the equilibrium value. Interestingly with this increase in this BL, O-Ha···π bond length decreases and charge is transferred to O-Ha bond. So, after the initial decrease charge on the O atom increases.
Change of charges (ΔQ) at Ha and Hb is decreasing continuously with the increase of N–H···O length, but the rate of change is different. Sudden change of charge at Ha and O is observed close to 3.40 Å. This is due to the development of a weak C2-H···O bond with the increase of H···O BL. This effect is prominently observed in O-Ha bonds signifying the difference in Ha and Hb atoms. Consequently, hybridization character changes. The change in % of the s-character at O and its charge changes consistently. In case of the N–H bond, the s-character at N continuously decreases which is natural as the h-bond length increases. The % of the s-character at the electronegative atom involving HB should decrease according to Bent’s rule [36].
In BAW1b (Fig. 1b), similar phenomena are observed apart from some sudden change. With the increase in N–H···O bond lengths, water shifts more towards the pi-electron cloud, and the C2-H···O bond is formed much more easily than in TAW1b. O-Hb increases more than O-Ha when N–H···O is about 2.10 Å as it comes closer to π-electrons cloud and the orientation of H2O changes. As N–H···O is shifted to a BL around 3.20 Å, H2O is shifted towards C2-H and C2-H···O HB is formed. Interestingly, as the shifts progress the O-Ha shifts to the side of the benzene ring, and O-Hb···π become more prominent than O-Ha···π which was more in earlier case. All these things are clearly and consistently indicated by the change of charge, O–H lengths, and % of s-character.
In “a” type complexes (e.g., TAW1a and TEW1a), OH bonds acted [26] as HB donors, while in “b” type one like TAW1b and BAW1b these bonds act both as a donor and acceptor. The relative strength of HBs changes with the type of complex formed. With the increase in N–H···O BLs the O-Hb bonds showed an increase in the “a” types [26], while this is not observed here. The involvement of that bond at higher N–H···O BLs might also be a reason to be probed later. The different kind of trend is also observed for “a” and “b” types of complexes in the changes in charges (ΔQ) over Ha and Hb atoms. ΔQ and change in % s-character at O atom in “a” type complexes were discussed [26] in detail and it was concluded that hyperconjugation predominates rehybridisation. Here, in these “b” type complexes ΔQ over both the H atoms decrease with the increase in N–H···O BLs, but as O–H bond length decreases % of s-character at O increases. At 2.10 Å in BAW1b complex, there is a change in orientation of water molecules, which is displayed as a sudden change in all the quantities in this complex. These indicate that rehybridisation predominates hyperconjugation in these complexes.
Table 1 lists the change in O-Ha and O-Hb bond lengths of water for all the monohydrates of THIQ computed at M06-2X/aug-cc-pVDZ level. The corresponding computations with M06-2X/6–311 G + + (2d, 3p) are in Table T1 in the supplementary file. The results are quite consistent. These tables also contain changes on NBO charges and the Ha-O-Hb angles. Changes are measured with respect to the values in bare water molecule. The O-Hb bond lengths always decreased for all “a” type complexes when O-Hb was free and O-Ha forming strong O–H···N HB [26]. The Ha-O-Hb angles increase for all “a” and “b” types of complexes other than two complexes [TEW1a and BEW1a] where a π-bond is associated with O–H···N h-bond. These changes in O-Hb BLs and Ha-O-Hb angles in these complexes are quite different from other complexes. These changes are also reflected in the atomic properties of involved individual atoms.
In these monohydrates of THIQ, we have lone pairs of N/O atoms as well as π electron clouds of the unsaturated rings interacting with various anti-bonding orbitals of the O–H/N–H/C-H bonds. The interaction energy is measured through ENBO. It is calculated as a second-order perturbation energy [18]. If we consider the lone pairs from the N atom and the anti-bonding orbital (σ*) of the OH bond, then it can be expressed as.
where q is the donor orbital occupancy and generally considered to be close to two, F is the Fock operator and \({\upepsilon }_{{\upsigma }_{\mathrm{OH}}^{*}}\mathrm{ and }{\upepsilon }_{{\mathrm{n}}_{\mathrm{N}}}\) are the energy eigenstates corresponding to the molecular orbitals \({\uppsi }_{\mathrm{n}}\;\mathrm{of\;nitrogen\;atom\;and}\;{\uppsi }_{{\upsigma }_{\mathrm{OH}}^{*}}\), respectively. This expression is suitably applied for other combinations of lone pairs and anti-bonding orbitals, respectively. Complexes having multiple HBs will have multiple contributions. Table 2 lists the computed values of ENBO for all the complexes detailing each individual contributions. This table also includes bond polarization, the amount of charge transfer, and values of the s-character of O atoms. According to this table, the s-character of the O atom increases in the respective complexes. Similarly, the s-character of the N atom increases in the respective complexes, where N–H···O h-bonds are involved, which validates Bent’s rule [36]. Table T2 in the supplementary file computes these parameters with 6–311 + + G (2d, 3p) basis. Results remained consistent as earlier.
