Abstract
Atypical spindle cell/pleomorphic lipomatous tumor (ASPLT) is a new entity of benign adipocytic tumor that spans a wide spectrum of histology from adipocytic to spindle cell/pleomorphic tumors. The latter non-adipocytic component rarely shows sarcomatous features although ASPLTs are not thought to dedifferentiate. A 78-year-old woman with ASPLT in the left thigh had a sarcomatous component with high mitotic activity and Ki-67 labeling index (LI) mimicking dedifferentiated liposarcoma. The adipocytic component consisted of various-sized adipocytic cells with few lipoblasts. The sarcomatous component consisted of a fascicular proliferation of atypical spindle cells with scattered large bizarre and multinucleated giant cells. Mitotic figures including atypical mitoses were frequently observed. Immunohistochemically, the tumor cells were positive for cluster of differentiation 34 but not mouse double minute 2 homolog (MDM2), cyclin-dependent kinase 4 (CDK4), or retinoblastoma (Rb) protein. Ki-67 LI in the sarcomatous component reached 40%. MDM2 and CDK4 genes were not amplified and 13q14 including the RB1 locus was deleted according to fluorescence in situ hybridization. The patient is alive with no evidence of local recurrence or distant metastasis 3.5 years after surgery. As ASPLT may exhibit morphological variation, it is important to rule out dedifferentiated liposarcoma with careful pathological examination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The current World Health Organization (WHO) classification of soft tissue and bone tumors defines atypical spindle cell/pleomorphic lipomatous tumor (ASPLT) as a new entity of benign adipocytic tumors [1, 2]. ASPLT encompasses several tumors that were previously known as atypical spindle cell lipomatous tumor [3], atypical spindle cell lipoma [4], and atypical pleomorphic lipomatous tumor [5]. Furthermore, some ASPLTs mimic spindle cell liposarcoma [6, 7]. The changing names notwithstanding, ASPLT is regarded as a benign tumor that does not show histological evidence of dedifferentiation and has a favorable clinical course. Histologically, ASPLT covers a broad spectrum of adipocytic and spindle cell tumors and often has a mixture of these components, resulting in a morphology similar to that of spindle cell/pleomorphic lipoma [8]. At the extreme adipocytic end of the spectrum, ASPLT is composed of mature adipocytes with scattered lipoblasts and resembles atypical lipomatous tumor/well-differentiated liposarcoma (ALT/WDLPS). However, at the extreme spindle cell end of the spectrum, ASPLT shows features of spindle cell tumor and sometimes exhibits a fibrosarcoma-like morphology [8]. Given the histological variety of ASPLT, the differential diagnosis of ASPLT ranges widely from typical adipocytic tumor to spindle cell tumor, including spindle cell sarcoma.
Although dedifferentiation is not likely to occur in ASPLT, there have been reports of rare ASPLTs that contain non-adipocytic, sarcomatous components mimicking the phenomenon of dedifferentiation [3, 9]. Cases similar to dedifferentiated liposarcoma (DDLPS) often include hyper-cellular components consisting of atypical spindle to pleomorphic cells. However, detailed pathological analyses by immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) have not been performed for such a sarcomatous component of ASPLT.
Here, we report a unique case of ASPLT that had a sarcomatous component showing high mitotic activity and Ki-67 labeling index (LI) and required exclusion of DDLPS in the differential diagnosis.
Case report
A 78-year-old woman had first noticed a soft tissue mass in her left thigh several years earlier. The mass gradually grew in size, and she eventually developed pain in the left thigh area overlying the mass. She visited a local hospital, where a malignant soft tissue tumor was suspected. She was subsequently referred to our hospital.
Magnetic resonance imaging (MRI) revealed a soft tissue mass measuring 122 × 60 × 40 mm that consisted of an adipocytic component and an adjacent non-adipocytic component. Axial T1-weighted images (T1WI) and T2-weighted images (T2WI) showed both an adipocytic component with high-signal intensity and an adjacent non-adipocytic component in the vastus intermedius muscle (Fig. 1a, b). The non-adipocytic component was enhanced with gadolinium on axial fat-saturation T1WI (Fig. 1c). A coronal short T1 inversion recovery image showed both fat- and non-fat-suppressed signals, suggestive of dedifferentiation (Fig. 1d). Based on these MRI findings, a diagnosis of DDLPS was suspected.
