Abstract
The mechanism of C-H bond activation of ethane was catalyzed by palladium halide cations (PdX+ (X = F, Cl, Br, H, and CH3)), which was investigated using density functional theory (DFT) at B3LYP level. The reaction mechanism was taken into account in triplet and singlet spin state potential energy surfaces. For PdF+, PdCl+, and PdBr+, the high spin states were the ground states, whereas the ground states were the low spin states in PdH+ and PdCH3+. The reaction of PdF+, PdCl+, and PdBr+ with ethane occurred via a typical “two-state reactivity” mechanism. In contrast, for PdH+ and PdCH3+, the overall reaction performed on the ground state PESs in a spin-conserving manner. The crossing points between two potential energy surfaces were observed and effectively decreased the activation barrier in PdX+/C2H6 (X = F, Cl, and Br). The minimum energy crossing points (MECP) were obtained used the algorithm in Harvey method. The natural valence electron configuration calculations were analyzed by natural bond orbital. The distribution and contribution of the front molecular orbital of the initial complexes could be further understand by the density of states. The feature of the bonding evolution in the main pathways was studied using topological analysis including localized orbital locator and atoms in molecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past few decades, the activation of the C-H and C-C bonds in alkanes catalyzed by transition metal ions has attracted considerable attention, due to its importance and widespread application in the energy transform, organic synthesis, and catalytic researches [1,2,3,4]. In the previous studies, chemists have discovered that some mononuclear transition metal ions M+ can activate the C-C and C-H bonds of alkanes. For example, methane could be activated by first-row and second-row transition metal cations [5, 6], C-C and C-H bonds of ethane activation by Fe+ [7], etc. However, some mononuclear transition metal ions were not or low reactivity to the C-H bonds of alkanes. To solve this problem, chemists have attempted adding ligands to increase the catalytic efficiency of these metal ions. The catalytic efficiency of the metal was effectively enhanced by the synergy between the transition metal ion and the ligand. For instance, Cr+ does not have any reactivity with small alkanes, whereas the diatomic CrCl+ does [8]; and CH4 cannot be activated by bare Ni+, but NiH+ brings about thermal bond activation [9]. Obviously, the metal combined with the ligand L by covalent bond changes considerably the catalytic activity of metals, and a suitably chosen ligand L can improve the reactivity of a reagent.
In 2007 and 2011, Schlangen et al. reported the reaction mechanism in gas-phase reactions of NiX+/RH couples (X = F, Cl, Br; R = CH3, C2H5, C3H7, n-C4H9) in experimental section and theoretical aspects, respectively [10, 11]. As Ni congener, palladium is a precious metal, 0.0000015% abundance of palladium in the Earth’s crust [12]. The reactivity of atomic palladium with alkanes was very low in the gas phase because its ground state cannot accomplish oxidative insertion, and its lowest excited state was also quite high in energy to deal with C-H bond activation [13]. Therefore, the addition of an appropriate ligand to enhance the catalytic activity of palladium had become an important issue. Compared with atomic Pd, theoretical studies and experiments have shown that the cationic palladium hydride has high activity toward C-H bonds of methane in the gas phase [14, 15]. Simultaneously, the dehydrogenation reaction of methane activation by [Pd(H)(OH)]+ has been theoretically investigated at our lab [16]. Theoretical studies on the activation of C-H bonds by PdX+ (X = F, Cl, Br, H, and CH3) had not been reported. To further insight into how halogen ligands affect the activity of palladium and the reaction mechanism with target molecules, in this paper, the reaction mechanism of PdX+-activated ethane C-H bond was systematically studied theoretically.
Computational methods
Theoretical calculation for all stationary points involved in the reaction of PdX+(X = F, Cl, Br, H, and CH3) with C2H6 has been surveyed by the density functional theory (DFT) [17,18,19,20]. For C, H, F, Cl, and Br, the large 6-311+G (d, p) basis set was applied [21]. The Pd atom was described by using the Stuttgart/Dresden relativistic effective core potentials (ECP) of SDD [22]. The vibrational frequency was calculated for each stationary point to confirm whether it was minima structure or transition state (TS). The minimum structure had only positive eigenvalues and TS had only one negative eigenvalue. Intrinsic reaction coordinate (IRC) calculations were performed to determine the correct link between the minima and the transition states (TS) [20]. The natural population analysis and the bonding properties for some structures have been made with natural bond orbital (NBO) analysis [23]. Density of states (DOS) of initial complexes was analyzed using the Multiwfn package [24, 25]. We used the single-point energy of the DFT to roughly locate crossing points (CPs) between two potential energy surfaces (PESs). The minimum energy crossing point (MECP) was obtained using the mathematical algorithm proposed by Harvey et al. [26]. In addition, the bonding evolution of the main reaction pathways was studied by localized orbital locator (LOL) [27] analysis and atoms in molecules (AIM) [28] analysis using the Multiwfn program. All of the theoretical calculations were implemented using the Gaussian 09 program [29].
