Abstract
One key strategy to further improve the power conversion efficiency (PCE) of organic solar cells (OSCs) is to incorporate various complementary functional groups in a molecule. Such strategies proved attractive for tuning the photovoltaic performances of the materials and can show a much higher absorption phenomenon with narrower band gaps. Despite the outstanding benefits, materials selection and their efficient modeling is also an extremely challenging job for the development of OSCs materials. In this manuscript, we proficiently developed an efficient series of small molecule-based non-fullerene acceptors (SM-NFAs) SN1-SN9 for OSCs and characterized by density functional theory (DFT) and time-dependent DFT (TD-DFT). The characteristics required to estimate electron and hole mobility, and open-circuit voltage (Voc) were investigated by optimizing the geometrical parameters, absorption spectra, exciton binding energy, frontier molecular orbitals (FMOs), electronic structures, and charge transfer rates. The outcomes of these materials showed that all newly constructed small-molecule-based non-fullerene acceptors exhibit broader and better absorption efficiency (λmax = 761 to 778 nm) and exciton dissociation, while much lower LUMO energy levels which may help to enhance the reorganizational energies. Further, a narrow bandgap also offers better photovoltaic properties. Hence, the designed molecules exhibited narrow bandgap values (Eg = 2.82 to 2.98 eV) which are lower than that of the reference molecule (3.05 eV). High Voc and photocurrent density values with lower excitation and binding energies eventually increase the PCEs of the OSC devices. The obtained results have shown that designed molecules could be effective aspirants for high-performance OSCs.
Graphical abstract

Highlights
-
1.
Half-moon-shaped fullerene-free acceptor molecules (SN1-SN9) are studied for organic solar cells applications.
-
2.
Significant lowering of energy gap with concomitant red-shifting of the absorption spectra is achieved with end-group engineering.
-
3.
The acceptors molecules show lower binding energy and excellent electron and hole reorganizational energies.
-
4.
All acceptor molecules have remarkable optoelectronic properties compared to reference R.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chalcogen-containing heterocycles are considered as potential candidates for enhancing the photovoltaic properties of organic solar cells. Highly efficient devices with high power conversion efficiency expressed strong backbone having non-covalent bonding which leads to a long and strong charge transfer route in these photovoltaic devices.
Nowadays, the field of organic solar cells (OSCs) has recognized a rapid considerable thanks to their higher PCEs. In organic solar cells, the large charge transfer behavior and incorporation of some inorganic counterparts significantly enhance the PCE [1,2,3]. Non-fullerene acceptor molecules are considered pivotal candidates for organic solar cells (OSCs) and tremendous research has been carried out on the development of non-fullerene acceptor molecules for OSC applications [4,5,6]. Non-fullerene acceptor molecules disclosed several advantages such as rapid chemical modifications, easy synthesis, low toxicity rate, high tunability, and low-cost development [7,8,9,10].
In recent years, the advancement in NFAs materials has gained significant attention. Recently, a very famous molecule Y6 had been synthesized and has shown high power conversion efficiency with different donor polymers [11]. Y6 has A′-DAD-A′ configuration, A′ were the end-capped acceptor molecules, and the core was a fused ring of benzothiadiazole molecule. The donor bridge was thienophene [3,2-b] thiophene group that was flanked with acceptor moieties [11, 12]. This combination provided the perfect backbone for important charge transfer within the whole molecule.
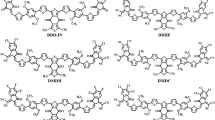
Recently, Chai and his co-workers have taken Y6 molecule and modified the donor groups with different chalcogen-containing heterocycles [13]. They used different units such as thienophene [3,2-b] furan ring, thienophene [3,2-b] thiophene, and thienophene [3,2-b] selenophene as donor units. Selenopheno [3,2-b] thiophene in these molecules act as donor unit and disclosed low bandgap, high red-shifting in absorption spectrum along with best PCE. It is believed that end-capped acceptor modifications have been proved as a unique and perfect strategy for enhancing the PCE of non-fullerene OSCs. In the past, different reports on end-capped engineering suggested that this strategy not only enhanced the PCE but also optimized the structures with a narrow bandgap. Therefore, motivated by these previous reports, our research group decided to perform end-capped modifications of recently synthesized selenopheno [3,2-b] thiophene containing BPS-4F (R) [13] molecule with novel end-capped units. After modifications, we have disposed of nine new novel fullerene-free acceptor molecules (SN1-SN9) having selenopheno [3,2-b] thiophene as donor unit.
Herein, we efficiently designed the new series of half-moon-shaped molecules (SN1-SN9) and studied their structural and physio-chemical relationship along with optoelectronic properties by using DFT and TD-DFT approaches. Various symmetrical characteristics such as the alignments of FMOs, excitation energies, the density of states (DOS), absorption spectrum, transition density matrix (TDM), and binding energies of (SN1-SN9) materials have been explored. Furthermore, Voc, reorganizational energies of electron and holes, and bandgap of the designed molecules (SN1-SN9) were studied extensively in comparison with reference (R). Moreover, complex studies of SN2/PTB7-Th and SN2/SZ5 are discussed as well. Results of these parameters proved that end-capped engineering is an interesting strategy for enhancing the photovoltaic properties of non-fullerene acceptor molecules. Therefore, we suggested a new type of half-moon-shaped acceptor molecules for future OSCs processing with high PCE.
Computational procedure
DFT and TD-DFT approaches have been employed on reference and designed molecules. The chemistry of molecules can be found in supporting information. A Gaussian 16 is used along with GAUSS VIEW 6.0 suit of a program for all of the characterizations. Firstly, the geometrical optimization of R was studied with four various functionals such as B3LYP [14], ωB97XD [15], MPW1PW91 [16], and CAM-B3LYP [17] at 6-31G(d,p) [18,19,20,21] level of DFT. Then, maximum absorption was determined using the above functionals at 6-31G(d,p) basis set by using organic solvent through conductor-like polarizable continuum model (CPCM). From experimental [13] and TD-DFT-based calculations of absorption maxima, it was found that B3LYP/6-31G(d,p) is the reliable method to reproduce the experimental datum.
