Abstract
Since the thermal stabilities of ionic liquids (ILs) are of significance for their application, an amine-functionalized IL 1,2-dimethyl-(3-aminoethyl) imidazolium tetrafluoroborate [aEMMIM][BF4] was chosen to study thermal decomposition mechanisms via the methods of FT-IR, 1H NMR, TGA, TGA-MS and density functional theory (DFT) calculations. Theoretical and experimental results indicated that amine-functionalization reduces the thermal stability of [aEMMIM][BF4] compared to its non-functionalized counterpart. Moreover, we found that [aEMMIM][BF4] follows a unimolecular nucleophilic substitution (SN1) decomposition (98.8 %), whereas the bimolecular nucleophilic substitution (SN2) decomposition (1.2 %) is unfavorable. The SN1 and SN2 reactions were fully optimized at B3LYP/6-311++G(d,p) level, and the energies of reactant (R), intermediates (IM), transition state (TS) and product (P) were obtained and analyzed by reaction mechanism. The energy of the intermediate is higher than that of the reactants by 18.92 kJ mol−1, and the energy of the TS is higher than that of the IM by 155.23 kJ mol−1. This result indicates that the IM are also more stable than the P2 product, thus the reaction is endothermic. The chemical nature of the covalent and hydrogen bonds was analyzed by vibrational modes analysis (VMA), nature bond orbital (NBO) and the theory of atoms in molecules (AIM).

Proposed thermal decomposition of [aEMMIM][BF4] via unimolecular ( SN1) and bimolecular( SN2) nucleophilic substitution mechanisms. The electrostatic potential surface (ESP) of the transition state illustrates that hydrogen bonds are generated when [BF4]− is close to [aEMMIM]+, and SN1 decomposition is much favorable than SN2 decomposition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ionic liquids (ILs) have many unique properties such as a very large liquid temperature range {e.g., 1-butyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide ionic liquid: −89 °C to 450 °C}, very low vapor pressure, low corrosivity and high chemical resistance. ILs can be made task-specific for a certain applications [1], including use as solvents in chemical synthesis and catalysis [2–8] and separation technology [9, 10], as electrolytes in electrochemical devices [11–13], and as lubricants or as heat-transfer fluids [14]. In addition, the huge range of possible combinations between cation and anion adds to the design flexability of ILs [15]. For example, an amino group confers basic properties; it can react with acidic groups such as carbon dioxide [16, 17], hydrogen sulfide [18, 19], sulfuric dioxide [20], and vinyl chloride [21].
However, ILs exhibit some thermal instability, which can reduce the efficiency of ILs as solvent or catalysts, and even lead to hazardous byproducts (e.g., HF) [22, 23]. Moreover, although ramped thermogravimetric analysis (TGA) measurements show that ILs seem to have high thermal stability, with long exposure times they can suffer a significant mass loss even at low temperatures [24]. Mass loss of ILs can be attributed to decomposition (e.g., elimination and nucleophilic substitution) and vaporization; both these factors become more severe at higher temperatures [24, 25]. Mass loss seriously hinders application of ILs. Therefore, investigations into the thermal stability of ILs are very necessary, and some researchers have reported in depth investigation of the thermal stability of conventional ILs [25].
An amine-functionalized IL, 1,2-dimethyl-(3-aminoethyl) imidazolium tetrafluoro-borate [aEMMIM][BF4], was designed and synthesized for better performance of CO2 capture based on our previous reports [24, 26]. Mu et al. [24] reported that CO2 could be captured by amine-functionalized ILs with 1:1 stoichiometry, both experimentally and in theoretical calculations. Amine-functionalized ILs might be widely used for CO2 capture in the future.
This study investigated the thermal stability of IL [aEMMIM][BF4]. We focused mainly on the decomposition mechanisms at elevated temperature. The proportion of vaporization and decomposition in mass loss was estimated based on TG-MS experimental results. The stable geometries and interaction modes of [aEMMIM][BF4] were obtained and characterized using density functional theory (DFT) calculations. Specific decomposition mechanisms can be observed and confirmed by transition state (TS) and intrinsic reaction coordinate (IRC) calculations. DFT calculations indicated that the energy barrier (130.92 kJ mol−1) of unimolecular substitution nucleophilicity (SN1) reaction is much lower than that (303.34 kJ mol−1) of bimolecular substitution nucleophilicity (SN2) decomposition, which explains why the SN1 reaction is more favorable (98.8 %). The chemical nature of the covalent and hydrogen bonds was analyzed by vibrational modes analysis (VMA), nature bond orbital (NBO) [27] and atoms in molecules (AIM) [28] methods. The results indicate that both SN1 and SN2 exist in the thermal decomposition of [aEMMIM][BF4].