If ENBO of these complexes is compared with binding energies (BE) [26], a discernible pattern is noticed. The maximum in both BE and ENBO appeared for “a” type of complexes. But, among the complexes within this category, the ones connected with the equatorial conformation of THIQ have the highest value in BE which might be arising from the multiple HBs associated with these kinds of structures. On the contrary, the axial conformations of bare molecule possess the highest values of ENBO. This might be due to the shorter HB lengths in these complexes producing largest amount of charge transfer.
The plots of the involved atomic orbitals in these two complexes are shown in Fig. 3. The participating atomic orbitals in TAW1b cluster are shown in Figs. 3a and b. The overlap is more pronounced in O⋅⋅⋅H HBs shown in Fig. 3a. While a close look at the weak HB involving π electron clouds of unsaturated ring displays the involvement of only C5 and C10 in TAW1b and C9 and C10 in BAW1b. Charge on both carbon atoms is found to decrease and the respective C–C BLs increase, as it is observed in O–H bonds in the acceptor site for both complexes. The change of charges and bond lengths are provided in Table T3 in the supplementary material. The H-bond distances for this category will henceforth be measured from the midpoint of that specific C–C bond of the ring. The respective plots in BAW1b complex are shown in Fig. 3c and d, where the O atom simultaneously acts as a donor as well as an acceptor.
Natural bond orbitals displaying the overlap between donor and acceptor orbitals of TAW1b complex for a N–H···O and b O–H···π hydrogen bonding interactions. Corresponding orbitals for the BAW1b complex are in c N–H···O and d O–H···π. Analysis has been performed with M06-2X/6–311 + + G (2d, 3p) level for an isovalue of 0.02
AIM results
This approach is successfully applied in numerous problems [9, 19,20,21, 37] to recognize the topological features of hydrogen bonding. In our earlier work [26] we had identified the presence of single and multiple h-bonds in TAW1a and TEW1a, respectively. Apart from computational works, the IR-UV double resonance experiments [28] also amply show a shift in NH stretching modes in these two complexes. The analysis of the shifts [26] clearly indicated the presence of additional hydrogen bonding in TEW1a cluster, which was later confirmed as O–H···π type. With this nice corroboration of our computational works, we are now in a position to apply these works for the entire set of monohydrates. Earlier, we had listed all the hydrogen bonds in all the complexes just from the optimized geometries of the quantum chemical computations. We were confident about the moderate to strong h-bonds, but not so for the weak ones and particularly those bonding with the π electron clouds of the unsaturated ring of THIQ. So, it becomes necessary to extend our work for all the complexes of THIQ to explore the quantitative nature of all the hydrogen bonds. In this respect, the AIM method comes handy and expects to ascertain our earlier observations.