Electron and hole reorganizational energies are sub-categorized into internal and external reorganizational energies. The internal reorganizational energy is associated with the internal changes of geometries and the effect of different internal factors that influence the internal geometries of molecules. At the same time, external reorganizational energies are associated with the polarization in the external environment. In this discussion, we have ignored the external reorganizational energy as it has a negligible contribution. For calculating internal and external reorganizational energy energies of the considered molecules, we have used the following equations [22,23,24].:
Hence, the cationic and anionic energies of neutral material optimization are \({E}_{0}^{+}\) and \({E}_{0}^{-}\), respectively. \({E}_{-}\) and \({E}_{+}\) are anionic and cationic energies of cation and anion geometries optimization, respectively. \({E}_{-}^{0}\) and \({E}_{+}^{0}\) define the energies of the neutral molecules optimized at anionic and cationic states. E0 is the neutral molecule energy at the ground state geometry. For the calculation of binding energy, different parameters like the first principle excitation energy (which comes from excitation energy calculation of a molecule in an excited state by employing the TD-DFT approach) and energy band gaps are considered. The following equation is proved to calculate calculating the binding energy:
where Eb corresponds to binding energy, Eg is the bandgap, and Eopt is the first principle excitation energy. Generally, the lower Eb value of a molecule allows an important charge transfer and thus high PCE.
Result and discussion
Basic optimization
Figure 1 shows the designed structures of (SN1-SN9) materials after modifying R as SM-NFAs for OSCs. Initially, the optimization of R was carried out at B3LYP, ωB97XD, CAM-B3LYP, and MPW1PW91 at 6-31G(d,p) basis set. Subsequently, the maximum absorption of R was obtained in chloroform solvent at the above-cited functionals and basis set. The absorption maxima of R was at 760 nm, 842 nm, 701 nm, and 811 nm at B3LYP/6-31G(d,p), ωB97XD/6-31 g(d,p), CAM-B3LYP/6-31G(d,p), and MPW1PW91/6-31G(d,p), respectively, as shown in Fig. 2. The experimental absorption maxima of R is obtained at 749 nm [13]. From these values, it is evident that the B3LYP/6-31G(d,p) level of theory revealed the best agreement with experimental data; therefore, this basis set and method have been used for further calculations.
Frontier molecular orbital analysis
Based on molecular orbital theory (MOT), the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) are considered as valance and conduction bands, respectively. Both orbitals are also known as frontier molecular orbitals (FMOs) [25,26,27,28]. The occurrence of FMOs is of crucial importance regarding charge transfer efficiency. The occurrence of FMOs is also a decisive factor for determining the charge transfer extent as it is directly linked with the performances of the materials. The energy difference between these molecular orbitals is described as the HOMO–LUMO energy gap (Eg). The Eg plays a significant role in deciding the charge transfer rate and also helps in estimating the PCE of a molecule for OSC [18, 22, 29,30,31,32,33,34,35,36,37]. A molecule that exhibits a narrow bandgap will lead to an important charge transfer rate. The optimized structures of designed (SN1-SN9) and R are shown in Fig. S1.
The alignment of FMOs of designed (SN1-SN9) and R are displayed in Fig. 3. The calculated HOMO energies of R and SN1-SN9 are given in Table 1. The deigned molecules (SN1-SN9) exhibit stabilized HOMO and LUMO orbitals which leads to reducing the bandgap energies. The bandgap is also another important tool that disclosed the OSC performance. Molecules with narrow band gaps are considered rich in charge carrier transfer. The HOMO and LUMO energy levels along with the bandgap values are tabulated in Table 1.
Designed molecules expressed narrow band gaps compared to R which indicated that our designed half-moon-shaped molecules are appropriate for NFA-based OSCs. Despite this, we realize that SN2 has the lowest bandgap value (Eg = 2.82 eV) because of the presence of incorporated 3-(dicyanomethylene)-2-methylene-1-oxo-2,3-dihydro-1H-indene-5,6-dicarbonitrile end-capped moiety. The second and third lowest band gap values are noted for SN8 and SN1 which is probably generated from the effect of 2-(2-methylene-3-oxo-2, 3-dihydro-1H-inden-1-ylidene) malononitrile combined with 1,1,1-trichloroethane and 2-(5,6-dichloro-2-methylene-1-oxo-1H-inden-3(2H)-ylidene) malononitrile end-capped acceptor moieties, respectively. The energy band gap of SN7 suggested that the addition of three fluorine atoms significantly causes the reduction of the bandgap energy. Similarly, the obtained band gap value of SN5 demonstrated that the incorporation of additional functional groups as end-capped units may decrease the band gap value. Molecules SN6 and SN3 displayed approximately similar bandgap values which might be due to the efficient 2-(2-methylene-3-oxo-2, 3-dihydro-1H-inden-1-ylidene) malononitrile with methyl acetate and methyl 6-cyano-3 (dicyanomethlene)-2-methylene-1-oxo-2,3-dihydro-1H-indene-5-carboxylate acceptor moieties. Molecule SN9 also exhibited a narrow bandgap (Eg = 2.98 eV) compared to R which could be explained by the addition of Si metal in the acceptor unit. As revealed, the designed molecules exhibited a narrow bandgap compared to R which is surely generated from the extra-functional addition within the acceptor moiety. The decreasing trend of the bandgap values is as follows: R > SN4 > SN9 > SN3 > SN6 > SN5 > SN7 > SN1 > SN8 > SN2. A narrow energy band gap allows easy transfer of charge from lower level to higher level orbital. The designed molecules exhibited a narrow band gap (2.82 to 2.98 eV) as compared to the reference molecule (3.05 eV). Similarly, designed molecules also exhibited red-shift in the absorption spectrum (761 to 778 nm) as compared to the reference molecule (760 nm). Further, designed molecules also expressed good values of excitation energy (1.60 to 1.89 eV). Among all designed molecules, SN2 expressed the narrowest band gap with high red-shifting. Moreover, it also expressed excellent excitation energy which suggested that SN2 show easy excitation in the excited state, thus enhancing the efficiency of the molecule for solar cell applications. From the preceding discussion and based on the bandgap order, we can conclude that the designed molecules (especially SN2) are suitable NFAs for OSCs.
