Abstract
Non-alcoholic steatohepatitis (NASH) is characterized by the presence of hepatic steatosis, oxidative stress, inflammation, and hepatocyte injury with or without fibrosis. In this study, we explored the effect of APD668, a GPR119 agonist alone or in combination with linagliptin, a DPPIV inhibitor, on the progression of steatohepatitis in a murine model of NASH with diabetes. A novel NASH model with diabetes was generated by administration of streptozotocin injection to neonatal C57BL/6 mice (2–3 days old) combined with a high-fat diet feeding from the age of 4 weeks. The plasma biochemical parameters, oxidative stress, inflammation and histopathological changes were assessed. APD668 alone showed reduction in plasma glucose (− 39%, P < 0.05) and triglyceride level (− 26%) whereas a combined treatment of APD668 with linagliptin resulted in a more pronounced reduction in plasma glucose (− 52%, P < 0.001) and triglyceride (− 50%, P < 0.05) in NASH mice. In addition, co-administration of APD668 with linagliptin demonstrated a significant decrease in hepatic triglyceride, NAS score, hepatic TBARS and hepatic TNF-α in NASH mice with diabetes. These findings suggest that GPR119 receptor agonists in combination with DPPIV inhibitors may represent a promising therapeutic strategy for the treatment of NASH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-alcoholic steatohepatitis (NASH) is the more severe form of non-alcoholic fatty liver disease (NAFLD) and is characterized by the presence of hepatic steatosis, oxidative stress, inflammation, hepatocyte injury with or without fibrosis [1]. NASH is closely associated with metabolic syndrome, which is a lifestyle-related disorder characterized by obesity, diabetes, dyslipidemia and hypertension [2]. At present, there is no approved drug for the treatment of NASH. Several pharmacotherapies have been attempted to treat NASH/NAFLD with limited overall benefit. Due to its high prevalence coupled with lack of approved treatments, there remains a strong unmet medical need in NASH patients [3].
Recently, G protein-coupled receptor 119 (GPR119) has emerged as a novel therapeutic target for the treatment of dyslipidemia and NASH [4, 5]. Previously, it has been reported that GPR119 agonists increased incretin (GLP-1 and GIP) levels [6,7,8] and improved circulating lipid levels in preclinical and clinical settings, suggesting that they act as anti-dyslipidemic agents [9,10,11]. Some GPR119 agonists have demonstrated anti-steatotic activity in obese and HFD/STZ diabetic mice [4, 12], anti-inflammatory activity in CDAA diet-induced NASH model [13] and inhibited activation of hepatic stellate cells (unpublished results) [14], suggesting that they also have anti-fibrotic activity. Similarly, DPPIV inhibitors are a class of oral anti-diabetic agents which improved hepatic steatosis, insulin resistance, oxidative stress, inflammation and fibrosis in murine models of NASH with or without diabetes background [15,16,17,18]. Moreover, it has been reported that patients with NASH have increased DPPIV activity [19, 20].
We recently reported that APD668 alone or in combination with linagliptin shows beneficial effects on various parameters in high trans-fat (HTF) diet-induced steatohepatitis in C57BL/6 mice [5, 21]. We speculated that the enhanced effect observed with the combination treatment could be due to (1) direct activation of GPR119 receptors present in liver and intestine, (2) enhanced active GLP-1 levels and (3) decreased degradation of GLP-1 in vivo through DPPIV inhibition in HTF diet fed mice. However, combination treatment showed non-significant additive effects on different parameters (except body weight gain) which could be due to maximal or near maximal activity observed with single agents per se in HTF diet mice [21]. Moreover, the effect of APD668, linagliptin or their combination on plasma triglyceride, hepatic inflammation and fibrosis markers has not been evaluated in HTF diet fed mice. Fujii et al. [22] have developed a STAM™ mice model which closely mimics the human NASH disease progression and also exhibits NASH characteristics within a short duration of time. Therefore, to further extend our investigation, we hypothesized that APD668 in combination with linagliptin at lower doses will likely show beneficial effects in NASH mice. To our knowledge, no study to date has investigated the effect of GPR119 receptor agonist alone or in combination with DPPIV inhibitor on the progression of steatohepatitis in a murine model of NASH with diabetes. Our findings suggest that combination of APD668 with linagliptin shows beneficial effects such as anti-steatotic, anti-oxidant and anti-inflammatory activity in NASH associated with diabetes in mice.
