Abstract
Accumulated evidence has shown that endocan, which was originally called endothelial cell-specific molecule-1, is an attractive prognostic factor in a variety of cancers. However, the relevance of endocan expression in human malignancies remains to be clarified. In the present study, the expression of endocan in cervical squamous neoplasia of the uterus, including low- and high-grade squamous intraepithelial lesions (LSIL and HSIL, respectively), as well as in invasive squamous cell carcinoma was examined by immunohistochemistry. Endocan was not sufficiently expressed in the normal cervical epithelium. Endocan expression was present in LSIL cases but was limited to basal and parabasal areas of the cells. HSIL cases exhibited strong expression of endocan with widely distributed expression toward the epithelial surface. In contrast, further strong expression of endocan was not observed in patients with invasive carcinoma. This study is the first study showing increased expression of endocan in precancerous dysplastic lesions and malignancy of the cervix. The data suggest that a high expression level of endocan potentially contributes to the development of cervical squamous neoplasia of the uterus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endocan, which was originally called endothelial cell-specific molecule-1 (encoded by the ESM1 gene), is a dermatan sulphate proteoglycan that was first cloned from a cDNA library of human umbilical vein endothelial cells [1]. In a physiological state, endocan is mainly secreted by endothelial cells in the lung and kidney [1, 2]. Recently, published studies have shown that endocan is expressed in various tissues, including the liver, gastrointestinal tract, and skin [2]. This protein is also synthesized by activated vascular endothelial cells in tumors [3]. In addition, endocan is aberrantly expressed and has been shown to be associated with a variety of diseases, including inflammation, vascular disorders, and different types of cancer [4,5,6,7]. Accumulating evidence has revealed that endocan promotes tumor formation and that deregulated expression of endocan is associated with aggressive tumor progression and poor prognosis [6,7,8]. Several recent studies have demonstrated that endocan expression is increased at the mRNA and/or protein levels in different types of tumors, including non-small cell lung cancer, breast cancer, bladder cancer, renal cell cancer, gastric and colorectal cancer, and ovarian cancer [6,7,8,9]. However, the relevance of elevated expression of endocan in cervical squamous neoplasia of the uterus remains to be clarified.
Retinoic acid (RA), an active metabolite of vitamin A, is a critical signaling molecule involved in the differentiation, proliferation, and apoptosis of a wide variety of cell types [10,11,12]. Our previous studies demonstrated that the RA-metabolizing enzyme CYP26A1 (cytochrome P450, family 26, subfamily A, polypeptide 1) promotes the survival and oncogenic potential of breast carcinoma cells, indicating a possible oncogenic function of CYP26A1 in breast carcinogenesis [13,14,15]. Consistent with these findings, enhanced RA metabolism and elevated CYP26A1 expression levels have been observed in various types of cancer [13, 16,17,18,19,20]. In a series of our published experiments, we performed high-resolution oligonucleotide-based microarray analyses on CYP26A1-overexpressing cancer cells [13]. Candidate genes that drive the cells into an oncogenic state were identified in those studies. Of these, we focused on endocan as a potential downstream target of CYP26A1 in the current study.
In the present study, the possible association between endocan expression and development of cervical squamous neoplasia of the uterus was examined by immunohistochemistry. Endocan expression was examined in the non-neoplastic (normal) cervical epithelium, precancerous cervical dysplastic lesions, including low- and high-grade squamous intraepithelial lesions (LSIL and HSIL, respectively), and in invasive squamous cell carcinoma (SCC).
Materials and methods
Patients and specimens
To examine the expression of endocan in cervical neoplasia of the uterus, 90 cases of archived formalin-fixed, paraffin-embedded tissue specimens prepared from surgically resected materials or biopsy samples were studied (Supplementary Table). Tissue specimens collected in Sapporo Medical University Hospital (Sapporo, Japan) during surgical resection or biopsy from 2018 to 2020 were used in the present study. Two independent board-certified surgical pathologists evaluated hematoxylin-eosin (H&E)-stained slides of all specimens and examined endocan expression by immunohistochemistry. Cervical neoplasia of the uterus was classified according to histological types using the World Health Organization guidelines [21].
