Abstract
Furfural is a valuable oxygenated compound derived from the thermal decomposition of biomass, and is one of the major problems of bio-oil upgrading. Due to its high reactivity, this compound requires further upgrading to more stable products such as furfuryl alcohol, 2-methylfuran (MF), furan, 2-methyltetrahydrofuran, and tetrahydrofuran. The thermochemical data and kinetic analysis of the reactions involved in the conversion of furfural were investigated by molecular modeling to guide experimental investigations in the process of designing efficient catalysts that allows the control of the reaction pathways in specific directions, towards the production of fuel precursors or chemicals. All calculations for reactants, intermediates, and products were performed using the long range corrected functional WB97XD, with the basis set 6–311+g(d,p), under the density functional theory framework. Thermochemistry results suggest that furfural hydrogenation to form furfuryl alcohol is spontaneous up to a temperature of 523 K, but beyond this temperature the reaction becomes a nonspontaneous process. By contrast, the decarbonylation of furfural was thermodynamically favored at temperatures greater than 523 K. Therefore, furan is a thermodynamically favored product, while furfuryl alcohol is kinetically preferred. Once furfuryl alcohol is formed, the hydrogenolysis path to produce methylfuran is favored kinetically and thermodynamically, compared to the ring-hydrogenation towards tetrahydrofurfuryl alcohol. Gas phase thermodynamic properties and rate constants of the reactions involved in the conversion of furfural were calculated and compared against existing experimental data. This study provides the basis for further vapor phase catalytic studies required for upgrading of furans/furfurals to value-added chemicals.

Furan is a thermodynamically favored product, while furfuryl alcohol is kinetically preferred.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Furfural is an oxygenated compound obtained by the acid-catalyzed dehydration of xylose, which is the main building-block of the hemicellulose fraction. It is also present in bio-oils produced from the fast pyrolysis of biomass [1,2,3]. Furfural is highly reactive and tends to polymerize, forming humins, which are responsible for catalyst deactivation. Thus, the prestabilization of furfural into key intermediates such as furan compounds (furan, 2-methylfuran, tetrahydrofuran) is required [1, 4].
The conversion of furfural involves different reaction pathways (see Scheme 1), such as hydrogenation, hydrogenolysis, and decarbonylation. Each of these routes leads to the production of fuel precursors (methylfuran, furan, methyltetrahydrofuran) and value-added chemicals (furfuryl alcohol, tetrahydrofurfuryl alcohol) that are conventionally obtained from petroleum-based feedstock. Due to the several routes available for furfural conversion, it is difficult to control the selectivity of the products. Therefore, it is necessary to understand the effect of operating conditions on the reaction pathways.
The hydrogenation of furfural leads to the formation of furfuryl alcohol (FOL), which can be hydrogenated to produce tetrahydrofurfuryl alcohol (THFOL) or converted into 2-methylfuran (MF) and water via C–OH hydrogenolysis as shown in Scheme 1 (Routes 3 and 1, respectively). The 2-methylfuran formed can undergo additional hydrogenation steps to generate methyl-tetrahydrofuran (MTHF). In the decarbonylation route (Route 2 in Scheme 1), furfural (FAL) is converted into furan and CO. Furan can be subsequently hydrogenated to produce tetrahydrofuran (THF).
From the point of view of fuel production, the decarbonylation and overhydrogenation routes, which produce THF and THFOL (Route 2 and 3 in Scheme 1) are not desirable, since the first loses C in the process and the latter requires a high hydrogen consumption and does not remove oxygen from the molecule [5, 6]. However, the products and intermediates obtained from these routes (THF, THFOL, furan, and FOL) are value-added chemicals that are commonly used as solvents and as starting materials for polyurethane manufacture. Conversely, the hydrogenation-hydrogenolysis route to produce MF is the most desired reaction path, since it removes oxygen from the molecule, keeping the C–C chain length, which is the major challenge of the hydrodeoxygenation reaction. Additionally, MF has been considered as a renewable fuel component for spark ignition (SI) engines because it has good fuel properties such as high octane number and low water solubility (RON = 131 and 7 g/L, respectively) [7, 8].
Since the composition of a typical fuel consists of hydrocarbons (C4–C12) such as paraffins (alkanes), cycloalkanes (naphthenes), and olefins (alkenes), oxygenated molecules are not desirable in a fuel because low heating value (LHV) decreases as the oxygen content increases. Neither hydrogenation (FAL to THFOL) nor decarbonylation (FAL to furan) are desirable reaction pathways. While the former does not remove O, the latter loses C in the process. For the above reasons, the hydrogenation-hydrogenolysis route to MF (via C–OH hydrogenolysis) is the most desirable.
