Abstract
Due to unpaired electrons, both radicals and superalkali are investigated widely. In this work, two interesting complexes (Li3O-PLY and Li3-PLY) were constructed by phenalenyl radical and superalkali atoms. Why are they interesting? Firstly, for Li3O-PLY and Li3-PLY, although the charge transfer between superalkali atoms and PLY is similar, the sandwich-like charge distribution for Li3O-PLY causes a smaller dipole moment than that of Li3-PLY. Secondly, their UV–vis absorption show that the maximum wavelengths for Li3O-PLY and Li3-PLY display a bathochromic shift compared to PLY. Moreover, Li3-PLY has two new peaks at 482 and 633 nm. Significantly, the β 0 values of Li3-PLY (4943–5691 a.u.) are much larger than that of Li3O-PLY (225–347 a.u.). Further, the β HRS values of Li3O-PLY decrease slightly while β HRS of Li3-PLY increase dramatically with increasing frequency. It is our expectation that these results might provide beneficial information for theoretical and experimental studies on complexes with superalkali and PLY radicals.

Two interesting complexes (Li3O-PLY and Li3-PLY) were constructed by phenalenyl radical and superalkali atoms. We explore their structures, Wiberg bond indices, interaction energies and the static first hyperpolarizabilities (β 0). The β 0 values of Li3-PLY (4943–5691 a.u.) were much larger than those of Li3O-PLY (225–347 a.u.).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenalenyl (PLY) radicals, considered as the building blocks for the assembly of novel molecule-based conductors and magnets, have attracted much attention from both theorists [1–5] and experimentalists [6–10]. For example, in 2006, Morita and coworkers [1] investigated the CSI-MS and NMR spectra of tri-tert-butylated biphenalenyl π-dimers for the first time. In 1999, Haddon and coworkers [6] reported the characterization of spiro-biphenalenyl radical—the first phenalenyl-based neutral radical molecular conductor. The phenalenyl radical consists of three adjacent benzene rings that share a central carbon atom with a highly symmetrical structure. (see Scheme 1)
On the other hand, superatom families have generated a great deal of theoretical [11–16] and experimental [17, 18]interest. Particularly, an important class of superatoms are superalkalis, which act as potential building blocks for nanostructural materials [19, 20]. Superalkalis have lower ionization potentials (IPs) than those (5.4–3.9 eV) of alkali atoms, which serve as stronger electron donors. In 1978, Kudo et al. [21] found the first hyperlithiated molecule—Li3O—in the equilibrium vapor over Li2O crystals. In 1979, Wu et al. [22] measured the ionization energy (IE) of Li3O as 3.54 ± 0.3 eV. In 2003, Alexandrova and Boldyrev [23] pointed out that Li3 can be viewed as a superalkali because the IP of Li3 is 4.08 ± 0.05 eV, i.e., lower than the IP of the Li atom (5.390 eV) [24]. Li3 is a special member of superalkali families; it has three valence electrons from the three Li atoms, but loses just one to form the stable Li3 + cation. Li3 + has two valence electrons, while most superalkali cations (e.g., Li3O+) do not have any available valence electrons in the Li atoms.
In this work, we employed PLY radical and superalkali atoms (Li3O and Li3) as building blocks to assemble two complexes, designated as Li3O-PLY and Li3-PLY. Although both Li3O and Li3 are superalkalis, when they bond to PLY, two different types of complexes are obtained. Frontier molecular orbital (FMO) analysis has shown that Li3-PLY has a larger diffuse electron cloud in highest-occupied molecular orbitals (HOMO; Scheme 2), which leads to larger β 0 values. Our investigation focused on the structures, bonding character, interaction energies and nonlinear optical (NLO) properties of Li3O-PLY and Li3-PLY.
Computational details
The hybrid meta exchange correlation functional (M06-2X) density function theory (DFT) method, has been used widely to optimize the geometries of PLY radical systems [25–30]. Besides, in our previous paper, the M06-2X method was chosen to optimize phenalenyl radical and azaphenalenyl radical [29]. Therefore, the geometrical structures of the molecules were obtained at the M06-2X/6-31 + G(d) level.