Results and discussion
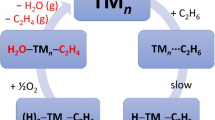
The mechanism of ethane activated by PdX+ has been considered in singlet and triplet state. The superscript prefixes “1” and “3” were defined as the singlet and triplet states, respectively. The structures of stationary points in the high and low spin states and the corresponding PESs are depicted in Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, and 11 (Fig. S1-S5 in support information). The scenario of the reaction paths is depicted in Scheme 1.
Electronic structures of reactants
The calculated results showed that 3PdX+ was ground state and the energy of 3PdX+ (X = F, Cl, and Br) was 24, 57 and 102 kJ/mol lower than 1PdX+, respectively. In contrast, 1PdX+ was the ground state for PdX+ (X = H and CH3). The energy of the triplet state 3PdX+ was 82 and 88 kJ/mol higher than the ground state, respectively. The energy level differences of the reactants between the singlet and triplet state became gradually bigger from PdF+ to PdCl+ and PdBr+, while PdH+ was very close to those of the PdCH3+. The natural valence electron configuration calculations were performed for PdX+ (X = F, Cl, Br, H, and CH3), which is shown in Table 1. From F to CH3, the electronic number of the 4d orbital was increased significantly, and the 5s orbit had almost no changed in the singlet state. In contrast, the number of 5s orbital electrons was changed obviously, whereas the 4d orbital did not in the triplet state.
As shown in Fig. 1 and Fig. S1, the bond distances of PdX+ (X = F, Cl, Br, H, and CH3) were 1.83, 2.19, 2.33, 1.48, and 1.99 Å (in the singlet state) and 1.88, 2.22, 2.32, 1.58, and 2.15 Å (in the triplet state), respectively. From H to Br, the distance of the Pd-X (H < F < CH3 < Cl < Br) bond gradually increased. The change in the bond distance of PdX+ was explained by the atomic radius increase from H to Br. From Figs. 1 and 7 (Fig. S1-S5), one can see that the bond distances of the low spin state PdX+ ions were shorter than the high spin state. The bond distance for PdX+ was determined by the spatial extent of the valence orbit (5s and 4d) [30]. The NBO analysis results indicated that the Pd-X bond was mainly composed of the p orbital of X with the 5s and 4d orbitals of palladium. However, since the less electron in 5s orbital of Pd in 1PdX+, the bond distance of Pd-X in the low spin state was shorter than that in the high spin state.
Initial complexes
The reaction started from catalyst PdX+ (X = F, Cl, Br, H, and CH3) combined with ethane to yield initial complexes IM1, in which the primary C-H bond was elongated (1.09 Å for free ethane), as shown Table 2. The binding energies of the formation of the initial complexes were 170, 153, 191, 102, and 70 kJ/mol (in singlet states) and 136, 99, 101, 62, and 77 kJ/mol (in triplet states), respectively. The initial complexes IM1 was found to had a η3 coordination, as seen Figs. 1 and 7, and Fig. S1-S5. Based on the distance of the activated C-H and the corresponding Pd-C in the initial complexes IM1 (see Table 2), it was concluded that there was an agostic Pd···H-C interaction prior to C-H cleavage.
To better understand the molecular orbital composition, we performed DOS analysis of initial complexes M1 (see Fig. S6) [31]. In the picture, fragment 1, fragment 2, fragment 3, and fragment 4 represented carbon, hydrogen, palladium, and ligand, respectively. The vertical dashed line indicated the position of HOMO level. The height of black curve represented the total density of states (TDOS), and we could clearly see the density of the energy levels distributed and strength everywhere. The curve of the partial density of states (PDOS) was valuable for visualizing the contribution of each atomic orbital (AO) to the molecular orbital (MO). For example, the PDOS-Pd curve was relatively high and close to the TDOS in the region of HOMO level, respectively. Therefore, we concluded that the d orbital of palladium had great contribution to the HOMO orbits.
Reaction mechanisms
Reactivities of PdX+ (X = F, Cl, and Br) with C2H6
Before the transition state 3TS1/2, there was a crossing phenomenon between the two PESs, as show in Figs. 2, 3, and 4. After the cross points, the reaction was carried out along the single PES in a spin-conserving manner. Since the stationary points of the triplet state were higher in energy than those of the singlet state, we were not going to discuss the triplet state PES further. Starting with the initial complexes IM1, the hydrogen atom H1 transferred from the α-carbon of ethane to PdX+ and afforded an intermediate 1IM2. In this process, H1 was transferred in two different ways (see Fig. 1).