Materials and methods
Experimental methods
Materials
[aEMMIM][BF4] was synthesized and purified by our previously reported procedure [17, 26]. The halogen ion content was undetectable (by AgNO3 precipitation) and less than 34 ppm (by inductively 30 coupled plasma optical emission spectrometry ICP-OES with a Varian Vista MPX); the metal ion content was less than 11 ppm (by inductively coupled plasma optical emission spectrometry ICPOES with a Varian Vista MPX).
TGA (TA Instruments, Q50–TG) was used to determine the short- and long-term thermal stability [30]. Verification of the operation temperature (T oper) of [aEMMIM][BF4] was carried out in TGA isothermally at 138 °C under inert atmosphere (N2) for 10 h. The results of three TGA experiments with [aEMMIM][BF4] were averaged, and deviations were below 0.2 %. The weight scope of TG was 1 g with a mass precision ± 0.1 μg. Temperature precision was ± 0.1 °C.
TGA and TGA–MS
TGA–MS (QIC20, HIDEN) was used to investigate the thermal decomposition mechanisms. As in our previous study [24], [aEMMIM][BF4] was heated in TGA–MS ramped from room temperature to 500 °C with a heating rate 10 °C min−1. After heating in the corresponding TGA, degradants of [aEMMIM][BF4] are detected by coupled MS with multiple ion detect (MID) mode.
IR spectra
The IR spectra of [aEMMIM][BF4] were measured on a Prestige-21 FTIR spectrometer (Shimadzu, Japan), equipped with a DTGS detector and a ZnSe attenuated total reflection (ATR) cell (ATR-8200H); 40 scans were performed, with a resolution of 4 cm−1 over the range of 4600 cm−1 to 400 cm−1.
Computational methods
All calculations in this work were carried out using the Gaussian03 program package [29]. All ground state, intermediate and TS geometries were located using the hybrid Becke 3-Lee-Yang-Parr (B3LYP) exchange-correlation functional with the 6-311++G(d,p) basis sets [31, 32]. No restrictions on symmetries were imposed on the initial structures, and therefore geometry optimization for the saddle points occurred with all degrees of freedom. The chosen basis set contains diffuse functions that are required for the correct description of intramolecular hydrogen bonds [33]. All the geometries were first optimized at the B3LYP/6-31G(d,p) level, and also characterized by frequency analysis; calculations were then performed at the B3LYP/6-311++G(d,p) level. Frequency analysis has verified the reasonableness of the optimized structures, in which only one imaginary frequency is found in TS, and no imaginary frequency is found in the reactant or product. Finally, the intrinsic reaction coordinate (IRC) pathways were used to confirm the energy profiles connecting the TS to the two desired minima of the proposed mechanism. Basis set superposition errors (BSSE) [34] were also considered in the interaction energy calculations, and corrected using the counterpoise (CP) method [35]. To confirm the existence and characteristics of the hydrogen bond, NBO [27] and AIM [28] calculations were carried out via the programs included in the Gaussian03 program package [29] at B3LYP/6-311++G(d,p) level. In AIM calculations, ρ(r) is used to describe the intensity of a bond, and the larger ρ(r) value corresponds to the stronger bond. The term ∇2ρ(r) describes the characteristics of the bond. If ∇2ρ(r) < 0, it is defined as a covalent bond, while closed-shell interactions such as van der Waals interactions, hydrogen and ILs are characterized by ∇2ρ(r) > 0. ∇2ρ(r) = λ1 + λ2 + λ3, where λI is an eigenvalue of the Hessian matrix.
Results and discussion
Experimental determination of the thermal stability of [aEMMIM][BF4]
The thermal stability of [aEMMIM][BF4] was investigated using isothermal TGA and ramped TGA. The onset temperature (T onset) usually indicates short-term thermal stability, and can be obtained by TGA with an isothermal mode, i.e., increasing the temperature by several degrees per minute to check the mass loss of sample with time. The T onset of [aEMMIM][BF4] is about 284 °C, with a heating rate of 10 °C/min in ramped mode. This is 114 °C lower than that of non-functionalized [BMMIM][BF4] (T onset = 398 °C) at the same heating rate of 10 °C/min, indicating that the amine-functionalized ILs are less stable than their non-functionalized counterparts. The specific decomposition mechanism will be discussed later in this paper.