Furthermore, to correct the basis set superposition error (BSSEs), the counterpoise (CP) procedure was used in calculations of interaction energies at the M06-2X/6-31 + G(d) level [31, 32]. The interaction energy (E int) was calculated using the following formula:
The Wiberg bond indices (WBI) were calculated at the M06-2X/6-31 + G(d) level. To calculate first hyperpolarizabilities (β 0), choosing the proper method is very important.
Specifically, considering precision and cost, the MP2 method has been proposed as the most suitable method to calculate first hyperpolarizabilities [33–35]. In the present work, the first hyperpolarizabilities were calculated at the MP2/6-31 + G(d) level. For comparison, we also used the methods of M06-2X and CAM-B3LYP.
The static first hyperpolarizability was noted as:
Where
In this work, the M06-2X method was also employed to evaluate NBO charge.
The hyper-Rayleigh scattering (HRS) response β HRS(−2ω,ω,ω) was evaluated using the NLO Calculator program [36, 37]. The β HRS(−2ω,ω,ω) is described as:
In addition, MP2 frequency-dependent values were estimated using the multiplicative approximation given by the following equation:
All calculations were performed with Gaussian 09 W program package [38].
Results and discussion
Geometric structures and Wiberg bond index
The geometrical structures for Li-PLY, Li3O-PLY and Li3-PLY with all real frequencies are given in Fig. 1. The frequency calculations confirmed that the optimized structures were at the minimum; geometric parameters are listed in Table 1. We also calculated PLY radical, Li3O and Li3 (see Fig. 1). The geometric parameters were in good agreement with previously reported studies [17, 23]. As shown in Fig. 1, the structure of Li3O is a planar triangle. However, for Li3O-PLY, the structure of Li3O changed to a triangle cone. For Li3-PLY, the Li–Li distances were equal (2.725 Å), indicating that the structure of Li3 is an equilateral triangle. However, the structure of the Li3 monomer is that of an isosceles triangle. Hence, the bond lengths and bond angles in the complexes differ from those in monomers, which indicates that the PLY has a significant impact on the structure of Li3O and Li3.
To further understand bond character, we evaluated the WBI by using the M06-2X/6-31 + G(d) method (Fig. 2, Table 2). For Li3O-PLY, the WBI value of the Li1 atom and carbon atoms in the labeled ring was 0.1195. Obviously, the three Li atoms located at the top of the three rings in the PLY plane are in the same chemical environment. Consequently, the total WBI was 0.359. Similarly, for Li3-PLY, the total WBI was 0.440, which is larger than that of Li3O-PLY. It is noteworthy that the increase order of WBI is inversely proportional to the distance between superalkali (Li3O, Li3) and PLY. This suggests that the bond in Li3-PLY is stronger than that in Li3O-PLY.
Natural bond orbital analysis and interaction energies
Table 3 lists the NBO charges of Li3O, Li3 and PLY. Clearly, the NBO charges of Li3O (0.802) in Li3O-PLY and Li3 (0.777) in Li3-PLY are close to +1. The charges of the PLYs are close to −1, which indicates electron transfer from Li3O /Li3 to PLY.
In order to investigate the stabilities of Li3O-PLY and Li3-PLY, the interaction energy (E int) was calculated at the M06-2X/6-31 + G(d) level of theory with CP correction, and the corresponding results are presented in Table 2. Furthermore, we used the same method to calculate the interaction energy of Li-PLY. The order of E int values was Li-PLY (−49.5 kcal mol−1) < Li3-PLY (−68.3 kcal mol−1) < Li3O-PLY (−72.7 kcal mol−1), indicating that Li3-PLY and Li3O-PLY are more stable in comparison with Li-PLY. Moreover, this result may help us understand the dramatic superalkali effect on E int values.