The present study was approved by the Ethics Committee (approval no. 4-1-44) and Institutional Review Board (study no. 312-230) of Sapporo Medical University (Sapporo, Japan). The Ethics Committee waived the requirement to obtain written informed consent from the patients for the use of human tissues owing to the retrospective nature of the study. The research was performed in accordance with the Declaration of Helsinki. The researchers involved in this study had no access to information that could identify individual participants during or after data collection.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue specimens were cut at 5 µm in thickness. Tissue sections were then deparaffinized in xylene, rehydrated through a graded series of ethanol and phosphate-buffered saline, and incubated in 3% H2O2 for 10 min to block endogenous peroxidase activity. After antigen retrieval by microwave heating (500 W, 95 ℃ for 15 min) in citrate buffer, the sections were incubated overnight at 4 ℃ with a primary monoclonal antibody against endocan (1:200, ab22491, abcam, Cambridge, UK). The expression of CYP26A1 (1:50, clone F27 P6 A1, sc-53618, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and expression of Ki-67 (1:100, clone SP6, ab16667, abcam) were also evaluated. The sections were then incubated with EnVision (Dako, Glostrup, Denmark) for 30 min at room temperature, and color was developed using 3,3'-diaminobenzidine tetrachloride (Sigma, St. Louis, MO, USA) as the chromogen. The slides were subsequently counterstained with Meyer’s hematoxylin. Appropriate positive and negative controls were used in each experiment, and the results were confirmed by independent duplicate assays.
Staining positivity for endocan in samples was quantitatively analyzed with consideration of both the staining intensity and the percentage of positive cells. A score was assigned on the basis of the percentage of positively stained tumor cells (proportion score) as follows: 10, staining in 100% of the cells; 9, staining in 99–90% of the cells; 8, staining in 89–80% of the cells; 7, staining in 79–70% of the cells; 6, staining in 69–60% of the cells; 5, staining in 59–50% of the cells; 4, staining in 49–40% of the cells; 3, staining in 39–30% of the cells; 2, staining in 29–20% of the cells; 1, staining in 19–10% of the cells, and 0, no or faint staining in < 9% of the cells. Another score was determined on the basis of the immunoreactivity intensity (intensity score) as follows: 3+ , strong; 2+ , moderate; 1+ , weak, and 0, negative. The immunoreactivity score of endocan was obtained by multiplication of the proportion and intensity scores.
Due to the heterogeneous staining pattern of endocan in cervical tumors, especially in non-invasive neoplasia of the uterine cervix, the epithelium was equally divided into three layers: one-third of the basal epithelium, middle one-third of the epithelium, and one-third of the surface epithelium. In invasive malignancy, three representative areas from tumors were arbitrary selected. The immunoreactivity score in each given layer or area of interest was determined and the final score was expressed as their average values.
Based on our previous study [13], positive staining of CYP26A1 was graded as percentage of tumor cells stained: 3+ (strong), > 50% positive cells; 2+ (moderate), 25–49% positive cells; 1+ (weak), 5–24% positive cells, and 0 (negative), no stained cells or < 5% positive cells. CYP26A1 expression was considered positive when the staining grade was ≥ 2+ and was considered negative when the staining grade was ≤ 1+ . For evaluation of Ki-67, the cells of interest were scored under a light microscope by counting the number of positive cells under low magnification (× 100) using Patholoscope software (Ver. 1.40, Mitani, Tokyo, Japan). The ratio (%) of Ki-67-positive cells in total tumor cells in the field of interest was expressed as Ki-67-labeling index. Finally, Ki-67 expression was considered positive or negative by the median value of total cases.
The observers were blinded to the clinical data during the evaluation. Consensus was reached by discussion of discordant cases.
Statistical analysis
All data were obtained from independent duplicate analyses and are presented as mean values. All data from each category were analyzed using unpaired two-tailed Student’s t test or Fisher’s exact test for two groups and the Kruskal–Wallis test for three or more groups to determine significance. Data analysis was carried out using EZR software (Ver. 1.27, Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [22]. A P value of < 0.05 was considered statistically significant.
Results
Expression of endocan in cervical neoplasia of the uterus
Previous studies showed that endocan is highly expressed in different types of cancer [6,7,8]. Thus, the expression of endocan in neoplasia of the uterine cervix was examined by immunohistochemistry (Fig. 1).