Materials and methods
Chemicals
APD668, a GPR119 agonist and linagliptin, a DPPIV inhibitor were obtained from ChemBo Pharma, Nanjing, China. All other chemicals were procured from Sigma-Aldrich, St Louis, MO. APD668 was suspended in 1% Tween-80 + 15% Gelucire 44/14 + 10% propylene glycol + 74% type 1 ultrapure water. Linagliptin was suspended in 1% Tween-80 + 99% methyl cellulose (1:99). Vehicle or APD668 or linagliptin was administered by oral gavage twice daily for 4 weeks.
Animal experiments and induction of NASH
Animals were housed in an air-conditioned room at a temperature of 22 ± 2 °C and a humidity of 50 ± 20%, with a 12 h light/dark cycle. The NASH model (hereafter termed NASH mice) was established according to the slightly modified protocol of Fujii et al. [22]. In brief, neonatal C57BL/6 male mice (2–3 days old) were injected with streptozotocin (STZ; 200 µg per mouse, s.c.) and then fed a high-fat diet (45% kcal fat, D12451, Research Diet Inc. NJ, USA) from 4 weeks after birth. NASH mice aged at 6 weeks were randomized based on body weight into four groups (n = 6/group) as follows: group (1) vehicle; group (2) APD668 (6.25 mg/kg); group (3) Linagliptin (6.25 mg/kg) and group (4) APD668 (6.25 mg/kg) + linagliptin (6.25 mg/kg) group. Male C57BL/6 mice (n = 6) of the same age fed a normal diet (Altromin diet, Germany) without STZ injections, were assigned to the normal control group. Body weight was measured weekly. Post 4 weeks of treatment (post 14 h of last dose), blood samples were collected from retro-orbital plexus under mild isoflurane anesthesia and the livers were isolated, immediately snap-frozen in liquid nitrogen, and stored at − 80 °C until further analysis.
Measurement of plasma biochemical and metabolic markers
Plasma ALT, triglyceride, glucose and cholesterol levels were measured using automated biochemical analyzer (Daytona, Randox Inc. UK). Plasma insulin was measured using commercially available ELISA kits (Mouse Ultrasensitive Insulin ELISA kit, ALPCO Diagnostics, USA).
Measurement of hepatic triglyceride and cholesterol
Lipids were extracted according to the method of Edvardsson et al. [23]. Frozen livers were homogenized in isopropanol (1 mL/50 mg tissue) and incubated at 4 °C for 1 h. The samples were centrifuged at 4 °C for 5 min. at 685 g. Plasma triglyceride and cholesterol levels in the supernatants were measured using automatic biochemical analyzer (Daytona, Randox Inc. UK).
Histological analysis
In brief, fresh liver tissue samples were fixed in 10% formalin and embedded in paraffin. The samples were cross-cut into slices of 4–5 µm and stained with hematoxylin and eosin (H&E) for evaluation of the steatosis. Finally, the stained sections were observed and photographed under a light microscope (with 200× magnification). All stained sections were evaluated in a blinded manner by two pathologists for NAFLD activity score (NAS) including the components of steatosis, inflammation and hepatocyte ballooning. The scoring system was as follows: steatosis grade (0–3; 0: < 5%, 1: 5–33%, 2: 33–66%, and 3: > 66%), lobular inflammation (0–3; 0: no foci, 1: few foci/200×, 2: many foci/200×, and 3: > 4 foci/200× scope), and ballooning (0–2; 0: no foci, 1: < 2 foci/200×, and 2: 2–4 foci/200× [24]).