Density of states analysis
The DOS analysis is a useful tool to determine the HOMO and LUMO positions within a molecule which in return measures the performance of a solar cell. DOS analysis is also of quite an important to explore the nature of different units of a molecule, i.e., the donor and acceptor part. Therefore, we have considered the DOS analysis to get in-depth information of designed (SN1-SN9) and R molecules and also to point out the position of stable HOMO and LUMO orbitals [38, 39]. DOS plots are illustrated in Fig. 4. In our case, the central unit of the studied molecules is an acceptor represented with A and attached to two donor units (D). The donor units are flanked with two efficient acceptor units (A′). Hence, in this way, a perfect A′-DAD-A′ backbone is formed for high charge transfer.
From Fig. 4, we can see the HOMO density distributed over the donor unit which suggested the strong electron-donating nature of selenopheno [3,2-b] thiophene units. However, the LUMO orbital density is dispersed over the central acceptor core unit and slightly over the end-capped acceptor units which indicated the electron-accepting nature of strong acceptor molecules. The distribution behavior of FMOs remains the same in all the studied molecules which indicated the efficient design of the new nine half-moon-shaped acceptor molecules (SN1-SN9). From DOS plots, we can determine that our designed (SN1-SN9) materials are suitable to be used as acceptors for OSCs.
Photovoltaic characteristics
The excitation energy, oscillating strength, absorption behavior (absorption spectrum), and related molecular orbitals assignments are considered the key features of a molecule. The photovoltaic properties of a molecule are very important for explaining the quality of a solar cell. Commonly, molecules that exhibit red-shifting in absorption spectrum are considered highly efficient molecules in terms of PCE. The absorption spectrum red shiftcate along with the absorption high intensity leads to a higher charge transformation from HOMO to LUMO and the possibility of enhancing the PCE of a solar cell. Therefore, the photovoltaic characteristics of the desi (SN1-SN9) and R design carried out in chloroform solvent. The calculated photovoltaic parameters are shown in Table 2.
In Table 2, we realized that the absorption of R is 760.98 nm (based on DFT) and 749 nm (based on experimental). The λmax values of the designed molecules are found at 762.83 nm (SN1), 778.21 nm (SN2), 765.19 nm (SN3), 761.96 nm (SN4), 766.71 nm (SN5), 769.11 nm (SN6), 770.01 nm (SN7), 776.92 nm (SN8), and 762.45 nm (SN9). From these results, it is evident that the designed (SN1-SN9) materials are highly red-shifted in absorption spectra compared to R. The highest red-shifting was detected for SN2 (λmax = 778.21 nm) generated from the efficient 3-(dicyanomethylene)-2-methylene-1-oxo-2,3-dihydro-1H-indene-5,6-dicarbonitrile end-capped acceptor unit. While the second and third highest red-shifting absorption is attributed to SN8 (λmax = 776.92 nm) and SN7 (λmax = 770.01 nm) which is probably originating from the addition of trichloro and tri-fluoro groups in end-capped acceptor moiety. SN5 (λmax = 766.71 nm) and SN3 (λmax = 765.19 nm) exhibited approximately similar absorption maxima which is explained by the presence of 2-(2-methylene-3-oxo-2, 3-dihydro-1H-inden-1-ylidene) malononitrile acetate and methyl 6-cyano-3 (dicyanomethlene)-2-methylene-1-oxo-2,3-dihydro-1H-indene-5-carboxylate end-capped acceptor groups, respectively. SN4 and SN1 also expressed close values of absorption maxima and a red-shifting compared to R. From the obtained results, it is concluded that SN1-SN9 is better than R in terms of red-shifting absorption. Thus, the designed half-moon-shaped materials are promising runners for efficient OSC devices.
Moreover, excitation energy could help in determining the photovoltaic performances of OSCs [40]. In general, lower reorganizational energies lead to a high PCE [41]. Likewise, low values of Ex also help to determine the extent of charge transformation originating from excited HOMO to LUMO [42,43,44,45,46,47]. Thus, materials that exhibit low excitation energies are considered as the most suitable molecules for high-performance photovoltaic devices [48]. Herein, we performed an excitation energy analysis that is shown in Table 2. Reference molecule (R) expressed an excitation energy value of 1.89 eV, while for the designed molecules, it is observed as SN1 (Ex = 1.77 eV), SN2 (Ex = 1.60 eV), SN3 (Ex = 1.82 eV), SN4 (Ex = 1.88 eV), SN5 (Ex = 1.89 eV), SN6 (Ex = 1.72 eV), SN7 (Ex = 1.85 eV), SN8 (Ex = 1.79 eV), and SN9 (Ex = 1.77 eV). Compared to R, it is revealed that SN1-SN9 exhibited low excitation energies indicating the formation of highly efficient OSCs. The decreasing trend of the excitation energy is as follows: R = SN5 > SN4 > SN7 > SN3 > SN8 > SN1 = SN9 > SN6 > SN2. Further, major molecular orbitals assignments are calculated. These outcomes showed that the designed (SN1-SN9) materials have the maximum charge density transition ability from HOMO to LUMO. The simulated absorption spectra are depicted in Fig. 5 which are recorded in chloroform solvent.
Reorganizational energy
The electron and hole mobility decide the overall performance of an OSC as it is inversely related to the reorganizational energy of the hole and electron of a material [49]. Usually, the lower reorganizational energies of hole and electron are considered for higher mobilities of hole and electrons, respectively. To sum up, the reorganizational energies of molecules play a major role in measuring OSC performances. Therefore, the reorganizational energies of hole and electron of all studied (SN1-SN9) molecules.