Experimental results have shown that, at high temperatures (503–573 K), noble-metal-based catalysts (Pd, Ru, and Pt) highly promote the furfural decarbonylation route [9, 10], while under mild reaction conditions (433–473 K), transition metal based catalysts such as Cu, Ni, and Co promote the furfural hydrogenation route [6]. Given the strong competition between the furfural hydrogenation and decarbonylation, recent studies on bimetallic catalysts have shown that it is possible to increase the selectivity to hydrogenation-hydrogenolysis products, i.e., towards MF, by the formation of alloys such as Ni-Fe and Cu-Co [5, 11]. It has been demonstrated in a previous work [12] that the incorporation of Fe onto a Pd/SiO2 catalyst reduces the decarbonylation rate, while increasing the selectivity to MF.
Considering the various possibilities for furfural conversion, computational chemistry offers a useful alternative to improve our understanding of the thermodynamic and kinetic feasibility of these reactions. In addition, a detailed thermochemical study can provide important molecular properties that are not readily available for elementary reactions using experimental techniques, e.g., activation enthalpies, rate constants, free energies, and reaction enthalpies, etc., information that is expected to be important for future development of efficient catalysts.
In this work, we carried out a computational study using the reactions observed in Scheme 1 with the aim of comparing these results with those obtained experimentally in a previous work [12]. The thermochemistry and kinetics of these reactions were evaluated. Accurate information on the energetics of these processes is an important aspect in the design of more efficient catalysts. To our knowledge, there are no previous studies related to the thermochemistry and kinetics of furfural reactions in gas-phase.
Computational methods
The reactions of furfural conversion in gas-phase were investigated using density functional theory (DFT) with the 6–311+g(d,p) basis set. To select an adequate level of theory, thermodynamic information obtained with the B3LYP and WB97XD functionals was compared with literature-reported values to study the accuracy in predicting reaction enthalpies (ΔH), entropy (ΔS), and Gibbs free energy (ΔG). Once an accurate level of theory was selected, geometry optimization and vibrational frequency calculations were carried out for the ground state multiplicity of each structure. The thermochemistry of all reactions was calculated at various temperatures in the range from 298 K to 573 K, keeping the pressure constant at 1 atm.
The first step of the calculations consisted of a full geometry optimization of all structures involved in the reaction pathways, such as reactants, intermediates, and products, with a subsequent frequency analysis to make sure that there were no imaginary eigenvalues and that each optimized structure corresponds to a minimum on the potential energy surface. To find the structures of the transition states (TS), the Synchronous Transit-guided Quasi-Newton method was used. Once the structure of the TS was found, an intrinsic reaction coordinate (IRC) calculation was performed to confirm that the TS structure connects to those of products and reactants. All calculations presented in this paper were carried out using the Gaussian 09 and Gauss View 5 software packages [13].
Results and discussion
Thermochemistry
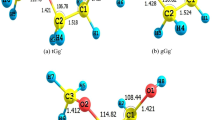
The normal-mode vibrational frequency calculation is calculated at T = 298 K and P = 1 atm, with the same level of theory used in the optimization. There is no sign of any imaginary frequency in any structure, thus affirming that all structures correspond to real minima on the potential energy surface. The corresponding molecular structures optimized at WB97XD/6–311+g(d,p) can be seen in Fig. 1.
Before calculating the thermodynamic properties involved in all reactions, and to select an adequate level of theory, the reaction enthalpy of FAL hydrogenation to FOL was calculated using two different functionals and compared with values reported in the literature. Specifically, B3LYP and WB97XD functionals were used, obtaining reaction enthalpies of −41.8 kJ mol−1 and − 59.1 kJ mol−1 at 298 K, respectively. By comparing these results with the experimentally reported value (−60.5 kJ mol−1) [14], it was noted that this reaction enthalpy was more similar to the result obtained with the WB97XD functional. Therefore, the WB97XD functional was selected to calculate the thermochemistry of all reactions shown in Scheme 1. A temperature range of 298 K to 573 K was considered to predict the thermochemical data, including enthalpies, entropies, and free energies at 1 atm. The results are listed in Table S1 of Supporting Information and will be analyzed in the following sections.
Conversion of FAL to MTHF—route 1
The conversion of FAL to MTHF consists of four steps, as shown in Scheme 1. The geometries of all TSs for this route are shown in the Supporting Information.
