Abstract
A theoretical approach was used to evaluate the antioxidant capacity of 20 flavonoids reported in Annona muricata leaves. The theoretical study was at the GGA level using the wB97XD functional and the cc-pvtz basis set. The calculations were performed in gas phase and implicit solvent phase. The flavonol robinetin (03c) and the flavanol gallocatechin (01c) are species that exhibited the best antioxidant capacity in the HAT, SEPT, and SPLET mechanisms. On the other hand, in the SET I mechanism, flavonol quercetin (03b) was the best, and in the SET II mechanism, the most favored species is the flavanol catechin (01a). However, these species do not achieve to overcome the antioxidant capacity presented by the Trolox.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, several research groups have been studying the relationship that might exist between the life quality of people and the components of the diet they ingest [1]. Likewise, it has been determined that the diet could have a link to the incidence of some diseases. Oxidative stress at the cellular level seems to be related to the emergence of noncontagious diseases such as cancer, diabetes, Alzheimer’s and Parkinson’s among others, or degenerative processes such as premature aging. These diseases and processes have a high social cost [2] for patients as well as for their relatives.
Oxidative stress is a biological disequilibrium caused by an excess or accumulation of free radicals in the organism [3]. From an electronic point of view, a free radical is a molecule or chemical species that possesses an unpaired electron in its last occupied orbital, which provides it a high instability and therefore an increase in its reactivity [4]. In order to stabilize these molecules, the organisms generate chain oxidation-reduction reactions that involve several biomolecules at cellular level, these structural modifications cause the biomolecules to lose their normal functionality, in such a way that cell damage is caused [5, 6]. Normally, free radicals are produced in the aerobic metabolism of any organism in tolerable amounts by the cell. However, the contamination, rhythm of life, and the intake of toxic products to which the population is continually exposed, increase the oxidative stress and therefore the amount of present free radicals [7].
The study of compounds capable of reducing oxidative stress, called antioxidants, has been increased in recent years [8]. These compounds may have a capacity for prevention, although it has not been proven conclusively. An antioxidant is a molecule capable of inhibiting a free radical (in this case they are called primary antioxidants) or of repairing the damage produced on other molecules (called secondary antioxidants) [2, 3]. In the body, antioxidants can be found endogenously. However, in many cases, its antioxidant capacity is decreased by the effect of some pathology [4]. Moreover, several types of analogous antioxidants are found as bioactive components in numerous plant species, increasing scientific interest, and currently, they present a high commercial demand in its form of nutraceuticals [1, 6]. It is for this reason that the pharmaceutical industry has focused on the search for more efficient antioxidants which implies that they exhibit high anti-radical activity and high stability after reacting [4, 9, 10]. These products can be found on the market as antioxidant supplements or nutraceutical products that are used both in prevention therapies and in complementary treatments [1, 8, 11].
A plant of tropical origin, commonly called Soursop (Annona muricata), in the alternative therapies, has been considered as a plant with medicinal properties [12, 13]. Due to this, the interest to study this plant in a systematic way has increased substantially. Therefore, the nutraceutical products derived from this plant have achieved wide acceptance in the world market of functional foods. However, most of the scientific information reported is still quite general [14,15,16]. Experimental reports have shown that the leaves of A. muricata are an important source of flavonoids [13, 14, 16,17,18,19,20]. Flavonoids are polyphenolic compounds that have a common phenyl benzopyran skeleton (see Fig. 1) and their structural classification depends on the presence of different substituents whose reactivity is subordinated to the present functional groups [1, 8, 21,22,23,24,25,26]. Normally, the flavonoids found in the leaves are in the form of glycosylated flavonoids or flavonoid glycosides [21, 27,28,29]. Additionally, it has been shown that they are able to act as antioxidants against reactive oxygen species (ROS), through an anti-radical activity, which is conferred by the phenolic OH groups, and the double bonds present in their fundamental chemical structure [22, 30,31,32,33,34]. Recent epidemiological studies [8, 21, 24], propose that a diet high in flavonoids and derivatives might be associated with a low incidence of diseases, as well as an increase in longevity [27, 35, 36].