Measurement of hepatic thiobarbituric acid-reactants (TBARS) levels
The hepatic content of thiobarbituric acid-reactants, considered to be a marker of lipid peroxidation was measured using an OxiSelect™ TBARS Assay Kit (Cell Biolabs, Inc. USA). Briefly, frozen liver tissues (50 mg/mL) were homogenized in PBS containing butylated hydroxytoluene (BHT, 10 µL of 100× BHT to 1 mL of sample volume) to prevent further oxidation. Then, liver homogenates were centrifuged at 10,000g for 5 min. and the supernatants were collected. The supernatants were directly assayed for estimation of TBARS levels and results were expressed as µM/50 mg of liver tissue.
Measurement of hepatic TNF-α levels
Briefly, frozen liver tissues (50 mg/mL) were homogenized in lysis buffer. Then, liver homogenates were centrifuged at 12,000g for 10 min at 4 °C and the supernatants were collected. The concentrations of TNF-α levels in the supernatant were analyzed using commercially available ELISA kits according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA). The results were expressed as pg/mg of total protein. Total protein was estimated using Bicinchoninic Acid Protein Assay Kit (Sigma-Aldrich, USA).
Measurement of hepatic hydroxyproline levels
For hepatic hydroxyproline measurement, 10 mg of frozen liver tissue was homogenized in 100 µL of deionized water. Then, liver homogenate was added in glass screw-thread vial containing 100 µL of concentrated HCl (10 N). Further, samples were incubated at 120 °C for 24 h on heat block and mixed periodically during incubation. If the hydrolyzed black residue is still present in the samples, transfer to a microcentrifuge tube and spin at 2740g for 3 min. The concentrations of hydroxyproline levels in the supernatant were analyzed using commercially available ELISA kits according to the manufacturer’s instructions (Chondrex, Redmond, WA, USA). The results were expressed as µg/mg of liver tissue.
Statistical analysis
All the results are expressed as mean ± S.E.M. One-way ANOVA using Tukey’s post hoc test was used for multiple comparisons. P < 0.05 was considered to be statistically significant. Data were analyzed using GraphPad version 7.02 of GraphPad Prism for Windows, GraphPad software, San Diego, California, USA.
Results
Effect of APD668, linagliptin or their combination on body weight and biochemical parameters
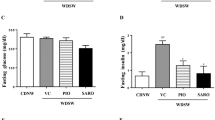
As shown in Table 1, the body weight tended to decrease in diabetic mice as compared to control group mice. At the end of study, none of the treatment groups showed a significant effect on body weight in mice. As expected, NASH mice showed a significant increase in plasma ALT, glucose, triglyceride and cholesterol levels as compared to control group mice (Table 1). APD668 or linagliptin monotherapy reduced elevated plasma glucose levels (39%, P < 0.05 and − 30%) and plasma triglyceride (− 26 and − 33%) in NASH mice. However, the effect was more pronounced upon treatment with combination of both drugs on plasma glucose (− 52%, P < 0.001) and plasma triglyceride (− 50%, P < 0.05), but failed to reach statistical significance as compared to monotherapy groups (Table 1). In addition, APD668 or linagliptin monotherapy non-significantly reduced elevated plasma ALT and cholesterol levels in NASH mice. Surprisingly, combination treatment failed to demonstrate more pronounced reduction in plasma ALT (− 16%) and cholesterol (− 13%) as compared to monotherapy with either APD668 (ALT: − 31% and cholesterol: − 18%) or linagliptin (ALT: − 21% and cholesterol: − 16%) in mice, as shown in Table 1. Moreover, NASH mice displayed hypoinsulinemia phenotype and APD668, linagliptin or combination treatment was unable to show increase in plasma insulin in mice (Table 1).