The topography of all the complexes is shown in Fig. 4. The existence of multiple HBs is distinguished by the presence of multiple BCPs and their respective associated parameters. In these cases, all the bond critical points (3, − 1) and ring critical points (3, + 1) are easily identifiable. The O–H···π interactions are also identified in the respective complexes. The presence and location of BCPs indicate the nature of this kind of intermolecular interactions as hydrogen bonding. The values of electron density\(\left({\uprho }_{\mathrm{C}}\right)\), its Laplacian\(\left({\nabla }^{2}{\uprho }_{\mathrm{C}}\right)\), total electron energy density (HC), kinetic electron energy density (GC), and potential electron energy density (VC) are shown in Table 3 for the H···N pair of interatomic atoms for all the complexes. This include (i) NH covalent bonds for N–H···O hydrogen bridges involving lone pair of O atom in the hydrogen bonding and (ii) O–H···N in hydrogen-bonded systems involving nitrogen lone pair. Table 4 includes the corresponding values involving O···H pairs of atoms. In the case of covalent NH bonds, shorter bond lengths in the range of 1.0091–1.0145 Å, a high value of electron density at BCP along with negative values of Laplacian are noted. In all the N–H and O–H bonds both HC and \({\nabla }^{2}{\uprho }_{\mathrm{C}}\) is negative, satisfying the ideas put forward earlier. On the other hand, in all the hydrogen bonds \({\nabla }^{2}{\uprho }_{\mathrm{C}}\) although remains positive, we find a difference in the nature of HC. Apart from the TEW1a cluster, in all other O–H···N hydrogen bonds, HC remains negative, indicating strong hydrogen bonding. But HC remains negative for all O–H···N hydrogen bonds in MP2 computation. H-bonds remain weak in all O–H···π bonds, confirmed by the positive nature of HC. One can also guess about the strength through the respective values of \({\uprho }_{\mathrm{C}} .\) Although TEW1a is one of the most stable monohydrates, its strength is enhanced through the presence of an additional O–H···π bond. Another interesting complex is BEW1a. Although BE is a transition state in bare THIQ, this complex has one of the largest binding energy among all the monohydrates, making it somewhat special [26]. In this case, O–H···N h-bond length is slightly smaller than TEW1a, which makes HC negative through the increase of \({\uprho }_{\mathrm{C}} .\) The variation of \({\nabla }^{2}{\uprho }_{\mathrm{C}}\) and HC with \({\uprho }_{\mathrm{C}}\) is shown in Fig. 5. Strong and weak h-bonds are clearly separated out in this plot. At a value of \({\uprho }_{\mathrm{C}}=0.0285\) we can observe a change over from weak to strong h-bonding with the transformation of the sign of HC. Considering primary bond lengths only, ρC is linearly proportional to bond lengths. This can also be inferred from Figure F3 in the supplementary file. Here, we can assume that strong non-covalent interactions are expected to appear for hydrogen bond lengths approximately shorter than 1.96 Å. Lower than this value of BL as well as the corresponding value of \({\uprho }_{\mathrm{C}},\) HC becomes negative, clearly indicating the predominance of VC over GC. For further verification of this statement, we plotted the different (GC and VC) contributions of HC separately with ρC in Fig. 6. Comparing Figs. 5 and 6, we can clearly identify that both \({\nabla }^{2}{\uprho }_{\mathrm{C}}\mathrm{ and }{\mathrm{G}}_{\mathrm{C}}\) show a linear variation with \({\uprho }_{\mathrm{C}}.\) HC and VC on the other hand cannot be ascribed to a linear plot. HC in particular shows a strong deviation in the weak h-bonds. The negative values of HC signify the accumulation of electron density in the inter-nuclear region displayed also by the increase in the value of\({\uprho }_{\mathrm{C}}\), which corresponds to the covalent nature [9] of the respective non-covalent O–H···N bonds. For further verification and removing any doubts on the methods of computations, we plot the same graphs with the AIM outputs from MP2 computations. This is also included in Figs. 5 and 6.
Variation of Laplacian (\({\nabla }^{2}{\uprho }_{\mathrm{c}}\)) and Total electron Energy Density (HC) with electron density (\({\uprho }_{\mathrm{c}}\)) at Bond Critical Point (BCP) for all the hydrogen-bonded monohydrates of THIQ. QTAIM computations are performed at a) M06-2X/6–311 + + G (2d, 3p) and b) MP2/6–311 + + G (2d, 3p) level of theory. All quantities are in atomic units (a.u.)
Variation of Electron Kinetic Energy Density (GC) and Electron Potential Energy Density (VC) with electron density (\({\uprho }_{\mathrm{c}}\)) at Bond Critical Point (BCP) for all the hydrogen-bonded monohydrates of THIQ. QTAIM computations are performed at (a) M06-2X/6–311 + + G (2d, 3p) and (b) MP2/6–311 + + G (2d, 3p) level of theory. All quantities are in atomic units (a.u.)
Some topological descriptors are shown in Table 5 computed with the help of the DFT method. Corresponding table with computations involving the MP2 method is in Table T4 in the supplementary file. The core valence bifurcation (CVB) indices [38] for N–H···O bonded complexes are positive and for O–H···N bonded complexes are negative indicating stronger interaction in the latter case. The ratio of − GC/VC at BCP for O–H···N bonded complexes ranges between 0.5 and 1 indicating partial covalent character [39]. Exception is being the TEW1a complex for computations at the DFT level. On the other hand, this ratio strongly correlates with the ordering of ENBO of those bonds. Similar pattern is observed in HC \({/\uprho }_{\mathrm{c}}\) ratio and more negative value indicates the stronger h-bonding with more covalent character.