Galano and coworkers [37] reported that the most important reaction mechanisms involving flavonoids and their derivatives are: (a) hydrogen atom transfer (HAT), (b) single electron transfer (SET), (c) sequential electron proton transfer (SEPT), and (d) sequential proton loss electron transfer (SPLET) [38,39,40,41].
HAT: this mechanism proposes that the flavonoid yields a hydrogen atom, which is captured by the free radical. The rupture is thought to be homolytic of one of the hydroxyl groups (see Eq. 1). As a result, the flavonoid is transformed into a phenoxyl radical. It is expected that this is a less reactive species than the initial radical. Likewise, the free radical is stabilized by the capture of hydrogen generated homolytically.
SET: this mechanism proposes that there might be two different pathways, which will depend on the capacity of the antioxidant, which could accept or donate an electron. Moreover, in both routes, the results of the reaction process are two ionized products. In the first path, the flavonoid yields an electron to the free radical (SET I), where the flavonoid is transformed into a radical cation, which can exhibit a lower reactivity, and the free radical is transformed into an anionic species (see Eq. 2). In the second route, the free radical yields an electron to the flavonoid (SET II), where the flavonoid is transformed into a radical anion that can exhibit a lower reactivity. Likewise, the free radical becomes a cationic species (see Eq. 3).
SEPT, this mechanism proposes two consecutive steps or stages. In the first step, the reaction is similar to the SET I mechanism, in which the flavonoid yields an electron to the free radical, forming a radical cation and an anion as reaction intermediates (see Eq. 4). In the second stage of the mechanism, the flavonoid radical cation formed in the previous step loses the proton of the hydroxyl group concentrating the electronic density of the radical, and since an anionic and a cationic species are present in the medium, they inevitably decay to a neutral species, resulting in the same reaction products of the HAT mechanism (see Eq. 5).
SPLET: this mechanism proposes two consecutive stages in its reaction process, but in reverse order to the SEPT mechanism. Thus, in the first stage, the flavonoid loses a proton due to the effect of the medium, in the way of a heterolytic rupture, forming a flavonoid anion as a reaction intermediate (see Eq. 6). In the second stage of the proposed mechanism, the flavonoid in its anionic form transfers an electron to the free radical (see Eq. 7), and finally, in the solution the ions of opposite charges are neutralized, resulting also in the same reaction products of the HAT mechanism.
On the other hand, although the evaluation of the antioxidant capacity of several compounds has been developed through reliable experimental methods, there are still limitations that the same practice can not avoid [32, 42, 43], such as the variation in the values of the antioxidant capacity, which fluctuate depending on the method. The most important problem, however, is that to determine the antioxidant capacity, we must isolate each one of the components, and that makes investigations very difficult. Many times, this has led to reporting only the values of the antioxidant capacity of mixtures present in the extracts of the plants.
In order to evaluate many of the proposed mechanisms, experimental results are limited. Through the use of computational calculations, the required data can be found with great precision with respect to the experimental data. Therefore, computational chemistry is gaining relevance in antioxidant research, as an innovative strategy shortening steps in experimental studies [44,45,46]. Computational methods based on calculations of all electrons, such as the density functional theory (DFT), make possible the calculation of physicochemical and thermodynamic properties related to the reaction mechanisms of a wide variety of compounds. [47,48,49,50,51,52,53]. In this way, computational chemistry is contributing to the analysis and evaluation of the antioxidant capacity of several systems, and thus enables us to understand the relationships that exist between structure and reactivity, which allows us to interpret and complement later in vitro and in vivo studies [41, 54,55,56,57,58,59]. In the present study, we have evaluated the antioxidant capacity of the flavonoids found in the Annona muricata (Soursop) leaves, through the use of quantum mechanical calculations.