The electron reorganizational energy of R is 0.0097, while for the designed (SN1-SN9) molecules, the reorganizational energy of electron is of 0.0083 (SN1), 0.0061 (SN2), 0.0083 (SN3), 0.0091 (SN4), 0.0077 (SN5), 0.0086 (SN6), 0.0081 (SN7), 0.0088 (SN8), and 0.0090 (SN9). It is found that SN1-SN9 has exhibited lower reorganizational energies of electrons in contrast to R, which suggested that the new half-moon-shaped fullerene-free acceptor molecules are potential candidates for highly efficient OSCs. The lowest reorganizational energy of electron was found for SN2 that is certainly originating from the efficient end-capped acceptor unit effect. Hence, we demonstrated that the end-capped acceptor modification disposes of a reliable tool for enhancing the performance of OSCs, while second and third lowest values of electron reorganizational energy were observed for SN5 and SN7 originating from the effect of 2-(2-methylene-3-oxo-2, 3-dihydro-1H-inden-1-ylidene) malononitrile acetate and 2-(2-methylene-3-oxo-2, 3-dihydro-1H-inden-1-ylidene) malononitrile combined with 1,1,1-trifluoroethane end-capped acceptor units. SN3 and SN1 disclosed similar values of electron reorganizational energies. Similarly, SN9 and SN4 expressed approximately the same values of electron reorganizational energies. The decreasing order of electron reorganizational energies is as follows: R > SN4 > SN9 > SN8 > SN6 > SN1 = SN3 > SN7 > SN5 > SN2. Thus, it is concluded that all designed molecules exhibited high charge mobilities compared to R. Based on these results, we can deduce that the designed (SN1-SN9) materials are fine electron transport molecules for efficient OSCs devices.
The hole reorganization energies were also calculated with the same method. The hole reorganizational energy of R is found at 0.0113. The designed SN1-SN9 materials present reorganization energies of 0.0101, 0.0089, 0.0105, 0.0112, 0.0109, 0.0118, 0.0091, 0.0098, and 0.0106, individually. Hence, these (SN1-SN9) molecules displayed lower and corresponding reorganization energy of hole as of R. In Fig. 6, the highest hole mobility was obtained for SN2 followed by SN7 and SN8, respectively. So, from the proceeding discussion, Fig. 6 and Table 3, it is illustrated that the designed (SN1-SN9) molecules are suitable hole and electron transport materials for high-performance OSCs.
Transition density matrix
Transition density matrix (TDM) investigations are widely employed to elaborate electron, hole, and hole-electron pair positions [50,51,52,53,54]. Further, TDM analysis disposes of an efficient evaluation for an OSC performance. Location of electron and hole inside a molecule considered crucial regarding the charge transfer analysis. Otherwise, TDMs are a helpful tool to estimate the unveiled nature of different parts of a molecule. The TDM plots have been calculated. Hydrogen does not involve in the overall charge transformation as its effect is ignored.
In this report, we separated the investigated (SN1-SN9) molecules into three portions, a central core which is an acceptor in nature represented with C, a donor bridge represented with D, and a terminal acceptor represented with A (Fig. 7). This molecular dissociation helps the readers to better understand the TDM plots. As shown in Fig. 7, it is indicated that electron density is located over the acceptor diagonal. The electronic density is also present at the center and terminal acceptors. Hence, it is deduced that the donor bridge donates electron density and also facilitates the energy transfer from the core to the donor to the terminal acceptor end. We discovered that electronic coherence is existing in all designed (SN1-SN9) molecules indicating that the end-capped modification represents a key approach for improving SCs parameters. The existence of electronic density in all designed (SN1-SN9) materials also proposed efficient end-capped modifications in these molecules. Therefore, after TDM analysis we can suggest that the designed (SN1-SN9) materials are promising candidates for efficient OSCs.
Binding energy (Eb) is yet another method to demonstrate the potential of SC materials [55]. The low value of Eb also presents a higher current charge density in a molecule [56]. Therefore, the Eb analysis of SN1-SN9 has been performed and results are summarized in Table 4.
As shown in Table 4, the R revealed Eb value of 1.16 eV, while SN1-SN9 expressed binding energy values of 1.13 eV (SN1), 0.92 eV (SN2), 1.14 eV (SN3), 1.11 eV (SN4), 0.96 eV (SN5), 1.07 eV (SN6), 1.08 eV (SN7), 1.09 eV (SN8), and 1.10 eV (SN9). The designed (SN1-SN9) materials displayed lower Eb, indicating their ability to dispose of a higher current density compared to R, while the lowest Eb is detected for SN2 and SN5 which could be originated from the addition of additional functional groups present over end-capped acceptors. As shown in Fig. 8, the designed (SN1-SN9) materials exhibited lower Eb values compared to R, whereas the decreasing trend of materials Eb is as follows: R > SN3 > SN1 > SN4 > SN9 > SN8 > SN7 > SN6 > SN5 > SN2. The recorded trend demonstrates that designed (SN1-SN9) materials, particularly SN2, could be a better material for OSC devices.
Open-circuit voltage (Voc)
The Voc of materials is another parameter to explore OSC performances. The Voc solar cell is measured at null voltage. The Voc is ascribed as the total current drawn by the device at null voltage [57]. It is a combination of two currents, i.e., photo-generated current and device saturated current. To measure Voc of any device, the following equation is employer [58]:
Here, e is the elementary charge, EHOMO is the HOMO orbital of the donor molecules which are PTB7-Th and SZ5 in this report, and ELUMO is the LUMO orbital of our studied molecules. PTB7-Th and SZ5 are commonly known as efficient donor polymers for OSCs. SZ5 was used in the experimental part as donor polymer; therefore, both donor polymers are considered for Voc analysis and charge transfer investigation (study of the complex).