We want to know how the different interactions change with the variation in BLs. TEW1a and TAW1b are considered where O–H···N, and N–H···O are the primary HBs. In both cases, a weak O–H···π interaction is also present. Then variations of HB bond lengths and associated bond angle as well as \({\nabla }^{2}{\uprho }_{\mathrm{c}}\), HC, GC, and VC with \({\uprho }_{\mathrm{c}}\) at BCP of O–H···N and N–H···O h-bonds are presented in Fig. F3 in the supplementary file. Figure F4(a), (b), and (c) correspond to the strong N–H···O and O–H···N interactions while Figure F4(d), (e), and (f) for the weak O–H···π interactions.
Figure F4(a) shows that with the increase in \({\uprho }_{\mathrm{c}}\) the H-bond length decreases rapidly for both types of interactions identically which might hint at identical strength in HBs in TAW1b and TEW1a. But the bond angles show a large variation amongst the two different HBs, where the latter shows more linearity in the participating atoms and amply indicates the latter has a higher binding energy. Here, the variation in bond angles provides a much clearer picture about their differences in bond strength. Figure F4(b) indicates that as the \({\uprho }_{\mathrm{c}}\) increases, the variation of \({\nabla }^{2}{\uprho }_{\mathrm{c}}\) and HC becomes prominent. The rate of change of \({\nabla }^{2}{\uprho }_{\mathrm{c}}\) with \({\uprho }_{\mathrm{c}}\) for N–H···O interaction is faster than that of O–H···N interaction. The corresponding variation of HC with \({\uprho }_{\mathrm{c}}\) of N–H···O interaction is slower than that of O–H···N interaction although HC became negative for approximately same value of \({\uprho }_{\mathrm{c}}\). At a lower value of \({\uprho }_{\mathrm{c}}\), similar variation is observed for GC and VC given in Figure F4(c). But as \({\uprho }_{\mathrm{c}}\) increases the variation of GC become much more than VC and the variation is faster for N–H···O interaction for both cases.
The change h-bond length with the change of \({\uprho }_{\mathrm{c}}\) is greatly affected by the π-bonding angle in the O–H···π interaction. The change of \({\nabla }^{2}{\uprho }_{\mathrm{c}}\), GC and VC with \({\uprho }_{\mathrm{c}}\) are almost linear in these types of HBs. But due to the formation of an additional C2-H···O in the TAW1b complex, the O–H···π bond angle changes and we find the change till \({\uprho }_{\mathrm{c}}\) = 0.0136 a.u. But the variation of HC is quite different for π-bond and it seems to affect significantly with bond angle.
We also plot the variation of these energy densities (GC, VC, and HC) with all the NH and OH bond lengths including covalent and non-covalent interactions in Figs. 7 and 8, respectively. Here, covalent interactions are clearly identified over the non-covalent interactions through the values of the respective energies. A close look at the figures indicates that NH covalent bonds are weaker than the OH covalent bonds. On the contrary, if we consider non-covalent interactions N···H bonds show larger strength than the O···H bonds. This can also be concluded from the respective bond lengths as shown in Table 3. We leave out the H···π bonds from this discussion as it is much weaker than the above ones.
In AIM theory, the strength of hydrogen bonding is further verified through the properties of hydrogen atoms in the complexes. In this way, the different types of hydrogen bonding were also identified [40] apart from the conventional ones. The topological criteria for the existence of hydrogen bonding were put forward by Popelier [24] which are already stated earlier. The criteria (i) to (iii) are already verified in the earlier discussions.