Computational details
We studied 20 flavonoids present in the leaves of A. muricata, reported by Coria-Téllez and coworkers [13], as well as the Trolox. The molecules were generated in the GaussView 6.0 program [60]. The calculations of quantum mechanics were carried out in Gaussian 16 [61]. The first step consisted of a semi-empirical optimization with AM1 [62]. Subsequently, a DFT approach was made at the GGA level, with the wB97XD functional [63] and the cc-pvtz basis set [64], this is basis set widely recommended in the literature when system have interactions of hydrogen bonds [65], and the vibrational frequencies were analyzed. The second step was to add implicit water (model SMD) [66] to the models optimized by DFT. It is necessary to introduce the solvent effect for systems in which we wish to study the antioxidant capacity. The third step analyzed intermediate species and products as shown in Eqs. 1 to 7, in gas and solvent phase. The intrinsic reactivity properties [38,39,40,41] of bond dissociation enthalpy (BDE) (Eq. 8), ionization enthalpy (IE) (Eq. 9), enthalpy associated with electronic affinity (EA) (Eq. 10), proton dissociation enthalpy (PDE) (Eq. 11), enthalpy associated with proton affinity (PA) (Eq. 12), and the electron transfer enthalpy (ETE) (Eq. 13), were evaluated. A Pearson linear correlation of BDE, IE, and ETE was performed with the experimental value of the percentage of antioxidant activity (%Aantiox) of some flavonoids reported by Burda and coworkers [67]. Likewise, the spin density distribution both numerically and graphically was determined for all the radical species of the present study, through a Hirshfeld population analysis.
Results and discussion
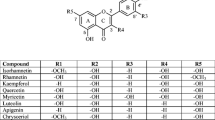
We worked with a total of 20 flavonoids; all of them are found in the leaves of the A. muricata. The base structure of the flavonoids under study is presented in Fig. 1. For better management, they were identified by means of an ID. In addition, the number and positions of the hydroxyl groups in the structures are shown, as well as the presence of saccharides (see Table 1). Of the 20 structures, three were flavanols, one flavanonol, eight flavonols, five flavones, and three isoflavones (see Fig. 2). Likewise, we worked with the Trolox (see Fig. 3).
From the optimized structures, the bond dissociation enthalpy (BDE) was determined for each of the hydroxyl groups present in the 20 species of study. The calculated values of the BDE are presented in Table 2, both in gas phase and in solvent phase. In this way, we can identify the hydrogen atoms most susceptible to a homolytic cleavage. The BDE values are given in kcal/mol. It can be observed that the solvent effect in some cases favors the process of homolytic cleavage, as well as disadvantage, without being an apparently determining element. In the Table 3, the species by ID with the lowest BDE value per flavonoid are shown, which implies that they are the species that can potentially suffer a homolytic cleavage; the BDE values are in kcal/mol. We can consider that we can identify by type of flavonoid, which is the structure that favors the homolytic cleavage, in the case of flavanols is the structure 01b is favored in the gas phase, but in the solvent phase, the species 01c is preferred. Likewise, in flavonols, it is the structure 03d in the gas phase and 03c in the solvent phase, those favored by its BDE value; in the flavones the structure 04a it is preferred both in gas phase and solvent; in the isoflavones the 05c in the gas phase and the 05b in the solvent phase is the favored structures. It should be noted that the Trolox in solvent phase has the lowest value of BDE.
In the Table 4, we show the lengths of the intramolecular hydrogen bond that are generated when the radical has been formed, both in gas phase and solvent. This interaction is formed between the oxygen belonging to the hydroxyl most susceptible to the homolytic cleavage (reactive site) and the hydrogen belonging to a nearby hydroxyl. Additionally, the bond length reduction values were found upon a homolytic cleavage of the flavonoids occurs. The bond reduction values in solvent phase were associated with their corresponding BDE values, finding an important inverse relation, with an r value equal to - 0.8565 (see Fig. 4). This implies that at a lower BDE value, the atoms will get closer. Therefore, flavonoids having a higher susceptibility to react by a homolytic cleavage tend to be prone to stabilize by an interaction with the neighboring hydrogen atoms with acid character. The BDE value and the approximation of a hydrogen atom with an acid character are indispensable to discuss the HAT type mechanism of reaction.