T onset generally overestimates thermal stability, so the long-term stability involved in the research and application of ILs should be characterized. Table 1 shows that, even at 220 °C, [aEMMIM][BF4] loses 20 % mass after 150 min heating in isothermal TGA. It can be seen that, contrasted with the higher onset temperature (i.e., T onset = 284 °C) of [aEMMIM][BF4], the instability is more obvious after heating for a long period. If [aEMMIM][BF4] were exposed to a lower temperature of 180 °C for 150 min, it would also present a mass loss of about 4 %, which is consistent with the conclusion drawn by Andreas Jess [36].
Rate constant and Arrhenius equation of thermal decomposition
From Table 1, it can be deduced that the isothermal decomposition of [aEMMIM][BF4] is a zero–order kinetic reaction (Eq. 1). The 1H NMR spectra of [aEMMIM][BF4] also indicate that all the decomposition products are volatile at relatively high temperatures.
Therefore, the decomposition rate constant (k) at a fixed temperature can be derived from the mass loss rate (dm/dt). The linear fitting graphs of mass loss (%) vs time at different temperatures are given in Fig. S1, and the decomposition rate constants (k) and correlation coefficients (R 2) of [aEMMIM][BF4] are shown in Table 2. It can be seen that the rate constants (k) are increased along with the increase of temperature, and the correlation coefficients (R 2) are decreased with increasing temperature, which may be due to the increase of temperature leading to a more complex thermal decomposition reaction.
Considering that the temperature range investigated is relatively narrow (i.e., 138–220 °C), the decomposition activation energy (E a) can be assumed to be constant. Therefore, the decomposition can be described by the Arrhenius equation (Eq. 2).
The lnk vs 1/T is given in Fig. 1, and the linear regression equation is y = −8686.4x + 15.25, with the standard errors of intercept and slope being 1.42831 and 0.64272, respectively. The activation energy for [aEMMIM][BF4] decomposition is 72.2 kJ mol−1 within the investigated range of temperature, and the correlation coefficients (R 2) is 0.97846. It can be seen that the thermal decomposition reaction is really a zero order kinetic reaction.
Determination of MS and 1H NMR spectra
Mass loss of [aEMMIM][BF4] after heating in TGA can be attributed to vaporization and decomposition. Gaseous impurities were excluded by drying [aEMMIM][BF4] in a vacuum oven and an isothermal N2 sweep in TGA. If vaporization of [aEMMIM][BF4] occurs, the corresponding m/z (140, i.e., m/z of the cation) in TG-MS should be detected. However, this was undetectable. The absence of [aEMMIM][BF4] in the MS shows that vaporization of [aEMMIM][BF4] is negligible.
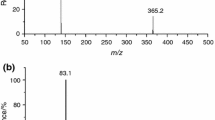
If the decomposition of [aEMMIM][BF4] in TGA is concurrent with an elimination reaction, the gaseous or liquid degradant [EMMIM][BF4] should exist. The gaseous degradant [EMMIM][BF4] (m/z = 210) was not detected by coupled MS, nor was the possible existence of liquid [EMMIM][BF4] in the TGA crucible after heating found in the 1H NMR spectra. These two lines of evidence indicate that the elimination mechanism of [aEMMIM][BF4] decomposition is negligible. The MS experimental results show that the decomposition mechanism for [aEMMIM][BF4] is a substitution nucleophilicity reaction. Cleavage of C8–N7 and C21–N4 by attack of the F atom in the anion favors an SN1 and an SN2 mechanism, respectively.
As expected, the corresponding MS peaks of volatile degradants by SN1 and SN2 are detected. The quantification of SN1 and SN2 could be determined roughly by the amount of their corresponding degradants. Specifically, the contribution of SN1 could be represented by the amount of 1,2-dimethyl imidazolium (MMIM m/z = 96), while the amount of 1-methyl-3-ethyl imidazolium (VMIM m/z = 108) indicated the contribution of SN2. The amounts of MMIM, VMIM, and other degradants are shown in Table 3, with the maximum concentration of degradant (MMIM) as the reference 100 %. Therefore, the contributions of SN1 and SN2 were calculated as 98.8 % and 1.2 %, respectively. The quantitative analysis also suggests that the SN1 mechanism dominates, while the SN2 mechanism subordinates within the SN decomposition mechanism for [aEMMIM][BF4].