The first step (I) of this route is the hydrogenation of FAL to produce FOL. The values of ΔH in Table S1 indicate that the hydrogenation of FAL to produce FOL is an exothermic reaction in the overall temperature range.
Considering that the temperature dependence of ΔH and ΔS is small, it is possible to describe the behavior of ΔG with temperature by a linear function (see Fig. 2), which is a useful approximation for the evaluated temperature range. In the case of the FAL hydrogenation to FOL, it was observed that the reaction spontaneity decreases at temperatures greater than 523 K (Fig. 2a). As seen in Table S1 the enthalpy and entropy of the FAL hydrogenation reaction are negative within the whole temperature range and, consequently, at high temperatures (T > 523 K), the term −TΔS becomes higher than the ΔH term, making the reaction nonspontaneous. Consistent with these results, the values of the equilibrium constant (ln Keq) in Fig. 3 also indicated that the equilibrium shifts towards reactants at 523 K. These results are in line with experimental reports in which high reaction temperatures (>473 K) yield furan as the main reaction product, decreasing the rate of hydrogenation [1]. Concluding this part, the hydrogenation of FAL to produce FOL is an exergonic process that proceeds spontaneously up to 523 K.
Temperature effect on ΔG for furfural conversion. a Furfural hydrogenation and decarbonylation to FOL and furan, respectively. b Furfuryl alcohol hydrogenolysis and ring-hydrogenation to MF and THFOL, respectively. c Hydrogenation of furan and MF, to THF and MTHF, respectively. d Overall routes for the conversion of furfural to MTHF, THF, and MF
The second step (II) of this route is the hydrogenolysis of FOL to produce MF with the elimination of one water molecule. Contrary to the FAL hydrogenation to FOL, this reaction is always spontaneous because the enthalpy of the reaction is negative and the entropy positive in the entire temperature range, as confirmed in the equation shown in Fig. 2a. In this case, the reaction is spontaneous in the forward direction to form the products with the indicated values of the equilibrium constant (see Table S3).
The next step of this route is MF hydrogenation, which proceeds in two steps. In step (III), MF is hydrogenated to produce DHMF, which is subsequently hydrogenated to produce MTHF in step (IV). The spontaneity of MF hydrogenation to DHMF decreases with increasing temperature. In particular, at temperatures greater than 473 K the reaction is not thermodynamically favored, probably due to a competition between the furan ring-hydrogenation and ring-opening. As in the hydrogenation of FAL to FOL, the values of ΔH and ΔS are negative within the whole temperature range and, therefore, at a temperature of 473 K the term −TΔS becomes higher than the ΔH term, making the reaction an endergonic process. Consequently, at temperatures greater than 473 K, the reaction will proceed spontaneously in the reverse direction (see values of the equilibrium constants in Fig. 3b).
Regarding the hydrogenation of DHMF to MTHF, the reaction is exergonic over the whole temperature range (see Fig. 2c) since it is a much more exothermic step than MF hydrogenation to DHMF. It is worth mentioning that the activation enthalpies for these hydrogenation steps are higher than those obtained for FAL hydrogenation to FOL (Table S2), indicating that saturation of the furanic ring is a difficult task compared to hydrogenation of the carbonyl group. This result is expected because the furanyl ring of furfural is more stable than that of the carbonyl group, thus only metals with strong affinities for C=C bonds are employed to produce hydrogenated products like MTHF, THFOL, and THF. In this context, noble metal catalyst (e.g., Pt, Pd, Ru) have a remarkable selectivity towards the hydrogenation of C=C double bonds, since the preferred adsorption of furfural is with the furanyl ring oriented essentially parallel to the metal surface. Therefore, this configuration makes the hydrogenation of the furan ring on the metal surface readily possible, decreasing the energy barriers and yielding the saturated compounds as the main reaction products.
Conversion of FAL to THF—route 2
According to Scheme 1, furan and FOL are the primary products from FAL decarbonylation and hydrogenation reactions, respectively. The thermochemistry data obtained for the FAL hydrogenation to MTHF was analyzed above. This section is focused on explaining the energetics of the conversion of FAL to THF. The overall reaction consists of three steps, as shown in Scheme 1.