In order to properly understand the SET mechanism, it is important to determine the ionization enthalpy (IE) values and the enthalpy associated to the electronic affinity (EA). These values are linked to the global structures, and we cannot discriminate by centers. These enthalpy values must be interpreted as in thermodynamics; We should interpret that the negative values of the EA, it is associated with releases energy in the process of capturing an electron; if positive, it is related to the energy required to obtain an electron. In the Table 5, the values for both enthalpies are presented, both in the gas phase and in the solvent phase. It should be noted that for the IE, all values in solvent phase tend to favor donor capacity, while for EA, the solvent phase makes them more resistant to receive an electron within the system. Regarding the IE, the values for the flavanols (01a–01c), in the solvent phase are really very similar, although in the gas phase there is a range of 3 kcal/mol, while for the flavonols (03a–03h), in the solvent phase they are in a range of 6 kcal/mol, and in gas phase with a range of 14 kcal/mol, flavones (04a–04e), in gas phase present values in a range of 11 kcal/mol and in solvent phase in a range of 12 kcal/mol. Finally, for isoflavones (05a–05c), the values oscillate between 4 kcal/mol in the solvent phase and 2 kcal/mol in gas phase. It is important to note that the IE value of the Trolox is the lowest in both gas phase and solvent phase. When we analyze the EA, the flavanols (01a–01c), in the solvent phase present values in a range of 30 kcal/mol, and the gas phase it is negative in the rank of 14 kcal/mol. In the event of the flavonols (03a–03h) in the solvent phase, present values in a range of 3 kcal/mol and the gas phase have a rank of 31 kcal/mol. For flavones (04a–04e), the values in the solvent phase have a range of 6 kcal/mol and the gas phase of 20 kcal/mol. Lastly, isoflavones (05a–05c) solvent phase, have a rank of 3 kcal/mol and in the gas phase of 5 kcal/mol. In the case of the Trolox, the value is negative in the gas phase and positive in the solvent phase, but in both cases, they are the lowest values in the data set.
In the SEPT mechanism, the IE values, and the proton dissociation enthalpy (PDE) values were determined, which are presented in the Table 6. In all cases, the values in the solvent phase are much higher than in the gas phase for the PDE, which implies that in the solvent phase, the radical cation species are much more stable, likely due that to the polar medium. In the case of the species 04e, there are no PDE values since it does not have hydroxyl groups.
Furthermore, it was determined the enthalpy associated with the proton affinity (PA) values and the electron transfer enthalpy (ETE) values, which are linked to the SPLET mechanism. These values are shown in Table 7, both for gas phase and for solvent phase. In most cases, the PA values are increased when the flavonoids are in the solvent phase. Oppositely, in the isoflavones and the Trolox, the decreases in the PA values tend apparently to favor a heterolytic rupture of the proton. On the other hand, the ETE values increase in all cases when the species are in the solvent phase. In addition, in flavanols, the species 1c is the one presenting a greater susceptibility to donate an electron from its anionic form, both in the gas phase and in the solvent phase. Likewise, in the flavonols, the species 03c is favored in both phases, together with 03a in solvent phase only. In the case of flavones, the structure 04a is the favored species in the gas phase, and the structure 04b is in the solvent phase. In isoflavones, the favored structure in both phases is the species 05b. It should be noted that the Trolox has the lowest ETE values in gas phase and solvent phase, in comparison with those obtained by the analyzed flavonoids when they are in the anionic form, being opposite to what happened with its PA values.
In the Table 8, the experimental percentages of antioxidant activity (%Aantiox) values are presented, which were reported for some of the flavonoids of the present job [67]. Additionally, we present the values of BDE, IE, and ETE calculated in solvent phase. The three properties reported in this article show an important inverse correlation with %Aantiox. It was found that the BDE values have the highest inverse relationship with the %Aantiox of the analyzed species, with a value of r equal to - 0.8816 (see Fig. 5), the correlation for the ETE values, present an r value of − 0.8775 (see Fig. 6). Finally, the IE values have a value of r equal to − 0.8629 (see Fig. 7). Thereby, it can to be considered that the BDE values are those best reflecting the antioxidant capacity of the studied flavonoids concerning the experimental results.