In one, H1 shifted to palladium from ethane via the three-centered late transition state 1TS1/2, resulted the high-energy intermediate 1IMa in 1PdX+/C2H6 (X = F, Cl) system. In 1IMa, the distance of the Pd-H bond was 1.50 Å, and NBO analysis revealed that the Pd-H1 bond was formed from 5s and 4\( {\mathrm{d}}_{{\mathrm{z}}^2} \) orbital of palladium and 1s orbital of hydrogen (see Fig. S7). After this oxidation addition (OA) reaction, the hydrogen atom migrated from palladium to halogen through reductive elimination (RE), and the stable intermediate 1IM2 (FHPd(C2H5)+ and ClHPd(C2H5)+) was formed. The exothermicity of the whole OA/RE process was 210 and 131 kJ/mol, respectively. In addition, the transition state 1TSa was − 114 kJ/mol lied below intermediate 1IMa by 2 kJ/mol when the zero-point energy correction was taken into account in 1PdCl+/C2H6 (see Fig. 3), indicated that the process was a low-barrier or even barrier-free transformation on the overall low spin reaction path. Another way, in 1PdBr+/C2H6, the hydrogen atom H1 shifted directly to form the intermediate 1IM2 via transition state 1TS1/2 with a barrier of 25 kJ/mol; this step was exothermic by 112 kJ/mol. In the process of σ-CAM, the Pd-X and C1-H1 bonds are concurrently broken, and two σ-bonds of Pd-C1 and X-H1 were formed simultaneously. In 1IM2, the Pd-C1 and C1-C2 bond lengths were 2.04 and 1.46 Å, respectively. This variation remained almost constant across from F to Br. But the Pd-X bond trends to lengthen along from F to Br. The intermediate 1IM2 was the global minimum structure on the singlet potential energy surface, and the energy of 1IM2 relative to the ground state reactants was − 356, − 230 and − 201 kJ/mol, respectively. As shown in Fig. 12, the π orbital of the four-membered ring was formed from p orbital of two C atoms and d orbital of Pd in 1IM2. This interaction results in increased stability of the intermediate 1IM2. After intermediate 1IM2, the reaction was divided into three paths, as shown in Scheme 1. Path 1, the loss of HX directly in the intermediate 1IM2 required energy of 63, 83, and 99 kJ/mol, respectively, yielded metal ethyl ions 1Pd(C2H5)+. The exothermicity of the loss of HX gradually decreased from F to Br relative to ground state reactants. Path 2 and 3 channels were distinguished based on the reaction mechanism of 1, 2- and 1, 1-elimination of hydrogen. The following reaction mechanism of path 2 and 3 was discussed in detail.
1, 2-Elimination mechanism
The second hydrogen atom H4 from β-carbon on the ethyl ligand shifted to the palladium via a four-member (Pd-C1-C2-H4) transitional structure 1TS2/3, given rise to the hydride complex 1IM3 (see Fig. 5). The second C-H activation energy barriers were 23, 27, and 28 kJ/mol, respectively. In intermediate 1IM3, the Pd, C1, and C2 atom formed an isosceles triangle. In the step, the distance of the C-C was shortened from 1.53 Å in an isolated ethane molecule to 1.38 Å. The changed of distance suggested that the C-C double bond has been formed (the distance of C-C bond in ethylene molecules was 1.33 Å). This step was endothermic by 9, 16, and 18 kJ/mol, respectively.
After the formation of the intermediate 1IM3, the reaction bifurcated to two routes (see Scheme 2). One route was the expulsion of HX directly from the newly formed complex 1IM3 with an activation barrier of 67, 80, and 95 kJ/mol, respectively. Another route was H1 back transfer from HX to the hydride ligand via transitional structure 1TS3/4 to afford the 1IM4 molecular complexes, as path ②. The energy barrier value in this step was 229, 136, and 109 kJ/mol, respectively. Compared with the loss of HX, dehydrogenation was relatively difficult. The reason was that the d orbital of the Pd in PdX+ interacted with the π orbital of the C=C bond and formed π bond in intermediate 1IM3 (see Fig. S9). In order to reach 1TS3/4, the intermediate 1IM3 needed to overcome more energy. And the energy barrier gradually decreased from F to Br relative to 1IM3. In the reaction mechanism of σ-CAM, Pd-H4 and X-H1 bonds were concurrently broken and then H-H and Pd-X bonds were formed simultaneously. The H2 elimination process was endothermic by 212, 132, and 108 kJ/mol, respectively. Finally, the elimination of H2 from the newly yielded 1PdX(H2)(C2H4)+ complex terminated this reaction pathway. In this process, it is necessary to overcome the dissociation energies of 72, 86, and 85 kJ/mol, respectively. Dehydrogenation was the rate-determining step of path 2. The final products of the reaction of PdX+ with ethane were the mixture of HX and H2.
1, 1-Elimination mechanism
In the second hydrogen transfer process, there was competition between α-hydrogen and β-hydrogen on the ethane. For the path 3, starting from the intermediate 1IM2, the second hydrogen atom H2 from the α-carbon migrated to palladium via transition state 1TS2/5, which lead to intermediate 1IM5 (see Fig. 6). H2 transfer occurred with the activation barrier of 113, 123, and 129 kJ/mol, respectively. The step was endothermic by 101, 117, and 125 kJ/mol, respectively.