The decarbonylation of FAL to furan becomes a spontaneous process at temperatures greater than 523 K as observed in Table S1. Positive and almost constant values of ΔH and ΔS were obtained in the evaluated temperature range (298–573 K) (see equation in Fig. 2a). Therefore, the term –TΔS becomes more negative as the reaction temperature increases, leading to a negative Gibbs free energy when the temperature is high enough. Since the FAL decarbonylation is an endothermic reaction, the increase in temperature must favor the forward reaction, which is in agreement with the values of the equilibrium constant (ln Keq is greater than 1, at temperatures greater than 523 K) in Fig. 3. This high endothermicity can be rationalized by the energy required for C–C bond cleavage to produce furan and CO.
Regarding activation enthalpies (see Table S2), FAL decarbonylation to furan has a higher activation enthalpy than FAL hydrogenation to FOL, which is expected as the first one requires a C–C bond cleavage with the formation of CO. These thermochemistry results are consistent with experimental reports. For example, Pushkarev et al. [15] reported that, at high temperatures (550–600 K), the decarbonylation reaction dominates, while at lower temperatures the hydrogenation activity increases, yielding FOL as the main reaction product. Similarly, Benson et al. [16] found that, at low temperatures (< 503 K), the yield of FOL is higher, while at high temperatures (> 503 K) the decarbonylation reaction dominates, producing more furan.
Once furan is formed, the molecule can undergo two additional hydrogenation steps to produce THF as is illustrated in Scheme 1. Similarly to the hydrogenation of MF to DHMF, the hydrogenation of furan to DHF (step II of the Route 2 in Scheme 1) becomes an endergonic reaction at temperatures greater than 473 K, and thus the reaction shifts towards the reactants (see Fig. 3). By contrast, the hydrogenation of DHF to THF is an exergonic process within the whole temperature range. Thus, this reaction proceeds spontaneously in the forward direction (see Fig. 2c). Experimental and computational studies have reported that, at high temperatures, the furan hydrogenation starts to compete with the furan ring-opening to form butanol [17], which may explain the decrease in the spontaneity of the process with temperature.
On the other hand, the results in Table S2 show that the hydrogenation of furan to DHF requires a higher activation enthalpy (378.9 kJ mol−1) than the hydrogenation of DHF to THF (353.0 kJ mol−1) and, consequently, the latter is kinetically favored. From an environmental point of view, the conversion of FAL to MF is a more desirable pathway than the production of furan, because MF has potential properties to be used as fuel additive and the decarbonylation route produces CO [8, 18].
Conversion of FAL to THFOL—route 3
The last evaluated route is the FAL hydrogenation to produce tetrahydrofurfuryl alcohol (THFOL). This route is composed of three steps: the first is the hydrogenation of FAL to FOL, as discussed above (Route 1). The next step is the hydrogenation of FOL to produce DHFOL. This reaction is spontaneous from 298 K to 473 K; above this temperature, the reaction is nonspontaneous (ΔG > 0) since the term –TΔS becomes positive and higher than the ΔH term. Along the same lines, the equilibrium constant values (Fig. 3b) decrease with temperature, suggesting a shift of equilibrium towards the reactants. Additionally, experimental results have shown that, at high temperatures, the conversion of FOL to 1,2-pentanediol is preferred over FOL ring-hydrogenation [19]. It can be noted from Table S1 that the hydrogenation of FAL to FOL is a spontaneous reaction up to 523 K, whereas the hydrogenation of FOL to DHFOL is nonspontaneous at 523 K. Therefore, this second hydrogenation is not a thermodynamically favored step because furanic ring aromaticity makes the structure more stable.
The last step of this route is the hydrogenation of DHFOL to THFOL. This reaction is spontaneous over the entire evaluated temperature range because the second hydrogenation step is a more exothermic reaction, and therefore, the term ΔH is always higher than the term −TΔS.
By comparing the activation enthalpies in Table S2, it must be noted that the hydrogenolysis of FOL to MF involves a lower activation enthalpy (328.6 kJ mol−1) than that for the FOL hydrogenation to DHFOL (374.9 kJ mol−1). The reason for this is that the furanic ring is a stable structure, and hence, it is easier to break the C–OH bond of FOL to form MF [5, 17]; the formation of MF from FOL is then the more kinetically favored route.
The activation enthalpy for the hydrogenation of DHFOL to THFOL is lower (357.3 kJ mol−1) than that for the FOL hydrogenation to DHFOL (374.9 kJ mol−1). Consequently, the hydrogenation of DHFOL to THFOL is kinetically favored.