Correlation between the calculated BDE s and the experimental %Aantiox reported in some flavonoids and Trolox by S. Burda and coworkers [67]
Correlation between the calculated ETE s and the experimental %Aantiox reported in some flavonoids and Trolox by S. Burda and coworkers [67]
Correlation between the calculated IE s and the experimental %Aantiox reported in some flavonoids and Trolox by S. Burda and coworkers [67]
The four mechanisms of antioxidant activity we will analyze only in solvent phase, because it best represents the experiment. Although in our results we did not include the free radical with which the antioxidant should react, the calculations were made for the hydroxyl radical and the superoxide anion radical verifying the feasibility of the reaction, but we do not include them in the present article. In the case of the HAT mechanism, taking into account the values of BDE and the length of the hydrogen bonds, the flavonoid that presents better characteristics as an antioxidant is the species 03c, followed by to species 01c, while difference of the BDE values respect to the Trolox is approximately 1.05 kcal/mol in absolute value, in both cases.
In the SET mechanism, according to the IE determined values, the flavonoid that has better capacity as an antioxidant through the SET I path is the species 03d, and according to the EA values, for the SET II path is the species 01a, although in both cases this capacity is lower than that of the Trolox.
Moreover, in the SEPT mechanism, the flavonoid that exhibits better antioxidant characteristics by the additivity of the enthalpies (IE and PDE) is the species 03c, and very close in value is found the 01c, in the global, it is the Trolox that exhibits a better enthalpic behavior for this mechanism.
In the SPLET mechanism (see Eqs. 6 and 7), according to the determined by the values of both mechanism stages, the flavonoid that has a higher potential as an antioxidant is the species 03c, and very close it is found the 01c.
Of the different mechanisms, we can rationalize that the favored species belong to the group of flavonols and flavanols, and they do not present saccharides in their structure. It should be noted that all the flavonoids mentioned exhibit lower antioxidant capacity than that presented by the Trolox. However, the activity of these species is still relevant in each mechanism. We could suppose a consortium activity of the different present species, so that, then, they could show a synergic effect within the organism.
Additionally, the analysis of correlations between the calculated properties and the experimental %Aantiox shows us that HAT is the predominant mechanism in the solvent phase, followed by the SPLET mechanism, and in last instead, the SET mechanism, these results agree with the reaction feasibility, this same reflect the BDE values, in comparison with the IE and the ETE values, so HAT was favored energetically over the rest of the mechanisms.
When a compound receives an electron or loses an electron, forming a cationic species, it will exhibit a better antioxidant property when the species itself is able to redistribute the remaining electron density, by the effect of adding an electron or losing an electron. Table 9 shows the numerical values for the spin density of the phenoxyl radical and the radical cation of the species with the highest antioxidant potential. The values we present are only for the atoms that exhibit a maximum value. The radical cation species better distribute the remaining spin density. In the case of phenoxyl radicals, they are also able to redistribute the remainder electronic density, but this capacity is always better in the radical cation form, as we can see from the graphics (Figs. 8 and 9). Depending on the nature of the species, we observed that the values could remain constant, increase or decrease in the solvent phase, not being able to find any particular trend.
Conclusions
The antioxidant capacity of the 20 flavonoids reported in the Annona muricata leaves was evaluated from a theoretical approach. It was found that in the solvent phase, flavonol robinetin (03c) and flavanol gallocatechin (01a) are the species that exhibited the best antioxidant capacity in the HAT, SEPT, and SPLET mechanisms. On the other hand, in the SET I mechanism, the best antioxidant species was the flavonol quercetin (03b), and in the SET II mechanism, the most favored species was the flavanol catechin (01a). However, these species fail to overcome the Trolox antioxidant capacity. Furthermore, an important correlation was found between the flavonoids that have a greater susceptibility to a homolytic cleavage (lower BDE values) and the interaction with another hydroxyl group (higher approximation between the atoms forming an intramolecular hydrogen bond). In addition, when the BDE, IE, and ETE values were related in solvent phase with some experimental antioxidant activity reported values, the best correlation was obtained with the BDE values, showing that HAT is the predominant mechanism, which also coincides with its energetic feasibility, compared to the rest of the mechanisms. Finally, according to the spin density distribution analysis, the radical cations showed a higher electronic delocalization capacity than the phenoxyl radicals. This property contributes to the stabilization of these species and is also a desired feature in the evaluation of antioxidant compounds.