Subsequently, the reaction was divided into two paths (see Scheme 3). In path ③, HX was liberated directly and 1Pd(H)(CHCH3)+ was formed by overcoming an activation barrier of 69, 75, and 83 kJ/mol, respectively. In path ④, H1 from HX back transferred to the hydride ligand via transition state 1TS5/6, and then an intermediate 1IM6 was formed. Corresponded activation barriers were 229, 136, and 109 kJ/mol, respectively. We noted in particular the features of the transition structure 1TS5/6 in which the migrated H1 atom interacted with three centers simultaneously, that is, the palladium, halogen, and H2 atoms. As required for an σ-CAM process, the atoms H1, H2, Pd, and X (X=F, Cl, Br) were coplanar in 1TS5/6. A similar trend was seen in the 1BrPd(C2H6)+ → 1(HBr)Pd(C2H5)+ and 1IM3 → 1IM4 transformation. Here, the H-H bond distance was 0.85 Å, which was very close to H-H bond distance (0.74 Å) of free H2. It was suggested that the Pd-H2 and H1-X bonds were broken and H-H bond was formed. During this reaction, dehydrogenation was the rate-determining step. In the last step, H2 and 1XPd (CHCH3)+ were formed by overcoming 66, 49, and 57 kJ/mol dissociation energy, respectively. In the following section, we would discuss the reaction of PdH+ and PdCH3+ with C2H6 in detail.
Reactivities of PdX+ (X = H and CH3) with C2H6
In the reaction of PdH+ and PdCH3+ with C2H6, the reaction mechanism and optimized structures on the two PESs were very similar to those for the reaction of PdX+/C2H6 (X = F, Cl, and Br) discussed above. Unlike PdF+, PdCl+, and PdBr+, the whole reaction of PdH+ and PdCH3+ with C2H6 proceeded on the ground state PES in a spin-conserving manner. Similar to PdX+ (X = F, Cl, and Br), we only discuss the reaction mechanism on the ground state PESs. Starting with the initial complexes 1IM1, the hydrogen atom H1 transferred from ethane to PdX+ in two ways and to yield an intermediate 1IM2. In the process of the C-H1 bond activation, there was no low energy barrier 1IMa → 1TSa → 1IM2 process on the 1PdH+/C2H6, whereas the barrier was 6 kJ/mol on the 1PdCH3+/C2H6. H1 transferred in 1PdCH3+ was very similar to that of 1PdF+ and 1PdCl+, with a total exothermicity of 76 kJ/mol, while 1PdH+ and 1PdBr+ were similar, this process exothermic 67 kJ/mol.
The intermediate 1IM2 was the global minimum structure on the whole PESs, which was 169 and 146 kJ/mol below the ground state reactants, respectively. After overcoming 61 kJ/mol in the 1PdH+ and 60 kJ/mol in the 1PdCH3+, H2 and CH4 were liberated. For the second C-H bond activation in the 1PdH+/C2H6 and 1PdCH3+/C2H6, there was also competition between α-H and β-H similar to that in the reaction of 1PdF+, 1PdCl+, and 1PdBr+ with ethane (path 2 and 3).
The β-C-H and the second α-C-H bond activation occurred in the same way as that of 1PdF+, 1PdCl+, and 1PdBr+. 1IM2 → 1TS2/3 → 1IM3 was the β-C-H bond activation process with the activation barrier of 56 and 29 kJ/mol, respectively. The step was endothermic by 52 for 1PdH+ and 14 kJ/mol for 1PdCH3+. Similar to 1PdX+/C2H6 (X = F, Cl, and Br), the Pd, C1, and C2 atom was an isosceles triangle in intermediate 1IM3. Finally, H2 and CH4 were liberated from 1IM3 with an activation barrier of 23 and 59 kJ/mol, respectively.
1IM2 → 1TS2/4 → 1IM4 was the second α-C-H bond activation process. The C-H activation occurred with the activation barrier of 136 and 120 kJ/mol, respectively. The step was endothermic by 130 and 116 kJ/mol, respectively. Subsequently, H2 and CH4 were liberated directly, and 1Pd(H)(CHCH3)+ was formed by overcoming dissociation energy of 40 and 53 kJ/mol, respectively. Similarly, the activation of β-C-H bond was also easier than that of the second α-C-H bond in 1PdH+ and 1PdCH3+. However, for PdX+/C2H6 (X = H and CH3), the hydrogen atom H1 back transfer from HX to the hydride ligand and combined with H2 or H4 on Pd to afford H2 was not observed. In paths 1 and 2, the energy of all points on the singlet state was lower than those of the reactants, indicated that these reactions were a barrier-free transformation. In path 3, the whole reaction was only endothermic 1 kJ/mol for 1PdH+ and 2 kJ/mol for 1PdCH3+. The final product was H2 for PdH+ and CH4 for PdCH3+.
Rate constant
From the potential energy profiles in Figs. 2, 3, and 4, it could be seen that the rate-determining transition 1TS3/4 was located below 1TS5/6 in the process of 1, 2-elimination in reaction of 1PdX+ (X = F, Cl, Br) with ethane. Compared with β-hydrogen transfer in pathway 2, the process of α-hydrogen in the pathway 3 had less competitive ability. And the process of HX loss was relatively easier than H2 elimination in the whole pathway 2. In 1PdX+/C2H6 (X = H, CH3) system, path 2 was also more favorable than path 3, as shown in Figs. 8 and 9. To estimate quantitatively the reactivity for several product formation processes of path 2 (Pd-X bond cleavage products, 1Pd(C2H5)+ + HX and 1PdH(C2H4)+ + HX, and without apparent Pd-X bond cleavage products 1PdX(C2H4)+ + H2), the rate constants have been evaluated based on conventional transition state theory (Eq. (1)) [32].
where kB is the Boltzmann constant, T is the thermodynamic temperature, h is the Plank constant, and ∆G≠ is the activation Gibbs free energy barrier. In T = 298 K, the rate constants of the rate-determining step in several products formation process have been calculated in Table 3.