We computed the charges on hydrogen atoms in isolated THIQ and water molecules and in the monohydrated complexes by integrating the electron density in the appropriate hydrogen atom regions partitioned by the AIM theory. All these results are listed in Table T5 in the supplementary material. It shows clearly that the hydrogen nuclei are deshielded on hydrogen bond formation. The amount of change ranges between 0.030 and 0.052 a.u. in all the monohydrates for O–H···N and N–H···O type of HBs. This is consistent with earlier studies on different hydrated complexes [40, 41]. The weak HBs involving pi electron clouds and C-H···O HBs show some discrepancies. The range of change in the magnitude of charge, first moment, volume, and energy depends on the involvement of the hydrogen atom with the h-bond acceptor. Similar variations were also observed in previous studies on hydrogen-bonded complexes [40, 41]. This table also includes the destabilization energy of the H atom. This value ranges between 0.001 and 0.162 a.u. indicating the changes in energy of H atoms participating in the complex formation. Here, also the stronger HBs show consistent data, but the weaker ones again show some unusual variations. The dipolar polarization is measured by the first moment of H atoms taking part in h-bonding. This table shows a decrease in this value, verifying the validity of the seventh criterion put forward by Popelier. In the same table, we also insert the changes observed in the computed values of hydrogen atom volume during the formation of complex from the values in bare THIQ and in the monohydrates. Interestingly, this also satisfies the criteria (vii), validating our earlier observations. In all the cases some complexes belonging to the weaker HBs show some discrepancies.
The strength of hydrogen bonds is also related through electron density and its Laplacian [41,42,43]. In Fig. 9, the sum of all the electron densities ΣρC and Σ∇2ρC are plotted with the corresponding BEs. The plots show a linear relationship. The correlation coefficient is also shown in the respective plots. The linear regressional analysis yields the following relation. Binding energy = 251.57 \(\sum {\uprho }_{\mathrm{c}}\) and Binding energy = 82.87 \(\sum {\nabla }^{2}{\uprho }_{\mathrm{c}}\) for DFT computations and the respective values for MP2 methods yield 217.29 \(\sum {\uprho }_{\mathrm{c}}\) and 69.18 \(\sum {\nabla }^{2}{\uprho }_{\mathrm{c}}\)
Relationship between binding energy and a total electron density \({(\uprho }_{\mathrm{c}})\), b total Laplacian of electron density (\({\nabla }^{2}{\uprho }_{\mathrm{c}}\)), at hydrogen bonds critical points for all THIQ:H2O complexes. All computations are performed with M06-2X/6–311 + + G(2d,3p) (black circle
 ) and MP2/6–311 + + G(2d,3p) (red circle
) and MP2/6–311 + + G(2d,3p) (red circle
 ) basis sets
) basis sets
Conclusions
The intermolecular non-covalent interactions in all the eleven monohydrates of THIQ are critically analyzed in this article. We successfully identified a variety of hydrogen bonds in the different complexes. The energy densities in the different complexes were partitioned by QTAIM approaches. Some of the hydrogen bonds show partial covalent character through the negative values of HC as well as the ratio of –GC/VC. All these are O–H···N type. The value of HC in the O–H···N bond of TAW1a has a value very close to zero. Corresponding value of \({\uprho }_{\mathrm{c}}=0.0285\) and bond length of around 1.96 Å may be considered as a turnover point to the partial covalent nature of H-bonds and vice versa. Poppeliar criteria are found to be satisfied in all the complexes. Although some of the weak HBs show some inconsistencies, rehybridization is found to be the dominant interaction in the “b” type complexes rather than hyperconjugation to determine the change of different properties of O-Ha and O-Hb bonds, ENBO and binding energies [26] are compared in all the complexes and their dependence with conformations of bare molecules are discussed. When an OH bond of water remained free in a complex then its bond length decreases from its value in bare water. This is observed in all cases when the OH bond acts as an HB donor only.