Static first hyperpolarizability
The static first hyperpolarizability (β 0) of Li3O-PLY and Li3-PLY are given in Table 4 at the MP2, M06-2X and CAM-B3LYP level with 6-31 + G(d) basis set. As shown in Table 4, it is clear that the β 0values calculated using the four methods are very similar. The β 0 value is 0 a.u. for an isolated PLY radical due to its centrosymmetric configuration. However, by doping Li3O and Li3 into PLY radical, we finally obtained two complexes (Li3O-PLY and Li3-PLY) without centrosymmetry. As we can see from Table 4, the β 0 values of Li3-PLY (4943–5691 a.u.) are much larger than the value of Li3O-PLY (225–347 a.u.). Interestingly, although two complexes are both constructed by PLY radical and superalkali atoms, the β 0 value of Li3-PLY is dramatically larger than that of Li3O-PLY; why?
To further understand the origin of the β 0 values, we consider the widely used two-level model [39, 40]:
Where ΔE, f 0 and Δμ are the transition energy, oscillator strength and difference in the dipole moments between the ground state and the crucial excited state, respectively. According to the above expression, β 0 is proportional to Δμ and f 0 but inversely proportional to ΔE [11–16]. The physical quantities in the two-level model may be helpful to qualitatively understand the variation in β 0 values.
In this work, the transition energies (ΔE) for Li3O-PLY and Li3-PLY were estimated by time-dependent (TD) M06-2X/6-31 + G(d) and listed in Table 4. The order of ΔE values is Li3O-PLY (3.28 eV) > Li3-PLY (1.95 eV). To explain the smaller ΔE value of Li3-PLY, we examined the HOMOs and related unoccupied molecule orbitals in Fig. 3. Clearly, Li3-PLY has a larger diffuse electron cloud in HOMO than that of Li3O-PLY, which makes transition easier. Therefore, the ΔE value for Li3-PLY is smaller than the value for Li3O-PLY, which leads to a larger β 0 value.
Furthermore, to gain insight into the detailed fragment contributions to the HOMOs of Li3O-PLY and Li3-PLY, we used the AOMix program [41, 42]. From Table 5, the HOMO of Li3O-PLY is contributed mainly by PLY radical, whereas the HOMO of Li3-PLY is contributed mainly by Li3, which is in accordance with the HOMOs in Fig. 3. Hence, the larger contribution of Li3 might decrease the ΔE value, whereas the larger contribution of PLY radical might increase the ΔE value. This also explains why the ΔE value of Li3-PLY is smaller than that of Li3O-PLY.
Absorption spectrum and frequency-dependent NLO properties
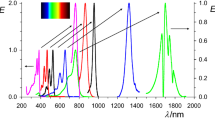
The absorption spectra of PLY, Li3O-PLY and Li3-PLY were simulated according to TD-M06-2X/6-31 + G(d) level and the results are plotted in Fig. 4. PLY, Li3O-PLY and Li3-PLY have obvious absorption peaks within the visible region. Moreover, for the maximum wavelengths, Li3O-PLY (λ max = 378 nm) and Li3-PLY (λ max = 376 nm) display slightly bathochromic shift, compared to PLY (λ max = 323 nm). Interestingly, the Li3-PLY has two new peaks at 482 and 633 nm. To further explore the new peaks, transition energies (ΔE), oscillator strengths (f 0), dominant excitation, and the configuration interaction of Li3-PLYare listed in Table 6 and the related FMO diagrams are shown in Fig. 4. At 482 nm, the transition of Li3-PLY occurs inside Li3. However, at 633 nm, the transition is from Li3 to PLY radical. Specially, the lowest energy absorption of Li3-PLY is red-shifted to 633 nm. It may be a critically important factor that Li3-PLY has the largest β value.