The Voc calculations by using PTB7-Th and SZ5 (donor polymer) blended with R and designed SN1-SN9 materials are shown in Fig. 9. First, we discussed the PTB7-Th/acceptor molecules, where the designed (SN1-SN9) materials displayed high Voc values compared to R which indicates that our designed (SN1-SN9) materials are competent candidates for OSCs. While the R exhibits a Voc of 1.85 V, the Voc values of the investigated molecules are 2.00 V (SN1), 2.08 V (SN2), 1.94 V (SN3), 1.91 V (SN4), 1.96 V (SN5), 1.95 V (SN6), 1.97 V (SN7), 2.02 V (SN8), and 1.92 V (SN9). The highest Voc is found for SN2 which could be explained by the effect of 3-(dicyanomethylene)-2-methylene-1-oxo-2,3-dihydro-1H-indene-5,6-dicarbonitrile end-capped group. Similarly, second and third highest Voc values are noted for SN8 and SN1 which is probably originating from the chloro groups in end-capped moieties. Molecules SN3, SN6, and SN5 exhibit close values of Voc which indicates that these (SN1-SN9) materials possess effective end-capped acceptor groups which help in improving the performances of materials.
In SZ5/Acceptor molecules, the Voc has been carried out at the same basis set. In these systems, the calculated Voc is found of 2.15 V (R), 2.30 V (SN1), 2.38 V (SN2), 2.24 V (SN3), 2.21 V (SN4), 2.26 V (SN5), 2.25 V (SN6), 2.27 V (SN7), 2.32 V (SN8), and 2.22 V (SN9). These materials displayed high values of Voc as compared to R which is the consequence of the adding of an extra-functional group within the end-capped acceptor units. The highest Voc is attributed to SN2 arising from the effect of 3-(dicyanomethylene)-2-methylene-1-oxo-2,3-dihydro-1H-indene-5,6-dicarbonitrile end-capped unit. Similarly, the second and third Voc values are assigned to SN8 and SN1 resulting from the presence of chloro group in the end-capped moiety. Molecules SN3, SN6, and SN5 exhibited close values of Voc indicating that these groups have effective end-capped acceptor units.
Based on these findings, it is evident that our designed half-moon-shaped (SN1-SN9) acceptor materials are potential runners for efficient OSC devices.
Molecular electrostatic potential analysis
We have also calculated molecular electrostatic potential (MEP) plots to estimate for designed (SN1-SN9) and R material, as illustrated in Fig. 10. The main determination of these calculations is to investigate the presence of various charge sites inside the materials. The investigations of MEP plots of SN1-SN9 and R were performed using an iso-surface value of 0.02e/Å3, respectively. The formed MEP plots showed colorful representation, where every color corresponds to some unique feature of the molecule. Hence, the positively charged moieties are accomplished to form the blue color representation, while the red color signifies the negative charge accumulation and the green color corresponds to neutral charge, i.e., the maximum possible potential area among the designed (SN1-SN9) materials and R.
Interestingly, we revealed similar charged sites for SN1-SN9 and R. As it can be seen in Fig. 10, the investigated molecules exhibit quite similar clouds densities. It was observed that similar electronic charge density spreads over all the newly designed molecules (SN1-SN9) as well as R. These investigations revealed the efficiency of our molecular modeling strategy to tune the photophysical and optoelectronic characteristics of the materials. Hence, regarding the compatibility of these newly designed (SN1-SN9) materials for an efficient charge transformation reaction, we are looking forward to their synthesis in the future to fabricate highly efficient solar cell devices.
Conclusion
In conclusion, we have developed a theoretical investigation based on a series of non-fullerene-based molecular systems to understand their photovoltaic and optoelectronic characteristics. The geometrical parameters, the absorption maxima, the FMOs, and the electronic structures of these materials were successfully analyzed by using DFT and TD-DFT approaches with various methods and basis sets. Interestingly, we realized that HOMO–LUMO molecular energy orbitals exhibited a noteworthy correlation as compared with experimentally verified molecule R. The designed materials (SN1-SN9) exhibited narrower energy gaps compared to R. The DOS analysis explained the distribution patterns of HOMO–LUMO levels. The photophysical studies of SN1-SN9 and R revealed that all the newly constructed materials are extremely red-shifted as compared to R ensuring our effective molecular modeling approach. Moreover, all the designed materials (SN1-SN9) exhibited Ex and Eb, which is helpful to produce higher photocurrent density values. The lower reorganization energies of electrons and holes denote higher charge mobilities. Our designed (SN1-SN9) materials produced much lower values of electron mobility which corresponds towards higher electronic mobilities as compared to R. In addition, we have also calculated the Voc of SN1-SN9 and R by considering the well-known polymers donor PTB7-Th and SZ5. TDM and electron–hole overlapping analysis uncovered the hidden charging site of the molecules which may provide a helpful tool to realize the electronic transition behavior of SC materials. Regarding these theoretical studies, we can conclude that our designed materials have a great ability to produce better photovoltaic and optoelectronic characteristics compared to the reference R. Moreover, we highly recommend these designed materials (SN1 to SN9) to be synthesized for fabricating efficient OSC devices.
Data availability
The data of this finding is available on request and requests should be made to corresponding author. In addition, optimized Cartesian coordinates of all studied systems are present in supporting information file.