Geometries and electrostatic potential analyses
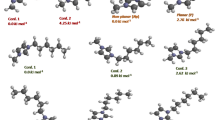
Geometries of [aEMMIM][BF4] were optimized by B3LYP/6-311 + G(d,p) level in order to give a visual understanding of the interactions before designing the initial geometries. The electrostatic potential (ESP) is a rigorously defined expectation quantity that is measured as a first-order interaction between the charge (electrons and nuclei) distribution and a positive unit charge at any point in space surrounding molecules and ions or solvents, and is a real physical property that can be determined experimentally by diffraction methods, as well as computationally [37]. Three stable conformers of [aEMMIM][BF4] were obtained, with energies of −863.906683, −863.899469 and −863.901161 a.u., called A, B and C, respectively. However, conformer A is more stable than both B and C. The structural parameters are shown in Table S1 (Electronic Supplementary Information), and the ESPs are given in Fig. 2. It can be seen that the ESP surface at sites close to the polar group is influenced by the stereo structure, and the charge density distribution is 3D plots of the ESP surface for the above ions. It indicates that the highly negative regions (red) of the [BF4]− anion are found around the polar F atom, and show high activity on the electronegative F atoms. In contrast, the highly positive regions (blue) in [aEMMIM]+ are localized on the hydrogen atom of the alkyl groups, which can be considered as a possible site for nucleoplilic attack of the F atom in [BF4]−. When the F atom in [BF4]− attacks the N atom that connects to ethylamine, due to the steric hindrance and amine action, the thermal decomposition is an SN1 reaction. And for the N atom, which connects to methyl, the thermal decomposition is an SN2 reaction.
Thermal decomposition mechanism of [aEMMIM][BF4]
The geometries of SN1 and SN2 reactions were fully optimized at B3LYP/6-311++G(d,p) level. The geometrical parameters involving the reactant ®), intermediates (IM), transition state (TS) and product (P) of [aEMMIM][BF4] are shown in Fig. 3. The potential energy surface (PES) profiles for SN1 and SN2 reaction are depicted in Figs. 4 and 5, respectively. For the SN1 reaction, when [aEMMIM][BF4] is heated, conformer A would be converted to conformer B (i.e., IM), and the energy of IM is higher than that of the R by 18.92 kJ mol−1. Fig. 4 also shows that the energy of P1 is higher than IM by 155.23 kJ mol−1. This result indicates that IM is more stable than the P1 product, and the reaction is endothermic.
From IRC calculation for TS1, we obtain two desired minima states of the process. This indicates that TS1 is a transition state, and has an imaginary frequency (−437.089 cm−1). It can be seen that F26, F28 and F29 in the [BF4]− anion form three hydrogen bonds with H16, H10 and H13 in the ethylamine group, with bond lengths of 1.772, 2.108 and 2.243 Å, respectively. Especially, F26 combined with H16 in NH2 is the shortest by 1.772 Å, which indicates that amine-functionalized ILs are less stable than their non-functionalized counterparts. This conclusion is in agreement with the above experimental results. Under the interaction of the above hydrogen bonds, the N7–C8 bond is extended gradually from 1.470 Å in IM to 2.166 Å in TS1, and is then broken in P1 and an SN1 thermal decomposition reaction takes place. The energy barrier is 130.92 kJ mol−1.
For SN2 reactions, when the [aEMMIM][BF4] is heated, conformer A also changes to conformer B (IM). From Fig. 5, we also find that the energy of P2 is higher than that of IM by 150.21 kJ mol−1. This result indicates that IM is more stable than P2. Compared with P2 in Fig. 3, the energy of P2 is as same as that of P1.
From frequency calculations of TS2, the imaginary frequency (−505.79 cm−1) is found. It can be seen that F26 in the [BF4]− anion forms only one hydrogen bond with H22 in the methyl group, with a bond length of 2.255 Å. This indicates that the SN2 reaction is more difficult than the SN1 reaction. Under the interaction of F26–H22 bonds, the N4–C21 bond is extended gradually from 1.465 Å in IM to 2.344 Å in TS, and is then broken in P2, and an SN2 thermal decomposition reaction takes place. The energy barrier is 322.26 kJ mol−1, which is much higher than that (130.92 kJ mol−1) of the above SN1 reaction. This explains why the contributions of SN1 and SN2 were calculated experimentally as 98.8 % and 1.2 %, respectively.