References
Lewis GN (1916) The atom and the molecule. J Am Chem Soc 38:762–785
Kollman PA (1977) Noncovalent interactions. Chem Rev 10:365–371. https://doi.org/10.1021/ar50118a003
Lopinski GP, Wayner DDM, Wolkow RA (2000) Self‐directed growth of molecular nanostructures on silicon. Nature 406:48–51. https://doi.org/10.1038/35017519
Autumn K, Sitti M, Liang YA, Peattie AM, Hansen WR, Sponberg S, Kenny TW, Fearing R, Israelachvili JN, Full RJ (2002) Evidence for van der Waals adhesion in Gecko State. Proc Natl Acad Sci USA 99:12252
Li D, Zhu Z, Sun DW (2020) Visualization of the in situ distribution of contents and hydrogen bonding states of cellular level water in apple tissues by confocal Raman microscopy. Analyst 145:897–907. https://doi.org/10.1039/C9AN01743G
Etim EE, Gorai P, Das A, Chakrabarti SK, Arunan E (2008) Interstellar hydrogen bonding. Adv Space Res 612870–2880. https://doi.org/10.1016/j.asr.2018.03.003
Pauling L, Corey RB, Branson HR (1952) The structure of proteins: two hydrogen-bonded helical configurations of the polypeptide chain. Proc Natl Acad Sci USA 37:205
Watson JD, Crick FHC (1953) A structure for deoxyribose nucleic acid. Nature 171:737
Grabowski SJ (2011) What is the covalency of hydrogen bonding? Chem Rev 111:2597–2625. https://doi.org/10.1021/cr800346f
Mahadevi AS, Sastry GN (2016) Cooperativity in noncovalent interactions. Chem Rev 116:2775–2825. https://doi.org/10.1021/cr500344e
Zwier TS (1996) The spectroscopy of solvation in hydrogen-bonded aromatic clusters. Annu Rev Phys Chem 47:205–241. https://doi.org/10.1146/annurev.physchem.47.1.205
Arunan E, Desiraju GR, Klein RA, Sadlej J, Scheiner S, Alkorta I, Clary DC, Crabtree RH, Dannenberg JJ, Hobza P (2011) Definition of the hydrogen bond (IUPAC recommendations 2011. Pure Appl Chem 83:1637–1641
Ghosh S, Wategaonkar S (2020) C-H···Y (Y=N, O, π) Hydrogen bond: a unique unconventional hydrogen bond. J Ind Inst Sc 100:101–125. https://doi.org/10.1007/s41745-019-00145-5
Banerjee P, Chakraborty T (2018) Weak hydrogen bonds: insights from vibrational spectroscopic studies. Int Rev Phys Chem 37(1):83–123. https://doi.org/10.1080/0144235X.2018.1419731
Weinhold F, Landis CR, Glendening ED (2016) What is NBO analysis and how is it useful? Int Rev Phys Chem 35(3):399–440. https://doi.org/10.1080/0144235X.2016.1192262
Alabugin IV, Manoharan M, Peabody S, Weinhold F (2003) Electronic basis of improper hydrogen bonding: a subtle balance of hyperconjugation and rehybridization. J Am Chem Soc 125(19):5973–5987. https://doi.org/10.1021/ja034656e
Joseph J, Jemmis ED (2007) Red-, blue-, or no-shift in hydrogen bonds: a unifiedexplanation. J Am Chem Soc 129(15):4620–4632. https://doi.org/10.1021/ja067545z
Grabowski SJ (2013) Non-covalent interactions - QTAIM and NBO analysis. J Mol Model 19(11):4713–4721. https://doi.org/10.1007/s00894-012-1463-7
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91:893. https://doi.org/10.1021/cr00005a013
Bader RFW (1990) Atoms in molecules, a quantum theory. Oxford University Press, Oxford
(2007) Quantum theory of atoms in molecules: recent progress in theory and application; Matta, C., Boyd, R. J., Eds.; Wiley-VCH: New York.
Grabowski SJ, Sokalski WA, Leszczynski J (2006) The possible covalent nature of N-H….O hydrogen bonds in formamide dimer and related systems: an ab initio study. J Phys Chem A 110:4772–4779. https://doi.org/10.1021/jp055613i
Kuznetsov ML (2019) Relationships between interaction energy and electron density properties for homo halogen bonds of the [(A)nY–XX–Z(B)m] type (X = Cl, Br, I), Molecules 24:2733 https://doi.org/10.3390/molecules24152733
Koch U, Popelier PL (1995) Characterization of C-H-O hydrogen bonds on the basis of the charge density. J Phys Chem 99:9747. https://doi.org/10.1021/j100024a016
Das S, Das L, Chakraborty A (2020) Conformers of 1,2,3,4 –tetrahydroisoquinoline in S0 and S1: an analysis through potential energy surface, hardness principles and vibrational spectroscopy. J Mol Struc 1207:127836. https://doi.org/10.1016/j.molstruc.2020.127836
Das S, Chakraborty A (2021) Conformer selective monohydrated clusters of 1,2,3,4 –tetrahydroisoquinoline in S0: I-Potential energy surface studies, vibrational signatures and NBO analysis. J Mol Struct 1225:129177. https://doi.org/10.1016/j.molstruc.2020.129177
Das S, Chakraborty A (2023) Computational investigation of the conformer selective complexes of 1,2,3,4-tetrahydroisoquinoline: ammonia (THIQ: NH3) in S0. J Mol Struct 134475. https://doi.org/10.1016/j.molstruc.2022.134475