The thermochemistry results obtained suggest that the hydrogenation of FAL to FOL is not thermodynamically favored at temperatures greater than 523 K, while above this temperature, the decarbonylation route becomes predominant. Figure 2a shows clear evidence of these results, i.e., the hydrogenation of FAL to FOL has negative values of ΔG up to a temperature of 523 K, but, above this temperature, the decarbonylation reaction becomes an exergonic process making furan the thermodynamically favored product. Nevertheless, the values of activation enthalpy in Table S2, suggest that FAL hydrogenation to FOL is more kinetically favored than the decarbonylation pathway.
The thermochemistry results of the subsequent reaction steps involved in the different routes show that hydrogenolysis of FOL to produce MF is a more thermodynamically feasible route than furan and FOL hydrogenation. Furthermore, the activation enthalpy required for the hydrogenolysis of FOL to MF is lower than that needed for the hydrogenation of furan and FOL, which leads to the production of DHF and DHFOL, respectively. Bearing in mind these results, and knowing that MF is a desirable fuel component, the hydrogenation-hydrogenolysis route of FAL would be experimentally enhanced at intermediate temperatures (373–473 K).
Contrasting computational with experimental results
Experimental results have shown that the conversion of FAL over noble-metal-based catalysts yields furan as the main reaction product, while over transition metals (e.g., Cu, Ni, and Co) FOL is preferred [6, 10, 20]. This behavior is associated with the electronic and structural properties of the catalysts, which are highly affected by the d-band character of the supported metals.
On noble-metal-based catalyst surfaces (e.g., Pd and Pt) FAL tends to form η2(C, O) surface intermediates, in which both C and O atoms of the carbonyl group interact with the metal. In the presence of hydrogen, this intermediate can be hydrogenated to form FOL. However, when the temperature is high enough, the η2(C, O) aldehyde may further convert into a more stable acyl surface species, in which the C atom of the carbonyl is strongly attached to the metal surface, leading to the decarbonylation reaction. By contrast, on transition-metal-based catalyst surfaces (e.g., Cu and Ag), FAL tends to adsorb through the carbonyl group in a η1(O)-aldehyde configuration, which leads to the formation of FOL [21, 22]. These experimental results suggest that the FAL reaction pathways depend not only on the operating conditions but also on the catalyst properties (e.g., hydrogenating function, acid and base properties of the catalytic support, oxophilicty).
With the aim of contrasting the thermochemistry results with previous experimental work performed at 523 K and 1 atm [12], the relative free energy and enthalpy profiles were evaluated at these reaction conditions for each of the routes showed in Scheme 1. The diagrams of relative free energy are shown in Figs. 4, 5, and 6; while the relative enthalpy profiles are shown in Figs. S1, S2, and S3.
In the previous experimental work [12], the gas-phase conversion of FAL over monometallic Pd and bimetallic Pd-Fe catalysts was studied at 523 K and 1 atm. It was found that the decarbonylation reaction dominates, yielding furan as the main reaction product. Interestingly, the rate of decarbonylation was diminished by adding Fe to the Pd/SiO2 catalyst, which caused a change in the reaction kinetics. This experimental work supports the thermochemistry data described above, since the calculated free energy profiles (Figs. 4 and 5), clearly suggest that the FAL decarbonylation is more favored thermodynamically (−7.38 kJ mol−1) than the FAL hydrogenation (−0.15 kJ mol−1). Similarly, Sitthisa et al. [5] found that at 483 K, FOL is the main reaction product over Ni/SiO2 catalyst, while, at higher temperatures, greater than 483 K, the decarbonylation becomes predominant, yielding furan in a great extent.
Likewise, a series of experimental studies [1, 23, 24] reported that the hydrogenation of FAL to FOL is favored at low reaction temperatures (< 473 K), whereas at high temperatures, the hydrogenation and decarbonylation become competitive reactions. These results are in agreement with the work by Sitthisa et al. [9], in which at 503 K and 1 atm, the selectivity to furan and FOL was the same (~45%) over a Cu/SiO2 catalyst.
By comparing Figs. 5 and 6, it can be observed that the hydrogenolysis of FOL to produce MF is more thermodynamically favored than the furanic ring-hydrogenation to THFOL. Consistent with these computational results, the experimental findings of a previous work [12] showed that, over the bimetallic Pd-Fe catalyst, the selectivity of MF was always higher than that obtained for THFOL. Furthermore, Sitthisa et al. [9] found that the yield of MF increases with temperature, from 18% at 483 K to 38% at 523 K, in the gas-phase conversion of FAL over Ni-Fe/SiO2 catalyst.