References
Zhang G, Zhao J, Chow PCY, Jiang K, Zhang J, Zhu Z, Zhang J, Huang F, Yan H (2018) Nonfullerene acceptor molecules for bulk heterojunction organic solar cells. Chem Rev 118(7):3447–3507. https://doi.org/10.1021/acs.chemrev.7b00535
Yan C, Barlow S, Wang Z, Yan H, Jen AK-Y, Marder SR, Zhan X (2018) Non-Fullerene acceptors for organic solar cells. Nat Rev Mater 3(3):18003. https://doi.org/10.1038/natrevmats.2018.3
Wadsworth A, Moser M, Marks A, Little MS, Gasparini N, Brabec CJ, Baran D, McCulloch I (2019) Critical review of the molecular design progress in non-fullerene electron acceptors towards commercially viable organic solar cells. Chem Soc Rev 48(6):1596–1625. https://doi.org/10.1039/C7CS00892A
Lin Y, Wang J, Zhang Z-G, Bai H, Li Y, Zhu D, Zhan X (2015) An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv Mater 27(7):1170–1174. https://doi.org/10.1002/adma.201404317
Zhao W, Li S, Yao H, Zhang S, Zhang Y, Yang B, Hou J (2017) Molecular Optimization enables over 13% efficiency in organic solar cells. J Am Chem Soc 139(21):7148–7151. https://doi.org/10.1021/jacs.7b02677
Jia T, Zhang J, Zhong W, Liang Y, Zhang K, Dong S, Ying L, Liu F, Wang X, Huang F, Cao Y (2020) 14.4% Efficiency all-polymer solar cell with broad absorption and low energy loss enabled by a novel polymer acceptor. Nano Energy 72:104718. https://doi.org/10.1016/j.nanoen.2020.104718
Song J, Li C, Zhu L, Guo J, Xu J, Zhang X, Weng K, Zhang K, Min J, Hao X, Zhang Y, Liu F, Sun Y (2019) Ternary organic solar cells with efficiency >16.5% based on two compatible nonfullerene acceptors. Adv Mater 31(52):1905645. https://doi.org/10.1002/adma.201905645
Gao J, Gao W, Ma X, Hu Z, Xu C, Wang X, An Q, Yang C, Zhang X, Zhang F (2020) Over 14.5% Efficiency and 71.6% fill factor of ternary organic solar cells with 300 nm thick active layers. Energy Environ Sci 13(3):958–967. https://doi.org/10.1039/C9EE04020J
Liu T, Ma R, Luo Z, Guo Y, Zhang G, Xiao Y, Yang T, Chen Y, Li G, Yi Y, Lu X, Yan H, Tang B (2020) Concurrent IMPROVEMENT In J SC and V OC in high-efficiency ternary organic solar cells enabled by a red-absorbing small-molecule acceptor with a high LUMO level. Energy Environ Sci 13(7):2115–2123. https://doi.org/10.1039/D0EE00662A
Yang W, Luo Z, Sun R, Guo J, Wang T, Wu Y, Wang W, Guo J, Wu Q, Shi M, Li H, Yang C, Min J (2020) Simultaneous enhanced efficiency and thermal stability in organic solar cells from a polymer acceptor additive. Nat Commun 11(1):1218. https://doi.org/10.1038/s41467-020-14926-5
Yuan J, Zhang Y, Zhou L, Zhang G, Yip H-L, Lau T-K, Lu X, Zhu C, Peng H, Johnson PA, Leclerc M, Cao Y, Ulanski J, Li Y, Zou Y (2019) Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core. Joule 3(4):1140–1151. https://doi.org/10.1016/j.joule.2019.01.004
Li S, Li C-Z, Shi M, Chen H (2020) New phase for organic solar cell research: emergence of y-series electron acceptors and their perspectives. ACS Energy Lett 5(5):1554–1567. https://doi.org/10.1021/acsenergylett.0c00537
Chai G, Zhang J, Pan M, Wang Z, Yu J, Liang J, Yu H, Chen Y, Shang A, Liu X, Bai F, Ma R, Chang Y, Luo S, Zeng A, Zhou H, Chen K, Gao F, Ade H, Yan H (2020) Deciphering the role of chalcogen-containing heterocycles in nonfullerene acceptors for organic solar cells. ACS Energy Lett 5(11):3415–3425. https://doi.org/10.1021/acsenergylett.0c01688
Civalleri B, Zicovich-Wilson CM, Valenzano L, Ugliengo P (2008) B3LYP Augmented with an empirical dispersion term (B3LYP-D*) as applied to molecular crystals. CrystEngComm 10(4):405–410. https://doi.org/10.1039/B715018K
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10(44):6615. https://doi.org/10.1039/b810189b
Adamo C, Barone V (1998) Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: the MPW and MPW1PW models. J Chem Phys 108(2):664–675. https://doi.org/10.1063/1.475428
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393(1–3):51–57. https://doi.org/10.1016/j.cplett.2004.06.011
Khan MU, Mehboob MY, Hussain R, Afzal Z, Khalid M, Adnan M (2020) Designing spirobifullerene core based three-dimensional cross shape acceptor materials with promising photovoltaic properties for <scp>high-efficiency</Scp> Organic Solar Cells. Int J Quantum Chem 120(22):1–15. https://doi.org/10.1002/qua.26377
Afzal Z, Hussain R, Khan MU, Khalid M, Iqbal J, Alvi MU, Adnan M, Ahmed M, Mehboob MY, Hussain M, Tariq CJ (2020) Designing indenothiophene-based acceptor materials with efficient photovoltaic parameters for fullerene-free organic solar cells. J Mol Model 26(6):137. https://doi.org/10.1007/s00894-020-04386-5
Hussain R, Hassan F, Khan MU, Mehboob MY, Fatima R, Khalid M, Mahmood K, Tariq CJ, Akhtar MN (2020) Molecular engineering of A-D–C–D–A configured small molecular acceptors (SMAs) with promising photovoltaic properties for high-efficiency fullerene-free organic solar cells. Opt Quantum Electron 52(8):364. https://doi.org/10.1007/s11082-020-02482-7
Khan MU, Mehboob MY, Hussain R, Afzal Z, Khalid M, Adnan M (2020) Designing spirobifullerene core based three-dimensional cross shape acceptor materials with promising photovoltaic properties for <scp>high-efficiency</Scp> organic solar cells. Int J Quantum Chem. https://doi.org/10.1002/qua.26377
Mehboob MY, Hussain R, Khan MU, Adnan M, Umar A, Alvi MU, Ahmed M, Khalid M, Iqbal J, Akhtar MN, Zafar F, Shahi MN (2020) Designing N-phenylaniline-triazol configured donor materials with promising optoelectronic properties for high-efficiency solar cells. Comput Theor Chem 1186:112908. https://doi.org/10.1016/j.comptc.2020.112908