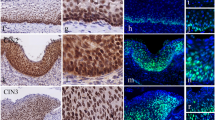
Endocan expression in cervical squamous neoplasia of the uterus. Representative images of hematoxylin–eosin (H&E) staining (A, C, F, I, and L) and immunohistochemistry of endocan (B, D, E, G, H, J, K, and M). Endocan expression was evaluated in the non-neoplastic (normal) squamous epithelium (A and B), in low-grade squamous intraepithelial lesion (LSIL) cases, also known as cervical intraepithelial neoplasia (CIN), such as CIN1 (mild squamous dysplasia; C, D, and E), in high-grade squamous intraepithelial lesion (HSIL) cases, such as CIN2 (moderate squamous dysplasia; F, G, and H) and CIN3 (severe squamous dysplasia; I, J, and K), and in invasive squamous cell carcinoma (L and M)
Endocan expression was negative or did not show strong positivity in non-neoplastic (normal) cervical tissues (Fig. 1A, B). Elevated expression of endocan was observed in squamous intraepithelial lesions, also known as cervical intraepithelial neoplasia (CIN). Endocan expression was present in CIN1, which shows mild squamous dysplasia, but it was restricted to the basal and parabasal areas of the cells (Fig. 1C, D, E). Endocan was strongly expressed in CIN2 (Fig. 1F, G, H), which shows moderate squamous dysplasia, and CIN3 (Fig. 1I, J, K), which shows severe squamous dysplasia and includes squamous cell carcinoma in situ, particularly in dysplasia with atypical cells occupying more than two-third of the epithelium thickness. In such cases, endocan-positive signals were detected in proliferating dysplastic cells. Lesions with marked histological atypia and dysplastic changes showed stronger cytoplasmic expression of endocan and a wide distribution of positive signals toward the epithelial surface.
Unexpectedly, endocan expression was not further increased in cervical malignancies such as invasive SCC. There were even some cases of invasive carcinoma that were negative for nuclear expression of endocan (3/12 cases, 25%) (Fig. 1L, M), while there was no case that was negative for endocan expression in non-invasive dysplasia such as LSIL and HSIL cases (Supplementary Table).
Quantification of endocan expression in cervical neoplasia of the uterus
Quantitative analyses of endocan expression by evaluating both the intensity and proportion scores were also performed. Positive signals of endocan were observed in cervical dysplasia, and the epithelium with dysplastic changes showed more positive areas and signals (Fig. 2).
Expression status of endocan in cervical squamous neoplasia of the uterus. A Quantitative analysis of nuclear and cytoplasmic endocan expression in non-neoplastic (normal) epithelium, squamous intraepithelial neoplasia, also known as cervical intraepithelial neoplasia (CIN), such as CIN1 (mild squamous dysplasia), CIN2 (moderate squamous dysplasia), and CIN3 (severe squamous dysplasia), as well as in invasive squamous cell carcinoma of the uterine cervix. B Quantitative analysis of nuclear and cytoplasmic endocan expression in low- and high-grade squamous intraepithelial lesions (LSIL and HSIL, respectively) of the uterine cervix. Differential expression of endocan in cervical neoplasias. Expression of nuclear and cytoplasmic endocan with CYP26A1 (C) or Ki-67 (D)-positive and -negative cervical neoplasia. The numbers within the bars represent the total number of cases. E CYP26A1 expression in cervical squamous neoplasia of the uterus. Representative images of immunohistochemistry of CYP26A1. The case in each category corresponds to the case that is presented in Fig. 1. a P < 0.05 vs normal epithelium; b P < 0.05 vs CIN1; c P < 0.05 vs CIN2; d P < 0.05 vs nuclear endocan, e P < 0.05 vs LSIL
Cases of non-invasive cervical neoplasias are divided into LSIL cases, which include CIN1, and HSIL cases, which include CIN2 and CIN3 [21]. LSIL cases showed significantly increased expression of endocan both in the nucleus and cytoplasm (Fig. 2A). HSIL cases with a higher degree of histological atypia were significantly highlighted by positive signals compared to LSIL cases (Fig. 2A, B). Although various mixed patterns of nuclear and cytoplasmic staining were observed in LSIL and HSIL cases, HSIL cases showed a significantly higher cytoplasmic expression level of endocan and causally showed a higher cytoplasmic/nuclear ratio, especially in CIN3 samples (Fig. 2B). Interestingly, invasive carcinoma did not show a further increase of endocan expression when compared to CIN3 cases and preferentially lost nuclear expression of endocan (Fig. 2A).