Vibrational mode analyses
The IR spectra of [aEMMIM][BF4] were measured on an FTIR spectrometer, and 40 scans were made with a resolution of 4 cm−1 over the range of 4600 cm−1 to 400 cm−1. The experimental IR spectra are given in Fig. S2. The experimental values of characteristic peak and theoretical frequencies of R, IM, TS, and P calculated by 3LYP/6-311++G(d,p) are shown in Table 4, respectively. In order to improve agreement with experimental data, the calculated wave numbers for the B3LYP method were scaled down to the calculated harmonic frequencies. Due to the part of electron correlation not taken into account in the functional, and the incompleteness of the basis set, the computed frequency values of theory contain systematic errors, and, therefore, a scaling factor of 0.9614 was considered to minimize B3LYP systematic errors [38]. The assignment of the calculated wave numbers was performed using the animation option of the GaussView 3.0 graphical interface, which gives a visual representation of the shape of the vibrational modes [39].
It is worth noting that an imaginary frequency (−437.089 cm−1), which is a symmetric stretching vibration between N7 and C8, appears in TS1. This clearly indicates rupture of the N7–C8 bond and formation of the C8–F28 bond. In TS2, an imaginary frequency (−505.79 cm−1) of a symmetric stretching vibration between C21 and F28 indicates formation of C21–F28 and breakage of C21–N4. From Table 4, it can be seen that the experimental results are in good agreement with the scaled calculated values, and the differences between the observed and calculated frequencies may be due to the fact that the calculations were actually applied to a single ion pair in the gaseous phase, in contrast to the experimental values recorded in the presence of intermolecular interactions. The experiment values of the frequencies were found to be limited by some experimental conditions. For example, there is no way to determine the vibrational spectrum in IM and TS experimentally, so the calculated frequency values are helpful in analyzing the vibration modes of ILs.
The highest vibrational frequencies are N–H2 asymmetric and symmetric stretching vibrations (see first and second rows in Table 4). Compared with experimental values, N–H2 symmetric stretching vibrations are red shifted from 3492 cm−1 to 3467 cm−1 and 3333 cm−1 for the R and P1 state, respectively. From Fig. 3 it can be seen that hydrogen bonds exist between F28 and F27 in [BF4]− and H16 in NH2, with bond lengths of 1.973 and 2.663 Å, respectively. The third and fourth rows in Table 4 show the vibrational modes are C17H3 asymmetric and symmetric stretching vibrations. It can also be found that C17H3 symmetric stretching vibrations are red shifted from 3078 cm−1 to 3099 cm−1, and 3094 cm−1 and 3098 cm−1 for R, TS1 and TS2, respectively. From Fig. 3 the changes are caused mainly by the hydrogen bond formations of F27⋯H20, F28⋯H18 and F27⋯H18, for R, TS1 and TS2, respectively. νs(C = C) and νs(C = N) of the imidazole ring are almost not varied, which explains that the influence of the thermal composition reaction on the imidazole ring is very small. The last two lines are ρ(alkyl C17-H), and the largest red shifts are from 1020 cm−1 and 955 cm−1 to 853 cm−1 and 848 cm−1 in TS2, which indicates that methyl is broken off from the imidazole ring, and CH3F will be formed.
NBO analyses
NBO theory was applied to obtain the charge distribution and intrinsic properties of the interactions among R, IM, TS and P [27]. The main charge distributions calculated at B3LYP/6-311++G(d,p) levels are given in Table 5. The complete results are shown in Table S2. As shown in Table 5, most of the positive charge is focused on the peripheral hydrogen atoms of the imidazole ring and the alkyl radical cations, while the [BF4]− anion preferentially approaches the positively charged groups, indicating that the electrostatic interaction between cations and [BF4]− is dominant for [aEMMIM][BF4]. The summations of atomic charges on the imidazole ring are −0.21284, −0.55864 and −0.71837 for IM, TS1 and P1, and −0.55052 and −0.7039 for TS2 and P2, respectively. This is because the imidazole ring is a Π5 6 conjugation, and a lone pair electron of N atoms takes part in the conjugation. When two alkyl radicals combine with 2 N atoms on the imidazole ring and form [aEMMIM]+, the imidazole ring adopts a Π5 5 conjugation. The thermal decompositions of SN1 and SN2 reaction occur, the conjugation on the imidazole ring changes from Π5 5 to Π5 6, and the charges on the imidazole ring are decreased along with IM, TS1 and P1 in order, respectively.