MRI findings of ASPLT with a non-adipocytic, sarcomatous component in the left thigh of a 78-year-old woman. a Axial T1-weighted magnetic resonance image. The lesion contained an adipocytic component with high-signal intensity (white arrowhead) and a juxtaposed non-adipocytic component with homogenous iso-signal intensity (white arrow) in the vastus intermedius muscle. b Axial T2-weighted magnetic resonance image. The lesion contained an adipocytic component with high-signal intensity (white arrowhead) and a juxtaposed non-adipocytic component with low to iso-signal intensity (white arrow) in the vastus intermedius muscle. c Axial enhanced fat-suppressed T1-weighted magnetic resonance image. The non-adipocytic component and the surrounding adipocytic component were enhanced with gadolinium. d Coronal short T1 inversion recovery magnetic resonance image. The lesion exhibited a fat-suppressed signal with septa (white arrowhead) and a juxtaposed non-fat-suppressed signal (white arrow) that was considered to indicate dedifferentiation. Diffuse surrounding edema was present (asterisk). These findings suggested a diagnosis of dedifferentiated liposarcoma
Histological examination of a needle biopsy specimen taken from the mass revealed a fascicular proliferation of atypical spindle cells with scattered large pleomorphic cells, suggesting spindle cell sarcoma. Mitotic figures were frequently observed, and the tumor cells showed a high Ki-67 LI. DDLPS was suspected based on the radiological and pathological findings. The mass was successfully resected with wide margins. The patient is alive with no evidence of local recurrence or distant metastasis as of 3.5 years after surgery.
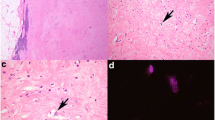
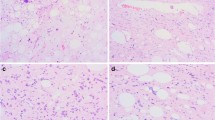
On gross examination, a well-demarcated, solid mass was located in the intermuscular space of the left thigh. On the cut surface, the tumor was composed of a yellowish adipocytic component and whitish multinodular non-adipocytic component (Fig. 2). Histologically, it was confirmed that these two components were adjacent to each other (Fig. 3a). The adipocytic component contained adipocytes of varying size with fibrous stroma (Fig. 3b). Small numbers of atypical spindle and larger pleomorphic cells with hyperchromatic nuclei were observed in fibromyxoid stroma. No ropy collagen bundles typical of spindle cell/pleomorphic lipoma were evident. Few lipoblasts were observed. By contrast, the non-adipocytic component showed sarcomatous features and was composed of a fascicular proliferation of atypical spindle cells with hyperchromatic, irregular-shaped, oval-to-spindle nuclei, and eosinophilic cytoplasm (Fig. 3c). Scattered atypical large tumor cells with bizarre nuclei and multinucleated giant tumor cells were seen. Mitotic figures including atypical mitoses were frequently observed (10/mm2) (Fig. 3d).
Histological findings of ASPLT with a sarcomatous component. a Histologically, the adipocytic (lower left) and sarcomatous (upper right) components were adjacent to each other. b The adipocytic component contained adipocytic cells of varying size with fibromyxoid stroma and few lipoblasts. c The sarcomatous component was composed of a fascicular proliferation of atypical spindle cells with hyperchromatic, irregular-shaped, oval-to-spindle nuclei and eosinophilic cytoplasm. Scattered atypical large tumor cells with bizarre nuclei and multinucleated giant tumor cells were also present. d Mitotic figures including atypical mitoses were frequently observed in the sarcomatous component (arrowhead)
On IHC, the tumor cells in both the adipocytic and sarcomatous components were strongly positive for cluster of differentiation 34 (CD34) (Fig. 4a) and vimentin, and were negative for cytokeratin AE1/AE3, epithelial membrane antigen, alpha-smooth muscle actin, desmin, muscle-specific actin (HHF35), mouse double minute 2 homolog (MDM2) (Fig. 4b), cyclin-dependent kinase 4 (CDK4), p53, and signal transducer and activator of transcription 6. Adipocytic cells were positive for S-100 protein. The majority of tumor cells in both the adipocytic and sarcomatous components showed loss of nuclear expression of retinoblastoma (Rb) protein (Fig. 4c). The Ki-67 LI of the tumor cells in the sarcomatous component reached approximately 40% (Fig. 4d).