Compared with the rate constants in several product formation processes, we concluded that the rate constant gradually decreased in the loss HX while gradually increases in the H2 elimination from F to Br. The results showed that the loss of HX was most favorable for 1PdF+/C2H6, but the elimination of H2 was easier for 1PdBr+/C2H6. In 1PdX+/C2H6 (X = H and CH3), only the products of Pd-X bond cleavage were observed. Also, the dissociation of product 1PdH (C2H4)+ + HX was easier than that of product 1Pd (C2H5)+ + HX.
To intuitively illustrate the efficiency of HX and H2 elimination, branching ratios of all products were presented. As shown in Fig. 13, the loss of HX was abundant for all systems, whereas the elimination of 1PdX(C2H4)+ + H2 was relatively most effective for 1PdBr+/C2H6. This was consistent with the results of the discussion above.
Gas-phase reactions proceed in closed containers, and all of these reactions occur at constant energy and angular momentum. Depuy reports, in the gas phase, the reaction that occurred through an ion-dipole complex that contains high energy [33]. In the reaction of PdX+ with ethane, the formation of encounter complex IM1 in the minimal energy reaction pathways (MERPs) contributed 146, 99, 101, 102, and 70 kJ/mol (for F, Cl, Br, H, and CH3) of heat to the closed system, respectively. And all energies were retained within the encounter complex IM1 since there were no solvent molecules to transfer it. This indicated that there was enough energy to overcome the reaction barrier. Therefore, we concluded from the above calculation results that ethane was effectively activated by PdX+ at room temperature.
MECP between PESs of different multiplicities
As shown in Figs. 2, 3, and 4, the crossing phenomenon between two PESs was observed at both the entrance and exit channels of reaction of PdX+ (X = F, Cl, and Br) with ethane. In PdF+/C2H6, the energy of 3IM1 was higher than 1IM1 by 10 kJ/mol, which implied that CPs existed at the entrance channel of reaction. For PdCl+ and PdBr+, the energy of 3IM1 was 3 and 12 kJ/mol lower than that of 1IM1, while 3TS1/2 was higher than 1TS1/2 by 115 and 99 kJ/mol, respectively, which indicated that CPs existed between 3IM1 and 3TS1/2. From Br to F, CPs was gradually close to the reactants. In order to locate the CPs between two PESs, we used the method of partial geometry optimization to calculate single-point energy. To more accurately locate the CP, the method of Harvey et al. was used to obtain the actual MECP, as shown in Fig. 14. This change of spin states between two PESs effectively avoided the energetically inaccessible transition structure 3TS1/2 on the ground state surface. This spin-crossing phenomenon could be called a “two-state reaction” in the organometallic chemistry [34]. After the systems crossing, the whole reaction on the singlet PESs proceeded in a spin-conserving manner. The minimal energy reaction pathway (MERP) started from the triplet state reactants via intersystem crossing mechanism, then proceeded along the low spin PESs to liberate HX and H2. For PdX+/C2H6 (X = H, CH3), the whole reaction proceeded in a spin-conserving manner on the singlet PESs, and there was no crossing phenomenon, as shown in Figs. 8 and 9.
Bonding evolution analysis
To further understand bond evolution of the main reaction pathways, LOL and AIM analyses were investigated. LOL was a function for locating high localization regions, defined by Schmider and Becke. Compared with the electron localization function (ELF) [35], LOL has similar description. Jacobsen reported that LOL could exhibit more accurate and clearer picture than ELF [36]. The value range of LOL is (0, 1). The LOL pictures for the stationary points in main paths were described in Fig. 15 and Fig. S11–12. The regions of white in pictures indicated that electron density exceeded the upper limit of color scale (0.8). In AIM analysis, the chemical bonding was described by the bond critical point (BCP) [37]. The bond strength and the atomic interaction at BCP were estimated by the electron density (ρb) and the Laplacian electron density (▽2ρb), respectively. BCP has been analyzed in terms of the values of ρb and ▽2ρb at the (3, − 1) critical points. The values of ρb and ▽2ρb were listed in Table S1–5. Positive and negative values of ▽2ρb indicated that electron density was depleted and concentrated.