Further, in order to supply more useful information for experimentalists, the HRS response β HRS(−2ω,ω,ω) was evaluated using the NLO Calculator program and coupled perturbed Hartree-Fock (CPHF) theory. In accordance with the method of calculating the static β values, we used Eq. (5) to estimate the MP2 frequency-dependent β values; the results are listed in Table 7. In accordance with the maximum absorption wavelengths of the systems, the frequency dispersion at wavelengths of 1064 nm (ω = 0.0428 au), 1340 nm (ω =0.0340 au), 1460 nm (ω =0.0312 au) and 1907 nm (ω =0.0239 au) were investigated. As shown in Tables 4 and 7, the static and dynamic β HRS(−2ω,ω,ω) values change in the same order as the β 0 values (Li3O-PLY < Li3-PLY). We also found that the frequency-dependent effect was more obvious for Li3-PLY than for Li3O-PLY. Interestingly, for Li3-PLY, there are two extremely large β HRS(−2ω,ω,ω) values (94,874 and 94,537 a.u.) at 1340 and 1046 nm. To explain this question, let us focus on the absorption spectra (Fig. 4): there are two strong peaks at 483 and 633 nm. As a result, the two-photon resonance of Li3-PLY is 966 and 1266 nm, which is close to 1064 and 1340 nm. It is possible that the two-photon resonance is the main reason for the very large β HRS(−2ω,ω,ω) values (94,874 and 94,537 a.u.) of Li3-PLY.
Conclusions
In the present work, we employed the PLY radical and superalkali atoms (Li3O and Li3) as building blocks to assemble two novel complexes, designated as Li3O-PLY and Li3-PLY. Our investigation focused on the structures, interaction energies, WBI and NLO properties of the two complexes. The key points of this work can be summarized as follows:
-
(1)
The interaction energies show that Li3O-PLY is more stable than Li3-PLY. Moreover, Li3-PLY and Li3O-PLY are both more stable in comparison with Li-PLY. This result may help us understand the dramatic superalkali effect on E int values.
-
(2)
The bonding characters were investigated by NBO analysis and WBI. The results demonstrate that the bond in Li3-PLY is stronger than the bond in Li3O-PLY.
-
(3)
Li3-PLY has a larger diffuse electron cloud in HOMO than that of Li3O-PLY, which makes transition easier. Therefore, the ΔE value for Li3-PLY is smaller than the value for Li3O-PLY, which leads to larger β 0 values. The order of the β 0 values is Li3O-PLY(225–347 a.u.) < Li3-PLY(4943–5691 a.u.).
-
(4)
The UV–vis absorption show that the maximum wavelengths for Li3O-PLY (λ max = 378 nm) and Li3-PLY (λ max = 376 nm) display a slight bathochromic shift compared to PLY (λ max = 323 nm).
We expect that this work could provide guidance for theoretical and experimental scientists attempting to design novel NLO materials with PLY and superalkali.
References
Suzuki S, Morita Y, Fukui K, Sato K, Shiomi D, Takui T, Nakasuji K (2006) Aromaticity on the pancake-bonded dimer of neutral phenalenyl radical as studied by MS and NMR spectroscopies and NICS analysis. J Am Chem Soc 128:2530
Huang J, Kertesz M (2007) Intermolecular covalent π−π bonding interaction indicated by bond distances, energy bands, and magnetism in biphenalenyl biradicaloid molecular crystal. J Am Chem Soc 129:1634
Craciun S, Donald KJ (2009) Radical bonding: structure and stability of bis(phenalenyl) complexes of divalent metals from across the periodic table. Inorg Chem 48:5810
Small D, Rosokha SV, Kochi JK, Head-Gordon M (2005) Characterizing the dimerizations of phenalenyl radicals by ab initio calculations and spectroscopy: σ-bond formation versus resonance π-stabilization. J Phys Chem A 109:11261