IHC and FISH of ASPLT with a sarcomatous component. a The tumor cells in the sarcomatous component were diffusely positive for CD34 on immunohistochemistry. b The tumor cells were negative for MDM2 on immunohistochemistry. c A majority of tumor cells showed no nuclear expression of Rb protein on immunohistochemistry, whereas the endothelial cells in small vessels and lymphocytes used as the internal control were positive for Rb protein. d Tumor cells in the sarcomatous component showed a high Ki-67 labeling index. e FISH revealed no MDM2 gene amplification. The nucleus contained two pairs of red (MDM2) and green (CEP12) signals and exhibited a normal diploid karyotype. No amplification of the red signal was found for the MDM2 gene. f FISH showed heterozygous deletion of 13q14 including the RB1 gene locus. The nucleus exhibited one red signal for 13q14 and two green signals
We performed FISH for MDM2 and CDK4 gene amplification and for 13q14 deletion, including the RB1 locus, using commercially available FISH probes: an MDM2 (12q15)/SE12 probe (Leica, Buffalo Grove, IL) for MDM2 gene amplification, an ON CDK4 (12q13)/SE12 probe (Leica) for CDK4 gene amplification, and the ZytoLight SPEC RB1/13q12 Dual Color Probe (ZytoVision GmbH, Fischkai 1, Bremerhaven, Germany) for 13q14 deletion. FISH copy numbers were evaluated by counting MDM2, CDK4, and chromosome 12 centromere copy numbers in 20 nuclei. An MDM2/CEP12 ratio > 2 and a CDK4/CEP12 ratio > 2 were considered to indicate amplification of the MDM2 and CDK4 genes, respectively. FISH revealed no MDM2 (Fig. 4e) or CDK4 gene amplification and the MDM2/CEP12 ratio and CDK4/CEP12 ratio were 1.0 and 1.1, respectively. We evaluated 50 nuclei and counted the percentage of 13q14 deletion signals. FISH detected 13q14 deletion in 80% of tumor cells, which was homozygous in 10% of cells and heterozygous in 62% (Fig. 4f) and showed a monosomy pattern in 8%.
These findings suggested that the tumor had the characteristic features of ASPLT as well as a sarcomatous component with high mitotic activity and an increased Ki-67 LI. DDLPS was ruled out based on the results of IHC and FISH. The final diagnosis in this case was ASPLT with a sarcomatous component mimicking DDLPS.
Discussion
The current WHO classification defines ASPLT as a benign adipocytic tumor that does not dedifferentiate and has a favorable clinical course [1]. However, there have been some reports of ASPLTs showing a phenomenon resembling differentiation or a dedifferentiation-like component histologically. In a cohort of 232 cases reported by Marino-Enriquez et al. [3], there were 2 cases with dedifferentiation-like features that were diagnosed as atypical spindle cell lipomatous tumor, atypical adipocytic neoplasm with myxoid spindle cell features, or spindle cell liposarcoma. In these 2 cases, the tumors were composed of mainly mature-looking adipocytes and scant spindle cells, which transitioned abruptly to non-lipogenic spindle cell areas with increased cellularity and nuclear atypia. In addition, Bahadir et al. identified a case of ASLPT that showed dedifferentiation-like features in a cohort of 20 ASPLT cases [9]. That tumor also contained adipocytic and sarcomatous spindle cell components with increased cellularity. The two components exhibited an abrupt transition. Interestingly, the sarcomatous spindle cell component had no mitotic activity despite its sarcomatous morphology and showed no MDM2 or CDK4 expression on IHC or MDM2 gene amplification by FISH [9]. It is not known whether the dedifferentiation-like morphology in these reported cases is a true dedifferentiation phenomenon because no additional information about cell proliferation activity was available.
In our present case, the sarcomatous component of the tumor showed not only morphologically sarcomatous features but also high mitotic activity with an increased Ki-67 LI mimicking dedifferentiation. The Ki-67 LI strictly reflects the proliferative activity of tumor cells and is an important objective indicator for assessing the histological grade of soft tissue sarcomas [10]. High-grade sarcomas, including DDLPS, tend to show a high Ki-67 LI. DDLPS was initially included in the differential diagnosis in our case, although the tumor cells were negative for MDM2 and CDK4 on IHC. Furthermore, the tumor cells did not show MDM2 or CDK4 gene amplification by FISH and showed loss of Rb protein expression on IHC and 13q14 deletion by FISH. Both the adipocytic and sarcomatous components of the tumor were found to exhibit diffuse CD34 positivity. Based on these findings, we considered this tumor to have the characteristics of ASPLT but not those of DDLPS. Hence, we finally diagnosed the case as ASPLT with a sarcomatous component mimicking DDLPS.
However, MRI in this case revealed an adipocytic component and a non-adipocytic component suggestive of dedifferentiation adjacent to each other with a clear boundary. This is a characteristic radiological feature of DDLPS [11]. Histologically, DDLPS is a high-grade adipocytic tumor that consists of an ATL/WDLPS component and a non-adipocytic sarcomatous component. The sarcomatous component is typically composed of high-grade spindle cell to pleomorphic sarcoma similar to fibrosarcoma and undifferentiated pleomorphic sarcoma [12]. The sarcomatous component does not usually contain adipocytic tissue and is attached to the adjacent ATL/WDLPS component. Therefore, the two components are clearly delineated according to whether or not they contain adipose tissue on MRI. Our initial diagnosis in this case was DDLPS based on the MRI findings and the results of pathological examination of a biopsy specimen. Distinguishing this unusual type of ASPLT from DDLPS would be difficult based on radiological findings alone. Therefore, it is important to combine imaging with careful pathological examination, especially when a small biopsy specimen is taken for a precise diagnosis.