Effect of APD668, linagliptin or their combination on hepatic steatosis and NAS score
As shown in Fig. 1a–c, there was a significant increase in liver-to-body weight ratio, hepatic triglyceride and cholesterol content in NASH mice compared to control group mice. The liver-to-body weight ratio tended to decrease in the combination treatment group, but not in monotherapy groups (Fig. 1a). Treatment with APD668 alone caused a reduction in hepatic TG (− 60%, P < 0.01) and TC contents (− 53%, P < 0.05) whereas linagliptin showed a non-significant reduction in hepatic TG (− 41%) and TC (− 34%) in mice. Nevertheless, the combination treatment caused a significant reduction only in hepatic TG (− 56%), but not in TC content (− 41%) as compared to vehicle treated NASH mice as shown in Fig. 1b, c. Furthermore, hematoxylin and eosin stained liver sections showed hepatic fat accumulation (P < 0.05), inflammatory infiltration (P < 0.01), hepatocyte ballooning (P < 0.01) and the aggregate NAS score (P < 0.001) were higher in vehicle treated NASH mice as compared to control group mice as shown in Fig. 2a, b and Supplementary Table S1. In this study, APD668 alone reduced hepatocyte ballooning grade (P < 0.05) and aggregate NAS score (P < 0.05) whereas linagliptin monotherapy caused non-significant reduction in aggregate NAS score as compared to vehicle treated NASH mice. However, combination of APD668 with linagliptin significantly decreased hepatic steatosis grade and aggregate NAS score compared to vehicle treated NASH mice (Fig. 2a, b and Supplementary Table S1).
Effect of APD668, linagliptin or their combination on liver-to-body weight ratio and hepatic steatosis in mouse model of NASH with diabetes. Mice were treated with vehicle or APD668 (6.25 mg/kg) or linagliptin (6.25 mg/kg) or their combination for 4 weeks twice daily. a Liver-to-body weight ratio, b hepatic triglyceride, c hepatic cholesterol. The results are represented as the mean ± SEM, n = 5–6 mice/group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs control group; *P < 0.05, **P < 0.01, ***P < 0.001 vs vehicle group
Effect of APD668, linagliptin or their combination on hepatic steatosis and NAS score assessed using histopathologic analysis in NASH mice. Mice were treated with vehicle or APD668 (6.25 mg/kg) or linagliptin (6.25 mg/kg) or their combination for 4 weeks twice daily. a Hematoxylin and eosin staining of liver sections from representative mice from each group (magnification, ×200), b NAFLD activity Score (NAS). N.D. not determined. The results are represented as the mean ± SEM, n = 3 mice/group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs control group; *P < 0.05, **P < 0.01, ***P < 0.001 vs vehicle group
Combined effect of APD668 and linagliptin on oxidative stress marker
As shown in Fig. 3a, a significant increase in hepatic TBARS levels was observed in NASH mice as compared to control group (8.46 ± 0.88 µM/50 mg of tissue vs 4.22 ± 0.38 µM/50 mg of tissue, P < 0.01). APD668 or linagliptin monotherapy showed a trend towards decrease in hepatic TBARS level in mice. On the other hand, co-administration of APD668 with linagliptin caused a significant reduction in hepatic TBARS levels compared to vehicle treated NASH mice (5.35 ± 0.38 µM/50 mg of tissue vs 8.46 ± 0.88 µM/50 mg of tissue, P < 0.05, Fig. 3a).
Effect of APD668, linagliptin or their combination on hepatic TBARS, inflammatory cytokine (TNF-α) and fibrosis marker (hydroxyproline) in NASH mice. Mice were treated with vehicle or APD668 (6.25 mg/kg) or linagliptin (6.25 mg/kg) or their combination for 4 weeks twice daily. The results are represented as the mean ± SEM, n = 5–6 mice/group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs control group; *P < 0.05, **P < 0.01, ***P < 0.001 vs vehicle group
Combined effect of APD668 and linagliptin on hepatic inflammatory cytokine
As shown in Fig. 3b, there was an increase in hepatic TNF-α level in NASH mice as compared to control group mice (1.92 ± 0.28 pg/mg of total protein vs 1.15 ± 0.09 pg/mg of total protein, P < 0.05). In this study, APD668, linagliptin and their combination significantly reduced elevated hepatic TNF-α levels as compared to NASH mice (Fig. 3b).