Nakano M, Takebe A, Kishi R, Fukui H, Minami T, Kubota K, Takahashi H, Kubo T, Kamada K, Ohta K, Champagne B, Botek E (2008) Intermolecular interaction effects on the second hyperpolarizability of open-shell singlet diphenalenyl radical dimer. Chem Phys Lett 454:97
Chi X, Itkis ME, Patrick BO, Barclay TM, Reed RW, Oakley RT, Cordes AW, Haddon RC (1999) The first phenalenyl-based neutral radical molecular conductor. J Am Chem Soc 121:10395
Itkis M, Chi X, Cordes A, Haddon R (2002) Magneto-opto-electronic bistability in a phenalenyl-based neutral radical. Science 296:1443
Cyrański MK, Havenith RWA, Dobrowolski MA, Gray BR, Krygowski TM, Fowler PW, Jenneskens LW (2007) The phenalenyl motif: a magnetic chameleon. Chem Eur J 13:2201
Ueda A, Wasa H, Suzuki S, Okada K, Sato K, Takui T, Morita Y (2012) Chiral stable phenalenyl radical: synthesis, electronic-spin structure, and optical properties of [4]helicene-structured diazaphenalenyl. Angew Chem-Int Edit 51:6691
Sarkar A, Itkis ME, Tham FS, Haddon RC (2011) Synthesis, structure, and physical properties of a partial π-stacked phenalenyl-based neutral radical molecular conductor. Chem Eur J 17:11576
Li Z-R, Wang F-F, Wu D, Li Y, Chen W, Sun X-Y, Gu FL, Aoki Y (2006) Royal crown-shaped electride Li3-N3-Be containing two superatoms: new knowledge on aromaticity. J Comput Chem 27:986
Wang B-Q, Li Z-R, Wu D, Wang F-F (2007) Structures and static electric properties of novel alkalide anions F-Li+Li- and F−Li3 +Li3. J Phys Chem A 111:6378
Tong J, Li Y, Wu D, Wu Z-J (2012) Theoretical study on polynuclear superalkali cations with various functional groups as the central core. Inorg Chem 51:6081
Tong J, Wu Z, Li Y, Wu D (2013) Prediction and characterization of novel polynuclear superalkali cations. Dalton T 42:577
Cochran E, Meloni G (2014) Hypervalence in monoxides and dioxides of superalkali clusters. J Chem Phys 140:204–319
Sun W-M, Fan L-T, Li Y, Liu J-Y, Wu D, Li Z-R (2014) On the potential application of superalkali clusters in designing novel alkalides with large nonlinear optical properties. Inorg Chem 53:6170
Yokoyama K, Tanaka H, Kudo H (2001) Structure of hyperlithiated Li3O and evidence for electronomers. J Phys Chem A 105:4312
Đustebek J, Veličković S, Veljković F, Veljković M (2012) Production of heterogeneous superalkali clusters LinF (n = 2–6) by knudesn-cell mass spectrometry. Dig J Nano Mater Bios 7:1365
Li Y, Wu D, Li Z-R (2008) Compounds of superatom clusters: preferred structures and significant nonlinear optical properties of the BLi6-X (X = F, LiF2, BeF3, BF4) motifs. Inorg Chem 47:9773
Tong J, Li Y, Wu D, Li Z-R, Huang X-R (2011) Ab initio investigation on a new class of binuclear superalkali cations M2Li2k+1+ (F2Li3 +, O2Li5 +, N2Li7 +, and C2Li9 +). J Phys Chem A 115:2041
Kudo H, Wu CH, Ihle HR (1978) Mass-spectrometric study of the vaporization of Li2O(s) and thermochemistry of gaseous LiO, Li2O, Li3O, and Li2O2. J Nucl Mater, J 78:380
Wu CH, Kudoa H, Ihle HR (1979) Thermochemical properties of gaseous Li3O and Li2O2. J Chem Phys 70:1815
Alexandrova AN, Boldyrev AI (2003) σ-Aromaticity and σ-antiaromaticity in alkali metal and alkaline earth metal small clusters. J Phys Chem A 107:554
Karplus M, Porter RN (1970) Atoms and molecules; an introduction for students of physical chemistry. Benjamin, New York
Zhong R-L, Zhang J, Muhammad S, Hu Y-Y, Xu H-L, Su Z-M (2011) Boron/nitrogen substitution of the central carbon atoms of the biphenalenyl diradical π dimer: a novel 2e–12c bond and large NLO responses. Chem Eur J 17:11773