References
Carocho M, Ferreira IC (2013) A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol 51:15–25
Uttara B, Singh AV, Zamboni P, Mahajan R (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7:65–74
Aruoma OI (1998) Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc 75:199–212
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K (2014) Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int 2014:1–19
Shahreza FD (2017) Oxidative stress, free radicals, kidney disease and plant antioxidants. Immunopathol Persa 3:1–6
Iuga C, Alvarez-Idaboy JR, Russo N (2012) Antioxidant activity of trans-resveratrol toward hydroxyl and hydroperoxyl radicals: a quantum chemical and computational kinetics study. J Org Chem 77:3868–3877
Procházková D, Bouṡová I, Wilhelmová N (2011) Antioxidant and prooxidant properties of flavonoids. Fitoterapia 82:513–523
Pisoschi AM, Pop A (2015) The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem 97:55–74
Gülċin I (2012) Antioxidant activity of food constituents: an overview. Arch Toxicol 86:345–391
Rao V, Balachandran B, Shen H, Logan A, Rao L (2011) In vitro and in vivo antioxidant properties of the plant-based supplement greens+. Int J Mol Sci 12:4896–4908
Moghadamtousi S, Fadaeinasab M, Nikzad S, Mohan G, Ali H, Kadir H (2015) Annona muricata (Annonaceae): a review of its traditional uses, isolated acetogenins and biological activities. Int J Mol Sci 16:15625–15658
Coria-Téllez A V, Montalvo-Gónzalez E, Yahia EM, Obledo-Vázquez E N (2018) Annona muricata: a comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab J Chem 11:662–691
Usunomena U, Paulinus ON (2015) Phytochemical analysis and mineral composition of Annona muricata leaves. IJRCD 1:38–42
Correa J, Ortiz D, Larrahondo J, Sanchez M, Pachon H (2012) Actividad antioxidante en guanábana (Annona muricata l.): una revisión bibliográfica. Bol latinoam Caribe plantas med aromát 11:111–126
George VC, Kumar DN, Suresh P, Kumar RA (2015) Antioxidant, DNA protective efficacy and HPLC analysis of Annona muricata (Soursop) extracts. J Food Sci Technol 52:2328–2335
Agu KC, Okolie PN (2017) Proximate composition, phytochemical analysis, and in vitro antioxidant potentials of extracts of Annona muricata (Soursop). Food Sci Nutr 5:1029–1036
Muthu S, Durairaj B (2015) Evaluation of antioxidant and free radical scavenging activity of Annona muricata. Euro J Exp Bio 5:39–45
Sujayil T, Dhanaraj T (2016) Determination of bioactive compounds in Evolvulus alsinoides leaf extract using GC-MS technique. Res J Life Sci Bioinform Pharm Chem Sci 2:34–38
Syed Najmuddin S, Alitheen N, Hamid M, Nik Abd Rahman N (2017) Comparative study of antioxidant level and activity from leaf extracts of Annona muricata Linn obtained from different locations. Pertanika J Trop Agric Sci 40:119–130
Xiao J (2017) Dietary flavonoid aglycones their glycosides: which show better biological significance. Crit Rev Food Sci Nutr 57:1874–1905
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2:53
Schäffner A R (2016) Flavonoid biosynthesis and Arabidopsis genetics: more good music. J Exp Bot 67:1203–1204
McIntosh CA, Owens DK (2016) Advances in flavonoid glycosyltransferase research: integrating recent findings with long-term citrus studies. Phytochem Rev 15:1075–1091
Heim KE, Tagliaferro AR, Bobilya DJ (2002) Flavonoid antioxidants: chemistry, metabolism and structure–activity relationships. J Nutr Biochem 13:572–584
De Beer D, Joubert E, Gelderblom W, Manley M (2002) Phenolic compounds: a review of their possible role as in vivo antioxidants of wine. S Afr J Enol Vitic 23:48–61