Effect of APD668, linagliptin or their combination on hepatic fibrosis marker
Furthermore, we investigated the effect of APD668, linagliptin or their combination on hepatic fibrosis marker, i.e., hepatic hydroxyproline level. As shown in Fig. 3c, a non-significant increase in hepatic hydroxyproline levels was observed in NASH mice as compared to control group mice (3.18 ± 0.39 µg/mg of tissue vs 1.91 ± 0.27 µg/mg of tissue). APD668, linagliptin and combination treatment demonstrate a marginal decrease in hepatic hydroxyproline levels compared with vehicle treated NASH mice, as shown in Fig. 3c.
Discussion
Recently, we have reported the beneficial effects of AP668 alone or in combination with linagliptin in high trans-fat diet-induced steatohepatitis in the context of obesity with mild hyperglycemia and insulin resistance in C57BL/6 mice [5, 21]. However, the major limitation of this model is that high trans-fat diet induces all stages of NAFLD for periods > 20 weeks including lack of hypertriglyceridemia in normal C57BL/6 mice [25]. It is well-known that no single animal model so far has managed to recapitulate the full spectrum of human NASH disease progression, but individual animal models can mimic particular characteristics of human disease [26]. Therefore, it was tempting to assess effect of APD668 alone or in combination with linagliptin in a murine model of NASH with strong hyperglycemia, hypertriglyceridemia, moderately elevated liver enzymes, inflammation and fibrosis.
In the present study, APD668 alone or in combination with linagliptin significantly decreased plasma glucose in mice. Importantly, combination treatment, but not monotherapy caused significant reduction in plasma triglyceride in NASH mice, as shown in Table 1. Furthermore, APD668 alone or in combination with linagliptin, significantly reduced hepatic triglyceride and this was further supported by histological analysis with significant reduction in NAS score in mice (Figs. 1b, 2a, b). On the contrary, combination treatment failed to show enhanced or additive reduction in plasma ALT and hepatic triglyceride in NASH mice. These findings are in contrast with HTF diet study, where we found enhanced effects on hepatic injury markers and hepatic triglyceride caused by APD668 in combination with linagliptin in HTF diet fed mice [21]. Recently, Yang et al. [4] for the first time demonstrated that GPR119 receptors were expressed in mouse and human hepatocytes and the expression of lipogenic genes (FAS and ACC) was upregulated in GPR119 KO hepatocytes. Authors also reported that MBX2982, a GPR119 agonist, inhibited hepatic steatosis via inhibition of lipogenesis genes (SREBP-1c, FAS, ACC and SCD-1) in obese mice. Another GPR119 agonist, DA-1241, also attenuated hepatic steatosis via AMPK-mediated SREBP-1c inactivation and further inhibited the downstream lipogenic signals [27]. Additionally, we also demonstrated that APD668 alone or in combination with linagliptin enhanced active GLP-1 levels in HTF diet fed mice [21] and it has been reported that GLP-1 plays a major role in improvement of fatty liver in rodent studies [28, 29]. Therefore, we hypothesized that anti-steatotic effect of APD668 could be due to combination of direct activation of GPR119 receptors in intestine and hepatic tissues and elevated GLP-1 (indirect effect) may simultaneously contribute to the reduction in triglyceride uptake and hepatic lipogenesis in NASH mice.
Furthermore, it has been suggested that oxidative stress-induced lipid peroxidation followed by inflammation is another possible mechanism in the progression of non-alcoholic steatohepatitis [30]. As shown in Fig. 3a, combination of APD668 with linagliptin significantly reduced hepatic thiobarbituric acid-reactive substances (TBARS) in NASH mice. Moreover, APD668, linagliptin and their combination significantly reduced hepatic TNF-α in mice (Fig. 3b). The mechanism related to inhibition of hepatic oxidative stress and inflammation by APD668 alone or in combination with linagliptin could be due to improvement in hepatic steatosis in NASH mice. Combination treatment also showed a trend towards reduction in hepatic hydroxyproline levels, suggesting that APD668, a GPR119 agonist may have potential to inhibit hepatic fibrosis in NASH mice, as shown in Fig. 3c. Previously, it has been suggested that linagliptin attenuated hepatic steatosis and inflammation through inhibition of lipogenesis genes (SREBP-1c, FAS and SCD-1), inhibition of macrophage infiltration as well as TNF-α, SOCS3 gene expressions in rodent studies [15, 17, 18].