Regarding the hydrogenation of MF and furan towards MTHF and THF, respectively, previous experimental works [5, 6, 12] have reported that these are very difficult routes since they usually require high hydrogen consumption and long reaction times. For instance, Pino et al. [12] reported that, on the monometallic Pd/SiO2 catalyst, FAL is highly converted into furan and to a lesser extent, into THF.
Interestingly, the direct production of THF from FAL is complicated, since the thermochemistry results in Table S1 suggest that the hydrogenation of furan to DHF is thermodynamically favored at low temperatures (< 473 K), while FAL decarbonylation to produce furan is favored at high temperatures (> 473 K). Therefore, the formation of THF is favored only at certain reaction conditions (e.g., by using hydrogen donor solvents, bimetallic catalysts) [1, 25]. This behavior lies in the fact that the conversion of furfural to furan liberates carbon monoxide, which has a high heat of adsorption on metal surfaces and therefore the hydrogen cannot be adsorbed simultaneously [26].
Kinetic results
Rate constants (k) were calculated for all routes shown in Scheme 1 by using the Eyring equation:
where kB is the Boltzmann constant, h is Planck’s constant, T is temperature, ΔG‡ is activation free energy, and R is the universal gas constant. Previous sections have already discussed the values of the activation enthalpies for some of the FAL conversion routes. This was done with the purpose of contrasting the thermodynamic results with the kinetics. However, in this section, the kinetic results will be discussed in more detail. The values of the rate constants for all reactions at the temperature range 298–573 K are shown in Table S3.
Figure 7 shows the behavior of the kinetic rate constants with temperature for the different steps involved in the gas-phase conversion of furfural (see Scheme 1).
From Fig. 7 it is observed that the kinetic rate constants (k) for all the evaluated reactions increase with temperature and the Arrhenius plots yield straight lines. At low temperatures (< 473 K) the rate of the FAL hydrogenation is faster than the decarbonylation, while at high temperatures (> 473 K), decarbonylation begins to be predominant according to the values of activation enthalpies in Table S2.
The rate constant for the FAL decarbonylation reaction is on the order of 10−53 (s−1) at 298 K, while at high temperature (573 K), the same rate constant is on the order of 10−20 (s−1) (Table S4). As expected, this result indicates that the rate of decarbonylation increases with temperature, which is in good agreement with previous experimental results [12].
Kinetic analysis at 523 K showed that the FAL hydrogenation is slightly faster (1.58 × 10−23 L molecule−1 s−1) than the FAL decarbonylation (6.11 × 10−24 s−1). Similarly, experimental studies [6, 12, 27] have shown that at 523 K the FAL hydrogenation strongly competes with the decarbonylation reaction; hence, the catalyst properties would have an important influence on the rate of these two reactions. For instance, at 523 K, over Pd based-catalysts, the decarbonylation of FAL is preferred over hydrogenation. By contrast, on the bimetallic Pd-Fe catalyst, hydrogenolysis to produce MF becomes predominant. Hence, depending of the furfural-catalyst interaction (types of adsorption), as well as the reaction conditions (temperature, pressure, and solvent), one reaction route will be more kinetically favored.
Table S4 shows that the hydrogenation of FAL to FOL involves higher rate constants than those required for the hydrogenation of FOL to DHFOL and DHFOL to THFOL. This can be explained by the fact that the FAL to FOL step needs a lower activation enthalpy (292.4 kJ mol−1) compared to FOL to DHFOL (374.9 kJ mol−1) and DHFOL to THFOL (357.3 kJ mol−1) steps. This means that, in contrast to the hydrogenation of the carbonyl group, saturation of the furanic ring is a difficult task, probably due to the furanic ring aromaticity, which makes it more stable. Therefore, FOL will be the kinetically and thermodynamically favored product.
In addition, the hydrogenation of FOL to THFOL exhibited smaller rate constants than FOL hydrogenolysis, which means that the latter is kinetically more feasible since it requires a smaller activation enthalpy (around 328.6 kJ mol−1) compared to FOL hydrogenation to DHFOL (374.9 kJ mol−1). This computational result agrees with a similar experimental observation by Albilali et al. [28].
Finally, when analyzing the rate constants for the MF and furan hydrogenation towards MTHF and THF, respectively, they are similar at high temperatures, with MF hydrogenation being the more kinetically feasible. Unlike the previous steps, these ring-hydrogenation steps are not experimentally desirable since they require high hydrogen consumption and the selectivity is usually quite low.