Second order perturbation stabilization energies E(2) denote the intensity of the donor–acceptor interactions. Some donor–acceptor interactions and their E(2) calculated at the B3LYP/6-311++G(d,p) levels are given in Table 6. The larger the value of E(2), the stronger the donor–acceptor capacity will be. Taking IM as an example, it can be seen that the largest value (79.51 kJ mol−1) of E(2) is the LP(N4,1) → σ*(C3-N7) interaction orbital, which means the strongest orbital interactions will occur between the electron donor of the lone pair LP(N4,1) and the sigma antibond orbital of the electron acceptor σ*(C3–N7). The next largest values of E(2) are 29.90 kJ mol−1 and 17.26 kJ mol−1, corresponding to LP(N4,1) → σ*(C1–C2) and σ(C2–N7) → σ*(C1–C2), respectively. The above interactions indicate the conjugation and stability of the imidazole ring. The values of 13.99 kJ mol−1 and 10.85 kJ mol−1 are those of orbital interactions of LP (F29, 2) → σ*(B25–F28) and LP(F28,3) → σ*(B25–F29), which shows the interaction of the hybridization orbital in [BF4]− anion. It is worth noting that the orbital interaction of LP(F28,1) → σ*(N14–H16) indicates the existence of the F28⋯H16-N14 hydrogen bond, and the value of E(2) is much smaller than those of the above covalent bond.
For TS1, besides the above interaction of the conjugation orbital on the imidazole ring and hybridization orbital in the [BF4]− anion, the important information is the LP electron of the nitrogen atom on the imidazole ring transferring to the ethylamine group. Three modes are shown in Fig. 6. First, a LP orbital on N7 interacts with the sigma antibond orbital of σ*(C8–H10), and the electron transfer happens, i.e., LP(N7,1) → σ*(C8-C10). As shown in Fig. 6a, the worst overlap, a symmetrical matching orbital and the largest energy gap (0.84 a.u.) can be seen, and the second order perturbation stabilization energies, E(2) is 0.84 a.u. Analysis of the second mode [LP(N7,1) → σ*(C8-C11)] is the same as that of the first mode, and E(2) is only 0.35 a.u. In the third mode [LP(N7,1) → σ*(C8-N14)], a LP orbital on N7 interacts with the sigma antibond orbital of σ*(C8–N14). E (2) value (53.29 kJ mol−1) of the third mode is much larger than those of mode 1 and 2, as shown in Fig. 3c. this represents symmetrical matching with maximum overlap and minimum gap (0.36 a.u.). Furthermore, it can be seen that the hybridized index of the nitrogen atom varies from sp1.00 in IM to sp2.01 in TS, which also plays an important role in the electron transfer reaction, and the value of the electron transfer is 0.12e.
For P1, except for the interaction of the conjugation orbital on the imidazole ring, the interactions E(2) of LP(F26,3) → LP*(B25,1), LP(F27,3) → LP*(B25,1) and LP(F29,3) → LP*(B25,1) are 44.53 kJ mol−1, 40.52 kJ mol−1and 43.22 kJ mol−1, respectively, which means that the B atom takes part in sp2 hybridization and forms three covalent bonds with the F atom.
To simplify NBO analyses of the SN2 thermal decomposition reaction, TS2 and P2 are the same as those of TS1 and P1, and thus will not be described here.