Tian Y-H, Huang J, Kertesz M (2010) Fluxional [sigma]-bonds of 2,5,8-tri-tert-butyl-1,3-diazaphenalenyl dimers: stepwise [3,3], [5,5] and [7,7] sigmatropic rearrangements via [small pi]-dimer intermediates. Phys Chem Chem Phys 12:5084
Tian Y-H, Kertesz M (2010) Is there a lower limit to the CC bonding distances in neutral radical π-dimers? The case of phenalenyl derivatives. J Am Chem Soc 132:10648
Sini G, Sears JS, Brédas J-L (2011) Evaluating the performance of DFT functionals in assessing the interaction energy and ground-state charge transfer of donor/acceptor complexes:tetrathiafulvalene − tetracyanoquinodimethane (TTF − TCNQ) as a model case. J Chem Theory Comput 7:602
Zhong R-L, Xu H-L, Sun S-L, Qiu Y-Q, Zhao L, Su Z-M (2013) Theoretical investigation on the 2e/12c bond and second hyperpolarizability of azaphenalenyl radical dimers: strength and effect of dimerization. J Chem Phys 139, 124314
Chen S, Sun S-L, Wu H-Q, Xu H-L, Zhao L, Su Z-M (2014) Superatoms (Li3O and BeF3) induce phenalenyl radicalπ–dimer: fascinating interlayer charge-transfer and large NLO responses. Dalton T 43:12657
Alkorta I, Elguero J (1998) Theoretical study of strong hydrogen bonds between neutral molecules: the case of amine oxides and phosphine oxides as hydrogen bond acceptors. J Phys Chem A 103:272–279
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Chen W, Li Z-R, Wu D, Li Y, Sun C-C, Gu FL (2005) The structure and the large nonlinear optical properties of Li@Calix[4]pyrrole. J Am Chem Soc 127:10977–10981
Xu H-L, Li Z-R, Wu D, Wang B-Q, Li Y, Gu FL, Aoki Y (2007) Structures and large NLO responses of new electrides: Li-doped fluorocarbon chain. J Am Chem Soc 129:2967
Muhammad S, Xu H, Liao Y, Kan Y, Su Z (2009) Quantum mechanical design and structure of the Li@B10H14 basket with a remarkably enhanced electro-optical response. J Am Chem Soc 131:11833
Dongdong Qi. NLO Calculator, Version 0.2. University of Science and Technology Beijing, Beijing 100083, China
Zhang L, Qi D, Zhao L, Chen C, Bian Y, Li W (2012) Density functional theory study on subtriazaporphyrin derivatives: dipolar/octupolar contribution to the second order nonlinear optical activity. J Phys Chem A 116:10249
Frisch MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09, revision A.02; Gaussian, Inc. Wallingford, CT
Oudar JL, Chemla DS (1977) Hyperpolarizabilities of the nitroanilines and their relations to the excited state dipole moment. J Chem Phys 66:2664
Oudar JL (1977) Optical nonlinearities of conjugated molecules. Stilbene derivatives and highly polar aromatic compounds. J Chem Phys 67:446
Gorelsky SI (2009) AOMix version 6.46: program for molecular orbital analysis, University of Ottawa. Available at: http://www.sg-chem.net/
Gorelsky SI, Lever ABP (2001) J Organomet Chem 635:187
Acknowledgments
The authors gratefully acknowledge the financial support from National Science Foundation of China (NSFC) (21003019, 21473026), the Science and Technology Development Planning of Jilin Province (201201062 and 20140101046JC), the Computing Center of Jilin Province provided essential support and H.-L.X. acknowledges support from the Hong Kong Scholars Program And Project funded by the China Postdoctoral Science Foundation (2014 M560227).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, S., Xu, HL., Sun, SL. et al. Superalkali atoms bonding to the phenalenyl radical: structures, intermolecular interaction and nonlinear optical properties. J Mol Model 21, 209 (2015). https://doi.org/10.1007/s00894-015-2750-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2750-x