We think that the surgical method and follow-up of such a unique case is controversial because of its rarity. It is considered that there is a possibility of wide resection for surgery if the preoperative diagnosis of ASPLT with a sarcomatous component was made. Concerning surgery in the present case, we had to perform wide resection because we suspected sarcoma that needed to be differentiated from DDLPS. We also think that a close follow-up is necessary because this tumor showed high mitotic activity and Ki-67 LI even if this tumor is categorized into a benign adipocytic tumor. In fact, we have a close follow-up similar to that of malignant tumors at 3-month intervals until 2 years after the primary tumor diagnosis, then at 6-month intervals until 5 years after the primary tumor diagnosis.
In conclusion, we encountered an unusual case of ASPLT in which a sarcomatous component with high mitotic activity and Ki-67 LI mimicked DDLPS. The tumor showed no MDM2 and CDK4 gene amplification on FISH, allowing us to exclude a diagnosis of DDLPS. As ASPLT may exhibit such morphological variation, it is important to differentiate it from DDLPS with careful pathological examination, including conventional IHC staining with MDM2, CDK4, and Rb protein antibodies.
References
Creytens D, Mario-Enriquez A (2020) Atypical spindle cell/pleomorphic lipomatous tumor. In: Antonescu CR, Blay JV, Bovee JVMG et al (eds) World health organization classification of tumours soft tissue and bone tumours, 5th edn. IARC Press, Lyon, pp 34–35
Kallen ME, Hornick JL (2021) The 2020 who classification: what’s new in soft tissue tumor pathology? Am J Surg Pathol 45:e1–e23
Marino-Enriquez A, Nascimento AF, Ligon AH, Liang C, Fletcher CD (2017) Aypical spindle cell lipomatous tumor: clinicopathologic characterization of 232 cases demonstrating a morphologic spectrum. Am J Surg Pathol 41:234–244
Creytens D, van Gorp J, Savola S, Ferdinande L, Mentzel T, Libbrecht L (2014) Atypical spindle cell lipoma: a clinicopathologic, immunohistochemical, and molecular study emphasizing its relationship to classical spindle cell lipoma. Virchows Arch 65:97–108
Creytens D, Mentzel T, Ferdinande L, Lecoutere E, van Gorp J, Atanesyan L, de Groot K, Savola S, Van Roy N, Van Dorpe J, Flucke U (2017) “Atypical” pleomorphic lipomatous tumor: a clinicopathologic, immunohistochemical and molecular study of 21 cases, emphasizing its relationship to atypical spindle cell lipomatous tumor and suggesting a morphologic spectrum (atypical spindle cell/pleomorphic lipomatous tumor). Am J Surg Pathol 41:1443–1455
Mentzel T, Palmedo G, Kuhnen C (2010) Well-differentiated spindle cell liposarcoma (‘atypical spindle cell lipomatous tumor’) does not belong to the spectrum of atypical lipomatous tumor but has a close relationship to spindle cell lipoma: clinicopathologic, immunohistochemical, and molecular analysis of six cases. Mod Pathol 23:729–736
Shioi Y, Hasegawa T, Otsuka K, Fujisawa K, Itabashi T, Kimura T, Wakabayashi G, Mue Y, Uesugi N, Sugai T (2010) Primary retroperitoneal spindle cell liposarcoma: pathological and immunohistochemical findings. Pathol Int 60:472–476
Creytens D (2020) What’s new in adipocytic neoplasia? Virchows Arch 476:29–39
Bahadir B, Behzatoglu K, Hacihasanoglu E, Koca SB, Sigirci BB, Tokat F (2018) Atypical spindle cell/pleomorphic lipomatous tumor: a clinicopathologic, immunohistochemical, and molecular study of 20 cases. Pathol Int 68:550–556
Hasegawa T, Yamamoto S, Yokoyama R, Umeda T, Matsuno Y, Hirohashi S (2002) Prognostic significance of grading and staging systems using MIB-1 score in adult patients with soft tissue sarcoma of the extremities and trunk. Cancer 95:843–851
Rizer M, Singer AD, Edgar M, Jose J, Subhawong TK (2016) The histological variants of liposarcoma: predictive MRI findings with prognostic implications, management, follow-up, and differential diagnosis. Skeletal Radiol 45:1193–1204
Thway K (2019) Well-differentiated liposarcoma and dedifferentiated liposarcoma: an updated review. Semin Diagn Pathol 36:112–121
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Consent for publish
Written informed consent was obtained from the patient for publication of this report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sugita, S., Sugawara, T., Emori, M. et al. Atypical spindle cell/pleomorphic lipomatous tumor with a sarcomatous component showing high mitotic activity and Ki-67 labeling index: report of a unique case mimicking dedifferentiated liposarcoma. Med Mol Morphol 55, 323–328 (2022). https://doi.org/10.1007/s00795-022-00327-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-022-00327-8