Khan MU, Hussain R, Mehboob MY, Khalid M, Ehsan MA, Rehman A, Janjua MRSA (2021) First theoretical framework of Z-shaped acceptor materials with fused-chrysene core for high performance organic solar cells. Spectrochim Acta A Mol Biomol Spectrosc 245:118938. https://doi.org/10.1016/j.saa.2020.118938
Khan MU, Hussain R, YasirMehboob M, Khalid M, Shafiq Z, Aslam M, Al-Saadi AA, Jamil S, Janjua MRSA (2020) In Silico modeling of new “Y-series”-based near-infrared sensitive non-fullerene acceptors for efficient organic solar cells. ACS Omega 5(37):24125–24137. https://doi.org/10.1021/acsomega.0c03796
Ahmed M, Imran M, Muddassar M, Hussain R, Khan MU, Ahmad S, Mehboob MY, Ashfaq S (2020) Benzenesulfonohydrazides inhibiting urease: design, synthesis, their in vitro and in silico studies. J Mol Struct 1220:128740. https://doi.org/10.1016/j.molstruc.2020.128740
Hussain S, ShahidChatha SA, Hussain AI, Hussain R, Mehboob MY, Gulzar T, Mansha A, Shahzad N, Ayub K (2020) Designing novel Zn-decorated inorganic B 12 P 12 nanoclusters with promising electronic properties: a step forward toward efficient CO 2 sensing materials. ACS Omega 5(25):15547–15556. https://doi.org/10.1021/acsomega.0c01686
Adnan M, Iqbal J, BiBi S, Hussain R, Akhtar MN, Rashid MA, Eliasson B, Ayub K (2017) Fine tuning the optoelectronic properties of triphenylamine based donor molecules for organic solar cells. Zeitschrift für Phys Chemie 231(6). https://doi.org/10.1515/zpch-2016-0790
Siddique SA, Siddique MBA, Hussain R, Liu X, Mehboob MY, Irshad Z, Adnan M (2020) Efficient tuning of triphenylamine-based donor materials for high-efficiency organic solar cells. Comput Theor Chem 1191:113045. https://doi.org/10.1016/j.comptc.2020.113045
Khan MU, Mehboob MY, Hussain R, Fatima R, Tahir MS, Khalid M, Braga AAC (2020) Molecular designing of high-performance 3D star-shaped electron acceptors containing a truxene core for nonfullerene organic solar cells. J Phys Org Chem. https://doi.org/10.1002/poc.4119
Hussain R, Khan MU, Mehboob MY, Khalid M, Iqbal J, Ayub K, Adnan M, Ahmed M, Atiq K, Mahmood K (2020) Enhancement in photovoltaic properties of N, N -diethylaniline based donor materials by bridging core modifications for efficient solar cells. ChemistrySelect 5(17):5022–5034. https://doi.org/10.1002/slct.202000096
Mehboob MY, Khan MU, Hussain R, Ayub K, Sattar A, Ahmad MK, Irshad Z, Adnan SM (2020) Designing of benzodithiophene core-based small molecular acceptors for efficient non-fullerene organic solar cells. Spectrochim Acta A Mol Biomol Spectrosc 118873. https://doi.org/10.1016/j.saa.2020.118873
Mehboob MY, Khan MU, Hussain R, Fatima R, Irshad Z, Adnan M (2020) Designing of near-infrared sensitive asymmetric small molecular donors for high-efficiency organic solar cells. J Theor Comput Chem 19(08):2050034. https://doi.org/10.1142/S0219633620500340
Hussain R, Imran M, Mehboob MY, Ali M, Hussain R, Khan MU, Ayub K, Yawer MA, Saleem M, Irfan A (2020) Exploration of adsorption behavior, electronic nature and nlo response of hydrogen adsorbed alkali metals (Li, Na and K) encapsulated Al12N12 nanocages. J Theor Comput Chem 19(08):2050031. https://doi.org/10.1142/S0219633620500315
Hussain R, Saeed M, Mehboob MY, Khan SU, Usman Khan M, Adnan M, Ahmed M, Iqbal J, Ayub K (2020) Density functional theory study of palladium cluster adsorption on a graphene support. RSC Adv 10(35):20595–20607. https://doi.org/10.1039/D0RA01059F
Hussain S, Chatha SAS, Hussain AI, Hussain R, Mehboob MY, Muhammad S, Ahmad Z, Ayub K (2020) Zinc-doped boron phosphide nanocluster as efficient sensor for SO 2. J Chem 2020:1–12. https://doi.org/10.1155/2020/2629596
Hussain S, Hussain R, Mehboob MY, Chatha SAS, Hussain AI, Umar A, Khan MU, Ahmed M, Adnan M, Ayub K (2020) Adsorption of phosgene gas on pristine and copper-decorated B 12 N 12 nanocages: a comparative dft study. ACS Omega 5(13):7641–7650. https://doi.org/10.1021/acsomega.0c00507
Bilal Ahmed Siddique M, Hussain R, Ali Siddique S, YasirMehboob M, Irshad Z, Iqbal J, Adnan M (2020) Designing triphenylamine-configured donor materials with promising photovoltaic properties for highly efficient organic solar cells. ChemistrySelect 5(25):7358–7369. https://doi.org/10.1002/slct.202001989
Hussain R, Mehboob MY, Khan MU, Khalid M, Irshad Z, Fatima R, Anwar A, Nawab S, Adnan M (2020) Efficient designing of triphenylamine-based hole transport materials with outstanding photovoltaic characteristics for organic solar cells. J Mater Sci. https://doi.org/10.1007/s10853-020-05567-6
Younas F, Mehboob MY, Ayub K, Hussain R, Umar A, Khan MU, Irshad Z, Adnan M (2020) Efficient Cu Decorated Inorganic B 12 P 12 nanoclusters for sensing toxic COCl 2 gas: a detailed DFT study. J Comput Biophys Chem 2150006. https://doi.org/10.1142/S273741652150006X
Zhao Z-W, Pan Q-Q, Geng Y, Wu Y, Zhao L, Zhang M, Su Z-M (2019) Theoretical insight into multiple charge-transfer mechanisms at the P3HT/nonfullerenes interface in organic solar cells. ACS Sustain Chem Eng 7(24):19699–19707. https://doi.org/10.1021/acssuschemeng.9b04842
Mehboob MY, Hussain R, Irshad Z, Adnan M (2021) Designing of U-shaped acceptor molecules for indoor and outdoor organic solar cell applications. J Phys Org Chem. https://doi.org/10.1002/poc.4210