Conclusions
This work presents a detailed thermochemical and kinetic study of the gas-phase conversion of FAL. The results suggest that low temperatures (298–473 K) favor the hydrogenation of FAL to FOL, while the rate of decarbonylation increases at relatively higher temperatures (greater than 473 K). At 573 K, the rate constant for FAL decarbonylation to produce furan and CO becomes greater than that for the FAL hydrogenation to FOL, which is consistent with experimental results.
The hydrogenolysis of FOL to MF requires lower activation enthalpy than the hydrogenation of FOL to THFOL. In the overall temperature range studied (298–573 K) the hydrogenolysis of FOL to MF was found to be thermodynamically and kinetically favorable over the FOL hydrogenation. The sequential hydrogenation steps of MF to MTHF showed that the first hydrogenation step is not thermodynamically favored at high temperatures (greater than 473 K), while the second hydrogenation (DHMF to MTHF) is both kinetically and thermodynamically favored in the evaluated temperature range.
The temperature variations played a significant role in controlling the thermodynamic and kinetic feasibility of the gas-phase conversion of FAL. Therefore, the computations discussed in the present work provide an initial basis to improve our understanding on how it is possible to control the operating conditions to promote a specific reaction pathway.
References
Yan K, Wu G, Lafleur T, Jarvis C (2014) Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew Sust Energ Rev 38:663–676. https://doi.org/10.1016/j.rser.2014.07.003
Won W, Maravelias CT (2017) Thermal fractionation and catalytic upgrading of lignocellulosic biomass to biofuels: process synthesis and analysis. Renew Energy 114:357–366. https://doi.org/10.1016/j.renene.2017.07.023
Waters CL, Janupala RR, Mallinson RG, Lobban LL (2017) Staged thermal fractionation for segregation of lignin and cellulose pyrolysis products: An experimental study of residence time and temperature effects. J Anal Appl Pyrolysis 126:380–389. https://doi.org/10.1016/j.jaap.2017.05.008
Bui TV, Crossley S, Resasco DE (2016) C–C coupling for biomass-derived furanics upgrading to chemicals and fuels. In: Cavani F, Albonetti S, Basile F, Gandini A (eds) Chemicals and fuels from bio-based building blocks. Wiley, New York, pp 431–494
Sitthisa S, An W, Resasco DE (2011) Selective conversion of furfural to methylfuran over silica-supported NiFe bimetallic catalysts. J Catal 284:90–101. https://doi.org/10.1016/j.jcat.2011.09.005
Sitthisa S, Resasco DE (2011) Hydrodeoxygenation of furfural over supported metal catalysts: a comparative study of Cu, Pd and Ni. Catal Letters 141:784–791. https://doi.org/10.1007/s10562-011-0581-7
Zheng H-Y, Zhu Y-L, Teng B-T et al (2006) Towards understanding the reaction pathway in vapour phase hydrogenation of furfural to 2-methylfuran. J Mol Catal A Chem 246:18–23. https://doi.org/10.1016/j.molcata.2005.10.003
Ma X, Jiang C, Xu H et al (2014) Laminar burning characteristics of 2-methylfuran and isooctane blend fuels. Fuel 116:281–291. https://doi.org/10.1016/j.fuel.2013.08.018
Sitthisa, S, Pham, T, Prasomsri, T, Sooknoi, T, Mallinson, R, Resasco D (2011) Conversion of furfural and 2-methylpentanal on Pd/SiO2 and Pd–Cu/SiO2 catalysts. J Catal 280:17–27
Vorotnikov V, Mpourmpakis G, Vlachos D (2012) DFT study of furfural conversion to furan, furfuryl alcohol, and 2-methylfuran on pd (111). ACS Catal 2:2496–2504. https://doi.org/10.1021/cs300395a
Srivastava S, Jadeja GC, Parikh J (2016) A versatile bi-metallic copper–cobalt catalyst for liquid phase hydrogenation of furfural to 2-methylfuran. RSC Adv 6:1649–1658. https://doi.org/10.1039/C5RA15048E
Pino N, Sitthisa S, Tan Q et al (2017) Structure, activity, and selectivity of bimetallic pd-Fe/SiO2 and pd-Fe/γ-Al2O3 catalysts for the conversion of furfural. J Catal 350:30–40. https://doi.org/10.1016/j.jcat.2017.03.016