Importantly, endocan expression in both the nucleus and cytoplasm was positively associated with CYP26A1 expression status (Fig. 2C). In addition, cytoplasmic expression of endocan was significantly associated with higher Ki-67 labeling of tumor cells (Fig. 2D). These observations are consistent with the results of our previous studies showing that CYP26A1 expression was elevated in precancerous dysplastic lesions of the uterine cervix (Fig. 2E) and that CYP26A1 expression was associated with an aggressive nature of the tumor [13,14,15].
Discussion
In the present study, we found that endocan is highly expressed in cervical squamous neoplasia of the uterus. Interestingly, no further elevated expression of endocan was observed in patients with invasive SCC. The findings suggest that dysregulated expression of endocan potentially contributes to cervical squamous neoplasia in an early phase during cervical carcinogenesis.
Our immunohistochemical analysis showed an interesting cellular localization pattern of endocan in cervical neoplasia with varying nuclear and cytoplasmic expression. The effects of nuclear endocan and cytoplasmic endocan in cancer remain unknown, whereas the altered location of endocan is potentially associated with the regulation of unidentified signaling pathways. While both nuclear endocan and cytoplasmic endocan might be involved in cervical carcinogenesis, cytoplasmic endocan seems to exert more distinct functions than nuclear endocan in patients with cervical squamous neoplasia does. Based on the progressively increasing expression of endocan in cervical squamous neoplasia and the strong cytoplasmic expression of endocan in HSIL and SCC cases, we speculated that cytoplasmic localization of endocan strongly promotes the development and/or progression of squamous neoplasia of the uterine cervix.
There are published studies showing that a high expression level of endocan exerts different functions during carcinogenesis in various tumors [2, 3, 6, 7]. For example, endocan has been shown to be associated with a variety of molecules that are deeply involved in oncogenic signaling in numerous types of cancer, including nuclear factor-kappa B, extracellular signal-related kinase 1, glycogen synthase kinase, and Akt1. In addition, it has been suggested that endocan expression promotes cell survival, cell cycle progression, migration, invasion, and epithelial–mesenchymal transition. In contrast, suppression of endocan expression and activity significantly inhibited these effects. Since the present study did not include in vitro data, further studies using cultured cells are needed to clarify the functional impacts of endocan on tumor cells.
It is well known that chronic infection with human papilloma virus (HPV), especially the high-risk variants HPV16 and 18, is the single most important etiologic factor in the pathogenesis of cervical carcinoma and its precursors [21, 23]. Interaction between HPV-encoded oncoproteins and p53 leads to rapid degradation of p53, which abrogates apoptosis and deregulates cell cycle progression. Consistent with the fact that p53 mutation was reported to be a rare event in cervical cancer [23], we found that p53 immunoreactivity was absent in the majority of cases of cervical neoplasia. In addition, we did not observe strong p16 immunoreactivity, which is an appropriate surrogate marker of HPV infection, in particular sets of cervical neoplasia. Furthermore, a positive correlation between p16 and endocan expression was absent. Therefore, it is likely that the lack of deregulated signaling of p53 and p16 and endocan expression may have independent effects on the development of cervical neoplasia. In support of this possibility, there are no published data showing increased expression of endocan in other HPV-associated malignancies such as head and neck SCC.
Although our results clearly demonstrated elevated expression levels of endocan, the underlying molecular mechanism in cervical neoplasia remains to be examined. Because of the significance of transcriptional complexity in the regulatory mechanism of endocan, dysregulated signaling resulting from a multifactorial process involving various genetic alterations in carcinogenesis of the uterine cervix might offer a possible explanation for the molecular mechanism. In addition, endocan expression may be regulated by the activity of different types of nuclear transcription factors that have yet to be identified as endocan regulators. In corollary, it is possible that the signaling pathway involving endocan expression is modulated by the cellular RA bioavailability due to a positive correlation between endocan and CYP26A1 expression status in cervical tumors.
Our published data obtained by oligonucleotide-based microarray analyses suggested that endocan is a potential downstream target of CYP26A1 [13]. Consistently, the present study clearly showed that cervical neoplasia, particularly endocan-expressing tumors, expressed elevated levels of CYP26A1. The data indicate a possible positive correlation between endocan and CYP26A1 expression. Since CYP26A1 has been shown to exert oncogenic functions, it is plausible for endocan expression to be potentially involved in the development and/or progression of cervical neoplasia [13,14,15,16,17,18,19,20]. Nevertheless, it is difficult to conclude that CYP26A1 and RA status of the individual given cells directly regulated the expression of endocan and that endocan directly mediated the effect of CYP26A1. Bioinformatics data also revealed the absence of an RA responsive element located upstream of the ESM1 gene. Given that we showed a positive correlation between endocan and CYP26A1 expression status, the possibility that the endocan-mediated oncogenic effects are governed by a regulatory mechanism that is independent of CYP26A1 cannot be excluded.