As shown in Fig. 15 and Fig. S11-S12, the low LOL value indicated that there was no covalent bond between Pd and H1 atom in 1IM1. This conclusion was confirmed by the AIM analysis; result showed that ρb between Pd and H1 atom was very low (ρb = 0.078, 0.088, 0.088, 0.079, and 0.70 au, respectively), and ▽2ρb value was positive. The first C-H bond was activated in two different ways. For F, Cl, and CH3, the LOL of 1TS1/2 showed that C1-H1 bond was broken and Pd-H1 bond begins to form. In 1TS1/2, the ρb of Pd-H1 bond significantly increased (ρb = 0.152, 0.150, and 0.152 au, respectively), and ▽2ρb of Pd-H1 bond was negative value, but the Pd-H1 bond was not yet formed. In intermediate 1IMa, Pd-H1 bond was completely formed. This is consistent with the AIM analysis, which showed that ρb values of Pd-H1 bond were 0.171, 0.166, and 0.166 au respectively. Compared with 1IMa, the ρb value of Pd-H1 and Pd-X was decreased while Pd-C1 increased in 1TSa, indicated that H1 was transferred from Pd to ligand. In 1TSa, AIM analysis showed that the ρb value of Pd-H1 bond was 0.152, 0.157, and 0.158, respectively; this result indicated that the Pd-H1 bond was broken. Another for Br and H, H1 was transferred directly from C1 to Br and H, respectively. AIM analysis showed that the ρb (0.148 and 0.151 au) of Pd-H1 in 1TS1/2 increased obviously compared with that in 1IM1. Subsequently, the second C-H bond was activated. In 1TS2/3, the ρb value of Pd-H4 increased obviously compared with 1IM2, and ▽2ρb was negative value, suggested that H4 atom was gradually transferred from C2 to Pd. In addition, the weak interaction between Pd and H4 also was observed in 1IM2, which was confirmed by the small ρb value (ρb = 0.087, 0.079, 0.075, 0.072, and 0.078 au, respectively). In 1PdX+/C2H6 (X = H and CH3), the reaction was terminated after the formation of intermediate 1IM3. In the 1PdX+/C2H6 (X = F, Cl, and Br) system, the ρb value of the H1-H4 bond was 0.180~0.186 au, indicated that H2 was formed in stage of 1TS3/4. The H1-H4 bond was formed completely in 1IM4; this was also confirmed by the AIM analysis.
Comparison the reactivity of PdX+(X = F, Cl, Br, H, and CH3) with atomic Pd and Pd+ toward C2H6 activation
In order to compare the reactivity of Pd, Pd+, and PdX+ with ethane, we theoretically studied the reaction mechanism of Pd and Pd+ with ethane at the same level as PdX+. The optimized structure of stable point on PESs of two spin states is shown in Fig. S13-S14. The calculated results showed that the ground states were 1Pd and 2Pd+, respectively. The energy of the excited state 3Pd and 4Pd+ was 79 and 277 kJ/mol higher than the ground states, respectively. The NBO valence electron populations of the high spin were calculated to be 5s1.004d9.00 and 5s1.004d8.00 for 3Pd and 4Pd+, respectively. In the reaction of atom Pd with C2H6, C2H6 cannot been activated by triplet 3Pd, because of its lowest excited state was quite high in energy to deal with C-H bond activation [30]. As shown in Fig. S13-S14, the whole reaction of 1Pd and 2Pd+ with ethane performed on the ground state PESs in a spin-conserving manner. Therefore, we only discussed the reaction mechanism Pd and Pd+ with ethane on the ground state PESs. The reaction was divided into the following three steps. Firstly, the first C-H bond of ethane was activated by 1Pd and 2Pd+ with the energy barriers of 50 and 67 kJ/mol, respectively. Our calculation for the formation of 1IM2 was endothermic by 19 and 64 kJ/mol, respectively. For the second C-H bond activation in the 1Pd/C2H6 and 2Pd+/C2H6, there was also competition between α-H and β-H similar to that in the reaction of 1PdX+ with ethane. 1IM2 → 1IM3 was the process of β-C-H bond activation; the energy barrier heights (relative to 1IM2) were 122 and 71 kJ/mol, respectively. The exothermicities of the step were 52 and 135 kJ/mol for 1Pd and 2Pd+, respectively. Finally, H2 was liberated by overcoming the dissociation energies of 54 and 26 kJ/mol, respectively. The α-C-H bond was activated in 1IM2 → 1IM4; the C-H bond activation barriers were 293 and 126 kJ/mol, respectively. The step was exothermic by 118 and 53 kJ/mol, respectively. Subsequently, H2 was liberated by overcoming the dissociation energies of 41 (for 1Pd) and 39 kJ/mol (for 2Pd+), respectively. Compared with β-C-H bond activation, the α-C-H bond activation has less competition ability in the reaction of 1Pd and 2Pd+ with ethane. We concluded that the main reaction path was 11IM1 → 1TS1/2 → 1IM2 → 1TS2/3 → 1IM3 in the reaction of 1Pd and 2Pd+ with ethane. The highest energy relative to the ground state reactants was 123 and 33 kJ/mol in the whole 1Pd and 2Pd+ with ethane reaction. Comparing the reactivity of atomic Pd and Pd+ with ethane, the highest energy in 1Pd/C2H6 was 90 kJ/mol higher than that of 2Pd+/C2H6. It was indicated that the 2Pd+ was easier to activate the C-H of C2H6 than the neutral atomic Pd.