AIM analyses
AIM has been applied widely to study the bond properties between pairs of atoms analyzed systematically using quantum theory [28]. The electron density [ρ(r)] and the Laplacian of the electron density [∇2ρ(r)] as well as the eigenvalues (λI) of the Hessian matrix among the R, IM, TS and P are shown in Table 7; values were obtained at the B3LYP/6-311++G(d,p) level. All the hydrogen bonds values for the two topological properties are in the range of the criteria proposed by Popelier [40], i.e., 0.014–0.139 a.u. for ρ(r) and 0.002–0.035 a.u. for ∇2ρ(r). Since ρ(r) is related to bond strength, for R, and ρ(r) of the F28–H16 bond is 0.1029 a.u., which is much larger than the summation of F26–H22, F26–H20 and F27–H10 bonds, which indicates that the energy level of R is lower than that of IM by 18.92 kJ mol−1. For IM1, it can be seen that ∇2ρ(r)s of the N7–C8 and N4–C21 bonds are −0.5493 and −0.5612 a.u., respectively. Since the values are negative, the bond is defined as a covalent bond. ∇2ρ(r) of N7–C8 has changed to 0.1085 a.u. in TS1, thus although a covalent bond component exists in N7–C8 bond, the bond has begun to break. For TS2, the N4–C21 bond is the same as that of N7–C8, but ρ(r) of N4–C21 is 0.09463 a.u. It is obviously smaller than that of N7–C8, which indicates that the strength of N4–C21 is weaker than that of N7–C8. This fact is in good agreement with the bond lengths of N4–C21 (2.314) and N7–C8 (2.166 Å), respectively. For P1 and P2, the N7–C8 and N4–C21 bonds have been broken completely, and, at the bond critical point (BCP), there is no way to obtain the information between N7 and N4 with C8 and C21, and just the weak hydrogen bonds are found in AIM.
Conclusions
The decomposition mechanisms of IL [aEMMIM][BF4] were investigated by 1H NMR, TGA, TGA-MS and DFT calculations. The following conclusions can be drawn. (1) The amine-functionalization reduces the thermal stability of [aEMMIM][BF4] compared to its non-functionalized counterpart because the ethylamine group plays a major role in the thermal stability of ILs. It can form a hydrogen bond with [BF4]−, and carries out the unimolecular substitution nucleophilicity reaction easily. (2) The experimental determination of MS and 1H NMR spectra and the theoretical calculation of the energy barriers show that [aEMMIM][BF4] thermal decomposition reactions have two channel reactions, i.e., SN1 and SN2. Moreover, SN1 decomposition (98.8 %) is much faster than SN2 decomposition (1.2 %). (3) SN1 and SN2 reactions were fully optimized at B3LYP/6-311++G(d, p) level, and the energies of IM, TS and P were obtained and analyzed by reaction mechanism. The energy of IM is higher than that of the reactants by 18.92 kJ mol−1 and the energy of the TS is higher than IM by 155.23 kJ mol−1. (4) Vibrational modes analysis explains that an imaginary frequency (−437.089 cm−1) appears in TS1, which is a symmetric stretching vibration between N7 and C8. This clearly shows the rupture of the N7–C8 bond and formation of the C8–F28 new bond. And in TS2 an imaginary frequency (−505.79 cm−1) of a symmetric stretching vibration between C21 and F28 indicates formation of C21–F28 and breakage of C21–N4. (5) NBO theories indicate that the interaction between a LP orbital on N7 with the sigma antibond orbital of σ*(C8-N14), E (2) value (53.29 kJ mol−1), is much larger than that of other modes. (6) For IM, AIM explains ∇2ρ(r)s of N7–C8 and N4–C21 bond are −0.5493 and −0.5612 a.u., respectively, which are defined as the covalent bond, while in TS1, ∇2ρ(r) of N7–C8 has been changed to 0.1085 a.u., and the N7–C8 bond can be confirmed to have a covalent bond component, but the bond has begun to break.