A limitation of the present study is that the oncological impact of endocan expression on prognosis was not included because of the non-invasive nature of cervical squamous intraepithelial neoplasia. Indeed, we evaluated 12 cases of invasive SCC, based on the results of our preliminary experiments showing that invasive cervical malignancy did not sufficiently express endocan in higher frequency. Similarly, we could not include cases of other histological types of cervical tumor such as glandular neoplasms and their precursors, i.e., adenocarcinoma and adenocarcinoma in situ. However, the Kaplan–Meier plotter demonstrated that cervical malignancy with elevated expression levels of endocan is significantly associated with poor overall survival (P < 0.001) [24]. The human protein atlas provides similar results (P < 0.001; Human Protein Atlas proteinatlas.org). Since our research suggested that endocan is involved in cervical squamous neoplasia, further study on endocan expression in invasive cervical neoplasms using a large set of invasive carcinoma samples should be carried out to obtain an understanding of the fundamental biological nature of endocan.
To our knowledge, this study is the first study showing elevated expression of endocan in cervical tumors and suggesting a potential role of endocan in the development of cervical squamous neoplasia. Therefore, endocan upregulation might be an unrecognized mechanism of the development and/or progression of squamous neoplasia of the uterine cervix.
Availability of data and materials
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
Lassalle P, Molet S, Janin A, Heyden JV, Tavernier J, Fiers W, Devos R, Tonnel AB (1996) ESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokines. J Biol Chem 271:20458–20464
Zhang SM, Zuo L, Zhou Q, Gui SY, Shi R, Wu Q, Wei W, Wang Y (2012) Expression and distribution of endocan in human tissues. Biotech Histochem 87:172–178
Sarrazin S, Adam E, Lyon M, Depontieu F, Motte V, Landolfi C, Lortat-Jacob H, Bechard D, Lassalle P, Delehedde M (2006) Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy. Biochim Biophys Acta 1765:25–37
Öztop N, Özer PK, Demir S, Beyaz S, Tiryaki TO, Özkan G, Aydogan M, Bugra MZZ, Çolakoglu B, Büyüköztürkn S, Nalçacı M, Yavuz AS, Gelincik A (2021) Impaired endothelial function irrespective of systemic inflammation or atherosclerosis in mastocytosis. Ann Allergy Asthma Immunol 127:76–82
Rocha SF, Schiller M, Jing D, Li H, Butz S, Vestweber D, Biljes D, Drexler HCA, Nieminen-Kelhä M, Vajkoczy P, Adams S, Benedito R, Adams RH (2014) sm1 modulates endothelial tip cell behavior and vascular permeability by enhancing VEGF bioavailability. Circ Res 115:581–590
Lu J, Liu Q, Zhu L, Liu Y, Zhu X, Peng S, Chen M, Li P (2022) Endothelial cell-specific molecule 1 drives cervical cancer progression. Cell Death Dis 13:1043
Yang J, Yang Q, Yu S, Zhang X (2005) Endocan: a new marker for cancer and a target for cancer therapy. Biomed Rep 3:279–283
Cui Y, Guo W, Li Y, Shi J, Ma S, Guan F (2021) Pan-cancer analysis identifies ESM1 as a novel oncogene for esophageal cancer. Esophagus 18:326–338
Kano K, Sakamaki K, Oue N, Kimura Y, Hashimoto I, Hara K, Maezawa Y, Aoyama T, Fujikawa H, Hiroshima Y, Yamada T, Tamagawa H, Yamamoto N, Ogata T, Cho H, Ito H, Shiozawa M, Yukawa N, Yoshikawa T, Morinaga S, Rino Y, Yasui W, Masuda M, Miyagi Y, Oshima T (2020) ESM-1 gene expression on outcomes in stage II/III gastric cancer patients who received adjuvant S-1 chemotherapy. Vivo 34:461–467
Durston AJ, Timmermans JP, Hage WJ, Hendriks HF, de Vries NJ, Heideveld M, Nieuwkoop PD (1989) Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature 340:140–144
Means A, Gudas LJ (1995) The roles of retinoids in vertebrate development. Annu Rev Biochem 64:210–233
Petkovich M, Brand NJ, Krust A, Chambon P (1987) A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature 330:444–450
Osanai M, Sawada N, Lee GH (2010) Oncogenic and cell survival properties of the retinoic acid metabolizing enzyme, CYP26A1. Oncogene 29:1135–1144