Despite the qualitative similarities among the reaction paths, there were significant differences in some aspects. NBO calculations showed that the ground states 1Pd and 2Pd+ had a valence electron population of 4d10 and 4d9 on Pd atom, respectively. Compared with atomic 1Pd, the population of the 4d orbitals of Pd in cations 2Pd+ and 1PdX+ was unfilled. For PdF+, PdCl+, and PdBr+, the high spin states were the ground states, while the ground states were the low spin states in Pd, Pd+, PdH+, and PdCH3+. In the ground states of PdF+, PdCl+, and PdBr+, the valence electron population of the 4d orbitals of Pd from F to Br gradually increased; similarly from Pd+ to PdH+, PdCH3+, and atomic Pd, the valence electron population of the 4d orbitals on Pd also increased in the ground states of Pd+ to PdH+, PdCH3+, and atomic Pd. The reaction of PdX+ (X = F, Cl and Br) with ethane started from triplet state and then through CPs point; the final proceeded in the singlet state PESs. This reaction was typical “two-state reaction.” In contrast, for 1Pd, 2Pd+, and PdX+ (X = H and CH3) with C2H6, the overall reaction was performed on the ground state PESs in a spin-conserving manner. In 1PdX+/C2H6 (X = F, Cl, and CH3), the first hydrogen atom H1 was transferred through the OA/RE reaction mechanism, whereas for 1PdX+/C2H6 (X =H and Br), H1 was transferred via the σ-CAM mechanism. Before intermediate 1IM3, the reaction mechanism of 1Pd, 2Pd+, and 1PdX+ ethane was very similar. In reaction of PdX+ (X = F, Cl, and Br) with C2H6, the hydrogen atom H1 back transfer combined with H2 or H4 on Pd to afford H2, whereas those of PdH+ and PdCH3+ were not observed. In the reaction of 1PdX+ with ethane, the activation of the first C-H bond was exothermic, and the second C-H bond was endothermic, whereas for 1Pd and 2Pd+, the first C-H bond activation was endothermic and the second C-H bond activation was exothermic. For PdX+/C2H6 (X = F, Cl, and Br), the main products were HX with a small amount of H2. In the reaction of Pd, Pd+, and PdH+ with C2H6, only H2 was liberated. For PdCH3+, only CH4 was produced. Dehydrogenation was the rate-determining step of reaction 1PdX+/C2H6 (X = F, Cl, and Br), the rate constants gradually increased from F to Br. In 1Pd, 2Pd+, and 1PdH+, the activation of the second C-H bond was the rate-determining step. From 1Pd to 2Pd+ and 1PdH+, the rate constants also gradually increased (rate constants were 1.08 × 10−9 for 1Pd and 0.45 for 2Pd+). The rate-determining step was the release of CH4 molecule at the PdCH3+/C2H6 system. Based on the above data, the order of reactivity of atomic Pd-, Pd+-, and PdX+-activated C-H bond of ethane has been listed as PdH+ > PdF+ > PdCH3+ >PdCl+ > PdBr+ > PdCH3+ > Pd+ >Pd.
The above mentioned was the activation process of C-H bond in all systems, while the activation of C-C bond was only observed in the 1Pd/C2H6 system, as shown in Fig. S15. In the activation of the C-C bond process, it was observed that no methane was formed while formation the complex CH3-Pd-CH3. The barrier height of the TS in C-C bond activation was 26 kJ/mol lower than that of C-H bond. It is indicated that the reaction efficiency of atomic Pd activation C-H bond of ethane was lower than that of activated C-C.
Conclusions
The reaction mechanism of PdX+ activation C-H bond of C2H6 was investigated by B3LYP method under DFT theory. The front molecular orbital of the initial complexes was analyzed by density of states (DOS). The d orbital of palladium and the p or s orbital of ligand had significant contribution to the HOMO orbits. The first hydrogen atom can be transferred in two different ways, OA/RE (for F, Cl and CH3) and σ-CAM (for Br and H). During the second hydrogen transfer, the cations PdX+ exhibited high bond selectivity for the activation of α-C-H and β-C-H bonds in ethane. In the second hydrogen transfer process, the α-hydrogen transfer process had less competitive abilities than that of β-hydrogen. CPs between the two PESs could be observed and effectively decreased the activation barrier in PdX+ (X = F, Cl, and Br). The most favorable reaction pathway started from triplet PESs and then proceeded through the singlet PESs to liberate HX and H2. In PdX+ (X = H and CH3) system, the whole reaction proceeded on the ground state PESs in a spin-conserving manner. Theoretical studies on the reactions PdX+ (X = F, Cl, and Br) with ethane had showed that the loss of HX was more facile than dehydrogenation. The final main products of the reaction of PdX+ (X = F, Cl, and Br) with ethane were HX and small amount of H2. In the reaction of PdH+ with C2H6, only H2 was liberated. For PdCH3+, only CH4 was produced. Adding appropriate ligands could enhance the catalytic efficiency of palladium; the order of reactivity of atomic Pd-, Pd+-, and PdX+-activated C-H bond of ethane has been listed as PdH+ > PdF+ > PdCH3+ >PdCl+ > PdBr+ > PdCH3+.