Xiao J, Capanoglu E, Jassbi AR, Miron A (2016) Advance on the flavonoid C-glycosides and health benefits. Crit Rev Food Sci Nutr 56:S29—S45
de Souza GL, de Oliveira LM, Vicari RG, Brown A (2016) A DFT investigation on the structural and antioxidant properties of new isolated interglycosidic O-(1→3) linkage flavonols. J Mol Model 22:100
Yang D, Xie H, Jia X, Wei X (2015) Flavonoid C-glycosides from star fruit and their antioxidant activity. J Funct Foods 16:204–210
Jiménez V M, Gruschwitz M, Schweiggert RM, Carle R, Esquivel P (2014) Identification of phenolic compounds in Soursop (Annona muricata) pulp by high-performance liquid chromatography with diode array and electrospray ionization mass spectrometric detection. Int Food Res 65:42–46
Weston LA, Mathesius U (2013) Flavonoids: their structure, biosynthesis and role in the rhizosphere, including allelopathy. J Chem Ecol 39:283–297
Zhang D, Liu Y, Chu L, Wei Y, Wang D, Cai S, Zhou F, Ji B (2013) Relationship between the structures of flavonoids and oxygen radical absorbance capacity values: a quantum chemical analysis. J Phys Chem A 117:1784–1794
Pietta P-G (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1042
Kabanda MM, Mammino L, Murulana LC, Mwangi HM, Mabusela WT (2015) Antioxidant radical scavenging properties of phenolic pent-4-en-1-yne derivatives isolated from Hypoxis rooperi. A DFT study in vacuo and in solution. Int J Food Prop 18:149–164
Sebastian RS, Enns CW, Goldman JD, Steinfeldt LC, Martin CL, Clemens JC, Murayi T, Moshfegh AJ (2017) New, publicly available flavonoid data products: valuable resources for emerging science. J Food Compost Anal 64:68–72
Ivey KL, Jensen MK, Hodgson JM, Eliassen AH, Cassidy A, Rimm EB (2017) Association of flavonoid-rich foods and flavonoids with risk of all-cause mortality. Br J Nutr 117:1470–1477
Galano A, Mazzone G, Alvarez-Diduk R, Marino T, Alvarez-Idaboy JR, Russo N (2016) Food antioxidants: chemical insights at the molecular level. Annu Rev Food Sci Technol 7:335–352
Amić D, Stepanić V, Luċić B, Marković Z, Marković JMD (2013) PM6 study of free radical scavenging mechanisms of flavonoids: why does O–H bond dissociation enthalpy effectively represent free radical scavenging activity. J Mol Model 19:2593– 2603
Galano A (2015) Free radicals induced oxidative stress at a molecular level: the current status, challenges and perspectives of computational chemistry based protocols. J Mex Chem Soc 59:231–262
Izadyar M, Kheirabadi RA (2016) theoretical study on the structure-radical scavenging activity of some hydroxyphenols. Phys Chem Res 4:73–82
Zheng Y-Z, Deng G, Liang Q, Chen D-F, Guo R, Lai R-C (2017) Antioxidant activity of quercetin and its glucosides from propolis: a theoretical study. Sci Rep 7:7543
Shahidi F, Zhong Y (2015) Measurement of antioxidant activity. J Funct Foods 18:757–781
Prior RL (2015) Oxygen radical absorbance capacity (ORAC): new horizons in relating dietary antioxidants/bioactives and health benefits. J Funct Foods 18:797–810
Om A, Kim JA (2008) quantitative structure–activity relationship model for radical scavenging activity of flavonoids. J Med Food 11:29–37
Amić D, Luċić B, Kovaċević G, Trinajstić N (2009) Bond dissociation enthalpies calculated by the PM3 method confirm activity cliffs in radical scavenging of flavonoids. Mol Divers 13:27–36
López-Munguía A, Hernández-Romero Y, Pedraza-Chaverri J, Miranda-Molina A, Regla I, Martínez A, Castillo E (2011) Phenylpropanoid glycoside analogues: enzymatic synthesis, antioxidant activity and theoretical study of their free radical scavenger mechanism. PLoS One 6:11–18