Guchhait N, Banerjee S, Chakraborty A, Nath DN, Patwari GN, Chowdhury M (2004) Structure of hydrated clusters of tetrahydroisoquinoline THIQ–(H2O) n = 1,3 investigated by jet spectroscopy. J Chem Phys 120:9514–9523. https://doi.org/10.1063/1.1711810
Chakraborty A, Guchhait N, Banerjee S, Nath DN, Patwari GN, Chowdhury M (2001) Spectroscopic investigation of tetrahydroisoquinoline in supersonic jet. J Chem Phys 115:5184–5191. https://doi.org/10.1063/1.1394742
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, New York
Frisch MJ, Head-Gordon M, Pople JA (1990) A direct MP2 gradient method. Chem Phys Lett 166:275–280
Walker M, Harvey AJA, Sen A, Dessent CEH (2013) Performance of M06, M06–2X and M06-HF density functionals for conformationally flexible anionic clusters M06 functionals perform better than B3LYP for a model system with dispersion and ionic hydrogen-bonding interactions. J Phys Chem A 117:12590–12600
McLean AD, Chandler GS (1980) Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J Chem Phys 72:5639–5648. https://doi.org/10.1063/1.438980
Gaussian09 (Revision B.1), Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakat-suji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T Jr, Montgomery JA, Peralta J E, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian, Inc., Wallingford CT
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Bent HA (1961) An appraisal of valence-bond structures and hybridization in compounds of the first-row elements. Chem Rev 61:275–311
Johnson ER, Keinan S, Sa’nchez PM, Contreras-Garcı’a J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506. https://doi.org/10.1021/ja100936w
Fuster F, Silvi B (2000) Does the topological approach characterize the hydrogen bond? Theor Chem Acc 104:13–21
Emamian S, Lu T, Kruse H, Emamian H (2019) Exploring nature and predicting strength of hydrogen bonds: a correlation analysis between atoms-in-molecules descriptors, binding energies, and energy components of symmetry-adapted perturbation theory. J Comput Chem 9999:1–14. https://doi.org/10.1002/jcc.26068
Cubero E, Orozco M, Hobza P, Luque FJ (1999) Hydrogen bond versus anti-hydrogen bond: a comparative analysis based on the electron density topology. J Phys Chem A 103:6394. https://doi.org/10.1021/jp990258f
Parthasarathi R, Subramanian V, Sathyamurthy N (2005) Hydrogen bonding in phenol, water, and phenol-water clusters. J Phys Chem A 109:843–850. https://doi.org/10.1021/jp046499r
Kuznetsov ML (2019) Relationships between interaction energy and electron density properties for homo halogen bonds of the [(A)nY–XX–Z(B)m] type (X = Cl, Br, I), Molecules 24:2733. https://doi.org/10.3390/molecules24152733
Kumar PSV, Raghavendra V, Subramanian V (2016) Bader’s theory of atoms in molecules (AIM) and its applications to chemical bonding. J Chem Sci 128(10):1527–1536. https://doi.org/10.1007/s12039-016-1172-3
Acknowledgements
AC gratefully acknowledges the support received from University Grants Commission through a research Project (F. No. 37-560/2009(SR)) for conducting this work. The authors also acknowledge the instrumental support from DST (Govt. of India) under the departmental FIST programme of the University of Burdwan (Grant no: SR/FST/PS-II-/2018/52(C)) and University Grants Commission (UGC) for departmental CAS (Grant no. F.530/20/CAS-II/2018(SAP-I)) scheme.
Author information
Authors and Affiliations
Contributions
Santu Das: literature survey, editing, software handling, data handling, presentation of figures. Abhijit Chakraborty: conceptualisation and visualisation of the problem, writing, reviewing and editing the manuscript, literature survey.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, S., Chakraborty, A. Non-covalent interactions in the monohydrated complexes of 1,2,3,4–tetrahydroisoquinoline. J Mol Model 29, 37 (2023). https://doi.org/10.1007/s00894-022-05438-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05438-8









 GC,
GC,
 VC, and
VC, and
 HC) at BCP (in a.u) with the change of N–H/(N···H) length (in Å) of different complexes
HC) at BCP (in a.u) with the change of N–H/(N···H) length (in Å) of different complexes
 GC,
GC,
 VC, and
VC, and
 HC) at BCP (in a.u) with the change of O–H/(O···H) length (in Å) of different complexes
HC) at BCP (in a.u) with the change of O–H/(O···H) length (in Å) of different complexes