References
Mu T, Han B (2014) Structures and thermodynamic properties of ionic liquids. In: Zhang S, Wang J, Lu X, Zhou Q (eds) Structures and interactions of ionic liquids, vol 151. Springer, Berlin, pp 107–139
Sun H, Cao B, Tian Q, Liu S, Du D, Xue Z, Fu H (2016) J Mol Liq 215:496–502
Cao B, Liu S, Du D, Xue Z, Fu H, Sun H (2015) J Mol Graph Model 64:5159
Cao B, Du J, Liu S, Zhu X, Sun X, Sun H, Fu H (2016) RSC Adv 6:10462–10470
Laali KK (2003) Ionic liquids in synthesis. Synthesis 2003(11):1752–1752
Holbrey JD, Rogers RD (2009) Ionic liquids: industrial applications for Green. Chemistry:446–458
Li R, Luan Y, Zou H, Du J, Mu T, Li Z (2012) RSC Adv 2(2):3049–3056
Sheldon R (2001) Chem Commun 23(23):2399–2407
Cheng X, Yang G, Mu T, Guo X, Wang X (2009) Clean Soil Air Water 37(3):245–248
Chen Y, Cao Y, Sun X, Yan C, Mu T (2013) Int J Greenhouse Gas Control 16(4):1320
Hagiwara R, Oi T, Yamauchi N, Nohira T, Matsumoto K, Tamba Y, Ito Y (2005) WO
Michel A, Frank E, Macfarlane DR, Hiroyuki O, Bruno S (2009) Nat Mater 8(8):621–629
Bacsa RR, Jeannette DG, Marc V, Andrea F, Bruno M, Bacsa WS, Chen P, Zakeeruddin SM, Michael G, Philippe S (2009) Adv Funct Mater 19(6):875–886
Brennecke JF, Maginn EJ (2001) AIChE J 47(47):2384–2389
Li Z, Li R, Mu T, Luan Y (2013) Chemistry 19(19):6005–6013
Cao Y, Chen Y, Lu L, Xue Z, Mu T (2013) Ind Eng Chem Res 52(5):2073–2083
Chen Y, Zhou XQ, Cao Y, Xue Z, Mu T (2013) Chem Phys Lett 574(22):124–128
Karadas F, Atilhan M, Aparicio S (2010) Energy Fuels 24(11):5817–5828
Heintz YJ, Sehabiague L, Morsi BI, Jones KL, Luebke DR, Pennline HW (2009) Energy Fuels 23(1):4822–4830
Wu W, Han B, Gao H, Liu Z, Jiang T, Huang J (2004) Angew Chem Int Ed 43(18):2415–2417
Cheng X, Mu T, Wang X, Guo X, Zou L (2008) J Chem Eng Data 53(12):2807–2809
Freire MG, Neves CMSS, Marrucho IM, Coutinho JOAP, Fernandes AM (2010) J Phys Chem A 114(11):3744–3749
Scammells PJ, Scott JL, Singer RD (2005) ChemInform 36(36):155–169
Chen Y, Cao Y, Shi Y, Xue Z, Mu T (2012) Ind Eng Chem Res 51(21):7418–7427
Earle MJ, Esperan JM, Gilea MA, Lopes JN, Rebelo LP, Magee JW, Seddon KR, Widegren JA (2006) Nature 439(7078):831–834
Sun H, Zhou XQ, Xue Z, Zhou ZY, Mu T (2014) Int J Greenhouse Gas Control 20(5):43–48
Glendening ED, Landis CR, Weinhold F (2012) Natural bond orbital methods. WIREs Comput Mol Sci 2:1–42. doi: 10.1002/wcms.51
Bader RFW (1985) Acc Chem Res 18(2):915
Frisch MJ, Trucks GW (1996) Progress 51(7):5556
Cao Y, Mu T (2014) Ind Eng Chem Res 53(20):8651–8664
Gordon MS, Binkley JS, Pople JA, Pietro WJ, Hehre WJ (1982) J Am Chem Soc 104(10):2797–2803
Becke AD (1988) Phys Rev A 38:3098–3100
Csonka GI, French AD, Johnson GP, Stortz CA (2009) J Chem Theory Comput 5(4):679–692
Duijneveldt FBV, Lenthe JHV (1994) Chem Rev 26(7):1873–1885
Boys SF, Bernardi F (2002) Mol Phys 19(4):553–566
Ekhtesasi MR, Sepehr A (2009) Phys Chem Chem Phys 11(41):9375–9381
Scott AP, Radom L (1996) J Phys Chem 100(41):16502–16513
Linstrom PJ, Mallard WG (eds) (2001) NIST StandardReference Database Number 69; National Institute of Standards andTechnology: Gaithersburg, MD
Yurdakul SE, Yas̨Ayan NÇ (2013) Opt Spectrosc 114(4):500–508
Popelier PLA (1998) J Phys Chem A 102(10):1873–1878
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21203250 and 21206085) and the Natural Science Foundation of Shandong Province (ZR2010BM024).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Highlights
• Thermal decomposition of [aEMMIM][BF4] was investigated experimentally and by DFT calculations.
• [aEMMIM][BF4] prefers to decompose via an SN1 rather than an SN2 mechanism.
• The chemical nature of covalent and hydrogen bonds was analyzed by VMA, NBO and AIM.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 491 kb)
Rights and permissions
About this article
Cite this article
Zhou, X., Cao, B., Liu, S. et al. Thermal reaction of the ionic liquid 1,2-dimethyl-(3-aminoethyl) imidazolium tetrafluoroborate: a kinetic and theoretical study. J Mol Model 22, 138 (2016). https://doi.org/10.1007/s00894-016-2996-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-016-2996-y