References
Labinger JA, Bercaw JE (2002) Understanding and exploiting C-H bond activation. Nature 417:507–514
Enger BC, Lødeng R, Holmen A (2008) A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl Catal A Gen 346:1–27
Roithová J, Schröder D (2010) Selective activation of alkanes by gas-phase metal ions† - Chemical Reviews (ACS Publications). Chem Rev 110:1170–1211
Zhu B, Guan W, Yan LK, Su ZM (2016) Two-state reactivity mechanism of benzene C-C activation by trinuclear titanium hydride. J Am Chem Soc 138:11069–11072
Sicilia E, Russo N (2002) Theoretical study of ammonia and metactivation by first-row transition metal cations M+ (M = Ti, V, Cr). J Am Chem Soc 124:1471–1480
Blomberg MRA, Siegbahn PEM (1994) Svensson M, Reaction of second-row transition-metal cations with methane. J Phys Chem 98:2062–2071
Holthausen MC, Fiedler A, Schwarz H, Koch W (1996) How does Fe+ activate C-C and C-H bonds in ethane? A theoretical investigation using density functional theory. J Phys Chem 100:6236–6242
Mandich ML, Steigerwald ML, Reents WD (1986) The effects of chloro substitution on the electronic structure of ClCr+, ClMn+, and ClFe+ and their reactivity with small alkanes. J Am Chem Soc 108:6197–6202
Roithová J, Schröder D (2010) Selective activation of alkanes by gas-phase metal ions. Chem Rev 110:1170–1211
Schlangen M, Schrçder D, Schwarz H (2007) Ligand and substrate effects in gas-phase reactions of NiX+/RH couples (X = F, Cl, Br, I; R = CH3, C2H5, nC3H7, nC4H9). Chem Eur J 13:6810–6816
Schlangen M, Schwarz H (2011) Probing elementary steps of nickel-mediated bond activation in gas-phase reactions: ligand- and cluster-size effects. J Catal 284:126–137
Shang R, Ilies L, Nakamura E (2017) Iron-catalyzed C-H bond activation. Chem Rev 117:9086–9139
Irikura KK, Beauchamp JL (1991) Electronic structure considerations for methane activation by third-row transition-metal ions. J Phys Chem 95:8344–8351
Schlangen M, Schwarz H (2007) Thermal activation of methane by group 10 metal hydrides MH+: the same and not the same. Angew Chem Int Ed 46:5614–5617
Li WQ, Geng ZY, Wang YC et al (2009) Density functional theory studies of thermal activation of methane by MH+ (M = Ru, Rh, and Pd). J Phys Chem A 113:1807–1812
Liu HQ, Geng ZY, Wang YC et al (2011) Theoretical investigation of thermal activation of methane by [Pd(H)(OH)]+. Comput Theor Chem 963:470–474
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72:650–654
Andrae D, Dolg M et al (1990) Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Chim Acta 77:123–141
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Lu T, Chen F (2012) Quantitative analysis of molecular surface based on improved Marching Tetrahedra algorithm. J Mol Graph Model 38:314–323
Harvey JN, Aschi M, Schwarz H, Koch W (1998) The singlet and triplet states of phenyl cation. A hybrid approach for locating minimum energy crossing points between non-interacting potential energy surfaces. Theor Chem Accounts 99:95–99
Schmider HL, Becke AD (2000) Chemical content of the kinetic energy density. J Mol Struct THEOCHEM 527:51–61
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, IshidaM NT, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian, Inc., Wallingford
Liu SL, Geng ZY, Wang YC et al (2012) Methane activation by MH+ ( M = Os , Ir , and Pt ) and comparisons to the congeners of MH+ ( M = Fe , Co , Ni and Ru , Rh , Pd ). J Phys Chem A 116:4560–4568
Malechi JG (2010) Synthesis, crystal, molecular and electronic structures of thiocyanate ruthenium complexes with pyridine and its derivatives as ligands. Polyhedron 29:1973–1979
Eyring H (1935) The activated complex in chemical reactions. J Chem Phys 3:107–115
DePuy CH (2002) Understanding organic gas-phase anion molecule reactions. J Organomet Chem 67:2393–2401
Mai BK, Kim Y (2015) The kinetic isotope effect as a probe of spin crossover in the C-H activation of methane by the FeO+ cation. Angew Chem Int Ed Engl 54:3946–3951
Savin A et al (1992) Electron localization in solid-state structures of the elements: the diamond structure. Angew Chem Int Ed Eng 31:187–188
Jacobsen H (2008) Localized-orbital locator (LOL) profiles of chemical bonding. Can J Chem 86:695–702
Li P, Niu WX, Gao T, Wang HY (2014) Gas-phase water activation by Th atom: reaction mechanisms and topological analysis. Int J Quantum Chem 114:760–768
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2490 kb)
Rights and permissions
About this article
Cite this article
Nie, YX., Zhang, XX., Yuan, YN. et al. Theoretical study on the activation of C-H bond in ethane by PdX+ (X = F, Cl, Br, H, and CH3) in the gas phase. J Mol Model 26, 91 (2020). https://doi.org/10.1007/s00894-020-04357-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04357-w




















 ), (b) 1PdH(C2H4)+ + HX (
), (b) 1PdH(C2H4)+ + HX ( ), and (c) 1PdX(C2H4)+ + H2 (
), and (c) 1PdX(C2H4)+ + H2 ( )
)