Primas H (2013) Chemistry, quantum mechanics and reductionism: perspectives in theoretical chemistry; Springer Science & Business Media, vol. 24
Cramer CJ (2013) Essentials of computational chemistry: theories and models. Wiley, New York
Lewars EG (2010) Computational chemistry: introduction to the theory and applications of molecular and quantum mechanics. Springer Science & Business Media, Berlin
Young D (2004) Computational chemistry: a practical guide for applying techniques to real-world problems. Wiley, New York
Jensen F (2017) Introduction to computational chemistry. Wiley, New York
Leach AR (2001) Molecular modelling: principles and applications. Prentice Hall, England
Orio M, Pantazis DA, Neese F (2009) Density functional theory. Photosyn Res 102:443–453
Ritchie TJ, McLay IM (2012) Should medicinal chemists do molecular modelling?. Drug Discov Today 17:534–537
Mladenović M, Mihailović M, Bogojević D, Matić S, Nićiforović N, Mihailović V, Vuković N, Sukdolak S, Solujić S (2011) In vitro antioxidant activity of selected 4-hydroxy-chromene-2-one derivatives—SAR, QSAR and DFT studies. Int J Mol Sci 12:2822–2841
Lespade L, Bercion S (2012) Theoretical investigation of the effect of sugar substitution on the antioxidant properties of flavonoids. Free Radic Res 46:346–358
Sarkar A, Middya TR, Jana AD (2012) A QSAR study of radical scavenging antioxidant activity of a series of flavonoids using DFT-based quantum chemical descriptors–the importance of group frontier electron density. J Mol Model 18:2621–2631
Sroka Z, żbikowska B, Hładyszowski J (2015) The antiradical activity of some selected flavones and flavonols. Experimental and quantum mechanical study. J Mol Model 21:307
Chen Y, Xiao H, Zheng J, Liang G (2015) Structure-thermodynamics-antioxidant activity relationships of selected natural phenolic acids and derivatives: An experimental and theoretical evaluation. PLoS One 10:1–20
Dennington R, Keith TA, Millam JM (2016) GaussView Version 6., Semichem Inc. Shawnee Mission KS
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery J A Jr., Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian∼ 16 Revision B.01. Gaussian Inc. Wallingford CT
Dewar MJ, Zoebisch EG, Healy EF, Stewart JJ (1985) Development and use of quantum mechanical molecular models. 76. AM1: a new general purpose quantum mechanical molecular model. J Am Chem Soc 107:3902–3909
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Kendall RA, Dunning TH, Harrison RJ (1992) Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J Chem Phys 96:6796–6806
Riley KE, Op’t Holt BT, Merz KM (2007) Critical assessment of the performance of density functional methods for several atomic and molecular properties. J Chem Theory Comput 3:407–433
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
Burda S, Oleszek W (2001) Antioxidant and antiradical activities of flavonoids. J Agric Food Chem 49:2774–2779
Acknowledgments
The authors acknowledge the Vice-Rectorate for Research of the Universidad Católica de Santa María for the financial support through the internal project 24975-R-2017.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper belongs to Topical Collection QUITEL 2018 (44th Congress of Theoretical Chemists of Latin Expression)
Rights and permissions
About this article
Cite this article
Manrique-de-la-Cuba, M.F., Gamero-Begazo, P., Valencia, D.E. et al. Theoretical study of the antioxidant capacity of the flavonoids present in the Annona muricata (Soursop) leaves. J Mol Model 25, 200 (2019). https://doi.org/10.1007/s00894-019-4083-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4083-7