Osanai M (2017) Cellular retinoic acid bioavailability in various pathologies and its therapeutic implication. Pathol Int 67:281–289
Osanai M, Takasawa A, Takasawa K, Kyuno D, Ono Y, Magara K (2023) Retinoic acid metabolism in cancer: potential feasibility of retinoic acid metabolism blocking therapy. Med Mol Morphol 56:1–10
Van heusden J, Wouters W, Ramaekers FC, Krekels MD, Dillen L, Borgers M, Smets G, (1998) The antiproliferative activity of all-trans-retinoic acid catabolites and isomers is differentially modulated by liarozole-fumarate in MCF-7 human breast cancer cells. Br J Cancer 77:1229–1235
Sonneveld E, van den Brink CE, van der Leede BM, Schulkes RK, Petkovich M, van der Burg B, van der Saag PT (1998) Human retinoic acid (RA) 4-hydroxylase (CYP26) is highly specific for all-trans-RA and can be induced through RA receptors in human breast and colon carcinoma cells. Cell Growth Diff 9:629–637
Iaassen I, Brakenhoff RH, Smeets SJ, Snow GB, Braakhuis BJ (2001) Metabolism and growth inhibition of four retinoids in head and neck squamous normal and malignant cells. Br J Cancer 85:630–635
Ozpolat B, Mehta K, Tari AM, Tari AM, Lopez-Berestein G (2002) All-trans-retinoic acid-induced expression and regulation of retinoic acid 4-hydroxylase (CYP26) in human promyelocytic leukemia. Am J Hematol 70:39–47
Shelton DN, Sandoval IT, Eisinger A, Chidester S, Ratnayake A, Ireland CM, Jones DA (2006) Up-regulation of CYP26A1 in adenomatous polyposis coli-deficient vertebrates via a WNT-dependent mechanism: implications for intestinal cell differentiation and colon tumor development. Cancer Res 66:7571–7577
WHO Classification of Tumours Editorial Board 2020 Female genital tumours. International Agency for Research on Cancer (WHO classification of tumours Lyon series, 5th ed.; vol.4)
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl 48:452–458
Straight SW, Hinkle PM, Jewers RJ, McCance DJ (1993) The E5 oncoprotein of human papilloma virus type 16 transforms fibroblasts and effects the downregulation of the epidermal growth factor receptor in keratinocytes. J Virol 67:4521–4532
Nagy A, Munkacsy G, Gyorffy B (2021) Pancancer survival analysis of cancer hallmark genes. Sci Rep 11:6047
Acknowledgements
Certain parts of this study were included in the Japanese language PhD thesis of the authors MS and AI at Sapporo Medical University School of Medicine.
Funding
No specific funding was received. This study was supported in part by education and research funds of Sapporo Medical University School of Medicine.
Author information
Authors and Affiliations
Contributions
MS, AI, and MO substantially contributed to the conception and design of this study. MS, AI, AT, KT, DK, and KM performed histological examination of the cervical neoplasia and performed immunohistochemistry. MS, AI, and MO confirmed the authenticity of all of the raw data obtained. MS and AI were major contributors to data analysis and interpretation of the data. MS and MO contributed to manuscript drafting and critical revisions on the intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that we have no conflicts of interests.
Ethics approval and consent to participate
The present study was reviewed and approved by the Institutional Ethics Committee (approval no. 4-1-44) and Institutional Review Board (study no. 312-230) of Sapporo Medical University. The Ethics Committee waived the requirement to obtain written informed consent from the patients for the use of human tissues owing to the retrospective nature of the study. The research was conducted in accordance with the Declaration of Helsinki. The researchers involved in this study had no access to information that could identify individual participants during or after data collection.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sato, M., Inoue, A., Takasawa, A. et al. Elevated expression of endocan in the development of cervical squamous neoplasia of the uterus. Med Mol Morphol 56, 187–193 (2023). https://doi.org/10.1007/s00795-023-00353-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-023-00353-0