YasirMehboob M, Zaier R, Hussain R, Adnan M, Muhammad Asif Iqbal M, Irshad Z, Bilal I, Ramzan Saeed Ashraf Janjua M (2022) In silico modelling of acceptor materials by end-capped and π-linker modifications for high-performance organic solar cells: estimated PCE > 18%. Comput Theor Chem 1208:113555. https://doi.org/10.1016/j.comptc.2021.113555
Mehboob MY, Hussain R, Jamil S, Ahmed M, Khan MU, Haroon M, Janjua MRSA (2022) Physical-organic Aspects along with Linear and nonlinear optical properties of benzene sulfonamide compounds: in silico analysis. J Phys Org Chem. https://doi.org/10.1002/poc.4313
Mehboob MY, Hussain R, Asif Iqbal MM, Irshad Z, Adnan M (2021) First principle theoretical designing of W-shaped dithienosilole-based acceptor materials having efficient photovoltaic properties for high-performance organic solar cells. J Phys Chem Solids 157:110202. https://doi.org/10.1016/j.jpcs.2021.110202
Mehboob MY, Hussain R, Irshad Z, Adnan M (2021) Designing of U-shaped acceptor molecules for indoor and outdoor organic solar cell applications. J Phys Org Chem 34(8). https://doi.org/10.1002/poc.4210
Mehboob MY, Hussain R, Khan MU, Adnan M, Ehsan MA, Rehman A, Janjua MRSA (2021) Quantum chemical design of near-infrared sensitive fused ring electron acceptors containing selenophene as Π-bridge for high-performance organic solar cells. J Phys Org Chem 34(8). https://doi.org/10.1002/poc.4204
Mehboob MY, Hussain R, Irshad Z, Adnan M (2021) Role of acceptor guests in tuning optoelectronic properties of benzothiadiazole core based non-fullerene acceptors for high-performance bulk-heterojunction organic solar cells. J Mol Model 27(8):226. https://doi.org/10.1007/s00894-021-04843-9
Benatto L, de Jesus Bassi M, de Menezes LCW, Roman LS, Koehler M (2020) Kinetic model for photoluminescence quenching by selective excitation of D/A blends: implications for charge separation in fullerene and non-fullerene organic solar cells. J. Mater. Chem. C 8(26):8755–8769. https://doi.org/10.1039/D0TC01077D
Atahan-Evrenk S, Atalay FB (2019) Prediction of intramolecular reorganization energy using machine learning. J Phys Chem A 123(36):7855–7863. https://doi.org/10.1021/acs.jpca.9b02733
Mahmood A, Khan SU-D, Rana UA, Tahir MH (2019) Red shifting of absorption maxima of phenothiazine based dyes by incorporating electron-deficient thiadiazole derivatives as π-spacer. Arab J Chem 12(7):1447–1453. https://doi.org/10.1016/j.arabjc.2014.11.007
Mahmood A, Yang J, Hu J, Wang X, Tang A, Geng Y, Zeng Q, Zhou E (2018) Introducing four 1,1-dicyanomethylene-3-indanone end-capped groups as an alternative strategy for the design of small-molecular nonfullerene acceptors. J Phys Chem C 122(51):29122–29128. https://doi.org/10.1021/acs.jpcc.8b09336
Mahmood A, Khan SU-D, Rana UA (2014) Theoretical designing of novel heterocyclic azo dyes for dye sensitized solar cells. J Comput Electron 13(4):1033–1041. https://doi.org/10.1007/s10825-014-0628-2
Mahmood A, Tang A, Wang X, Zhou E (2019) First-principles theoretical designing of planar non-fullerene small molecular acceptors for organic solar cells: manipulation of noncovalent interactions. Phys Chem Chem Phys 21(4):2128–2139. https://doi.org/10.1039/C8CP05763J
Mahmood A (2019) Photovoltaic and charge transport behavior of diketopyrrolopyrrole based compounds with A-D–A–D–A skeleton. J Clust Sci 30(4):1123–1130. https://doi.org/10.1007/s10876-019-01573-0
Adnan M, Iqbal J, BiBi S, Hussain R, Akhtar MN, Rashid MA, Eliasson B, Ayub K (2017) Fine tuning the optoelectronic properties of triphenylamine based donor molecules for organic solar cells. Zeitschrift für Phys Chemie 231(6). https://doi.org/10.1515/zpch-2016-0790
Leblebici SY, Chen TL, Olalde-Velasco P, Yang W, Ma B (2013) Reducing exciton binding energy by increasing thin film permittivity: an effective approach to enhance exciton separation efficiency in organic solar cells. ACS Appl Mater Interfaces 5(20):10105–10110. https://doi.org/10.1021/am402744k
Scharber MC, Mühlbacher D, Koppe M, Denk P, Waldauf C, Heeger AJ, Brabec CJ (2006) Design rules for donors in bulk-heterojunction solar cells - towards 10 % energy-conversion efficiency. Adv Mater 18(6):789–794. https://doi.org/10.1002/adma.200501717
Rafiq A, Hussain R, Khan MU, Mehboob MY, Khalid M, Shehnaz A, Alam MM, Imran M, Ayub K (2022) Novel star-shaped benzotriindole-based nonfullerene donor materials: toward the development of promising photovoltaic compounds for high-performance organic solar cells. Energy Technol 1:2100751. https://doi.org/10.1002/ente.202100751
Author information
Authors and Affiliations
Contributions
Muhammad Yasir Mehboob: validation, visualization, formal analysis, writing-original draft.
Muhammad Usman Alvi, and Junaid Yaqoob: conceptualization, data curation.
Riaz Hussain: resources, supervision, software.
Muhammad Usman Khan: software, investigation, writing-review & editing.
Muhammad Adnan and Muhammad Khalid: review the final draft, edit, answer some question, and regenerated the spectrum.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mehboob, M.Y., Hussain, R., Khan, M.U. et al. Efficient designing of half-moon-shaped chalcogen heterocycles as non-fullerene acceptors for organic solar cells. J Mol Model 28, 125 (2022). https://doi.org/10.1007/s00894-022-05116-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-022-05116-9