Frisch MJ, Trucks GW, Schlegel HB, GES, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H et al (2009) Gaussian 09, Revis. B.01. Gaussian, Inc., Wallingford
Kudchadker S-A, Kudchadker A-P (1975) Thermodynamic properties of oxygen compounds III. Benzaldehyde and furfural (2-furaldehyde). Thermochim Acta 12:432–437. https://doi.org/10.1016/0040-6031(75)85074-X
Pushkarev VV, Musselwhite N, An K et al (2012) High structure sensitivity of vapor-phase furfural decarbonylation/hydrogenation reaction network as a function of size and shape of Pt nanoparticles. Nano Lett 12:5196–5201. https://doi.org/10.1021/nl3023127
Benson TJ, Daggolu PR, Hernandez RA et al (2013) Catalytic deoxygenation chemistry: Upgrading of liquids derived from biomass processing. Adv Catal 56:187–353. https://doi.org/10.1016/B978-0-12-420173-6.00003-6
Wang S, Vorotnikov V, Vlachos DG (2014) A DFT study of furan hydrogenation and ring opening on pd(111). Green Chem 16:736–747. https://doi.org/10.1039/C3GC41183D
Leitner W, Klankermayer J, Pischinger S et al (2017) Advanced biofuels and beyond: chemistry solutions for propulsion and production. Angew Chemie Int Ed 56:5412–5452. https://doi.org/10.1002/anie.201607257
Zhang B, Zhu Y, Ding G et al (2012) Selective conversion of furfuryl alcohol to 1,2-pentanediol over a Ru/MnOx catalyst in aqueous phase. Green Chem 14:3402–3409. https://doi.org/10.1039/c2gc36270h
Taylor MJ, Jiang L, Reichert J et al (2017) Catalytic hydrogenation and Hydrodeoxygenation of furfural over pt(111): a model system for the rational design and operation of practical biomass conversion catalysts. J Phys Chem C 121:8490–8497. https://doi.org/10.1021/acs.jpcc.7b01744
Vargas-Hernández D, Rubio-Caballero JM, Santamaría-González J et al (2014) Furfuryl alcohol from furfural hydrogenation over copper supported on SBA-15 silica catalysts. J Mol Catal A Chem 383–384:106–113. https://doi.org/10.1016/j.molcata.2013.11.034
Nagaraja BM, Siva Kumar V, Shasikala V et al (2003) A highly efficient cu/MgO catalyst for vapour phase hydrogenation of furfural to furfuryl alcohol. Catal Commun 4:287–293. https://doi.org/10.1016/S1566-7367(03)00060-8
Jiménez-Gómez CP, Cecilia JA, Durán-Martín D et al (2016) Gas-phase hydrogenation of furfural to furfuryl alcohol over cu/ZnO catalysts. J Catal 336:107–115. https://doi.org/10.1016/j.jcat.2016.01.012
Jiménez-Gómez CP, Cecilia JA, Márquez-Rodríguez I et al (2017) Gas-phase hydrogenation of furfural over Cu/CeO2 catalysts. Catal Today 279:327–338. https://doi.org/10.1016/j.cattod.2016.02.014
Wang C, Luo J, Liao V et al (2018) A comparison of furfural hydrodeoxygenation over pt-co and Ni-Fe catalysts at high and low H2 pressures. Catal Today 302:73–79. https://doi.org/10.1016/j.cattod.2017.06.042
Zeitsch KJ (2000) The chemistry and technology of furfural and its many by-products. Elsevier, Amsterdam, pp 184–185
Sitthisa S, Sooknoi T, Ma Y et al (2011) Kinetics and mechanism of hydrogenation of furfural on cu/SiO2 catalysts. J Catal 277:1–13. https://doi.org/10.1016/j.jcat.2010.10.005
Albilali R, Douthwaite M, He Q, Taylor SH (2018) The selective hydrogenation of furfural over supported palladium nanoparticle catalysts prepared by sol-immobilisation: effect of catalyst support and reaction conditions. Catal Sci Technol 8:252–267. https://doi.org/10.1039/C7CY02110K
Acknowledgments
The authors thank the project “Sustainable products from biomass” financed by Newton Institutional Link Funds, Colciencias, and Universidad de Antioquia UdeA (FP44842-241-2017). Natalia Pino gratefully acknowledges to the Universidad de Antioquia for her Doctoral scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 357 kb)
Rights and permissions
About this article
Cite this article
Pino, N., López, D. & Espinal, J.F. Thermochemistry and kinetic analysis for the conversion of furfural to valuable added products. J Mol Model 25, 26 (2019). https://doi.org/10.1007/s00894-018-3908-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3908-0