The present study had some limitations. In contrast to HTF diet study, combination treatment failed to show significant synergistic or additive effect in reduction of biochemical parameters, hepatic steatosis, NAS score, oxidative stress, inflammation and fibrosis markers as compared to monotherapy groups in NASH mice. These differential findings could be due to differences in murine models of NASH or due to small sample size failed to detect statistical significant differences. Thus, further investigative studies are required to confirm the significant differences between combination and monotherapy groups by increasing the sample size in this murine model of NASH with diabetes.
In summary, our findings demonstrated that co-administration of APD668 with linagliptin significantly improved hyperglycemia, hypertriglyceridemia and hepatic steatosis in NASH mice. Also, combination treatment significantly reduced hepatic TBARS and TNF-α levels in mice. Therefore, these findings together with our recent reports in HTF diet mice model suggest that GPR119 agonists in combination with DPPIV inhibitors may be useful for the management of non-alcoholic steatohepatitis.
Abbreviations
- NASH:
-
Non-alcoholic steatohepatitis
- NAFLD:
-
Non-alcoholic fatty liver disease
- GLP-1:
-
Glucagon-like peptide-1
- GIP:
-
Gastric inhibitory peptide
- DPPIV:
-
Dipeptidyl peptidase IV
- HFD/STZ:
-
High-fat diet/streptozotocin
- CDAA:
-
Choline-deficient, l-amino acid-defined
- KO:
-
Knockout
- AMPK:
-
AMP-activated protein kinase
- SREBP-1c:
-
Sterol regulatory element binding protein-1c
- SCD-1:
-
Stearoyl-CoA desaturase
- FAS:
-
Fatty acid synthase
- SOCS3:
-
Suppressor of cytokine signaling 3
- ALT:
-
Alanine aminotransferase
- HTF:
-
High trans-fat
- TG:
-
Triglyceride
- TC:
-
Total cholesterol
- TBARS:
-
Thiobarbituric acid reactants
- NAS:
-
NAFLD activity score
References
Farrell GC, Rooyen DV, Gan L, Chitturi S (2012) NASH is an inflammatory disorder: pathogenic, prognostic and therapeutic implications. Gut Liver 6:149–171
Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A (2013) Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 10:1544–1560
Perazzo H, Dufour J (2017) The therapeutic landscape of non-alcoholic steatohepatitis. Liver Int 37:634–647
Yang JW, Kim HS, Im JH, Kim JW, Jun DW, Lim SC, Lee K, Choi JM, Kim SK, Kang KW (2016) GPR119: a promising target for nonalcoholic fatty liver disease. FASEB J 30:1–12
Bahirat UA, Shenoy RR, Goel RN, Nemmani KVS (2017) APD668, a G protein-coupled receptor 119 agonist improves fat tolerance and attenuates fatty liver in high trans-fat diet induced steatohepatitis model in C57BL/6 mice. Eur J Pharmacol 801:35–45
Katz LB, Gambale JJ, Rothenberg PL, Vanapalli SR, Vaccaro N, Xi L, Polidari DC, Vets E, Sarich TC, Stein PP (2011) Pharmacokinetics, pharmacodynamics, safety and tolerability of JNJ-38431055, a novel GPR119 receptor agonist, in healthy male subjects. Clin Pharmacol Ther 90:685–692
Katz LB, Gambale JJ, Rothenberg PL, Vanapalli SR, Vaccaro N, Xi L, Sarich TC, Stein PP (2012) Effects of JNJ-38431055, a novel GPR119 receptor agonist, in randomized, double-blind, placebo-controlled studies in subjects with type 2 diabetes. Diabetes Obes Metab 14:709–716
Moss CE, Glassa LL, Diakogiannakia E, Paisa R, Lenaghanb C, Smithc DK, Wedin M, Bohlooly-Y M, Griblle FM, Reimann F (2016) Lipid derivatives activate GPR119 and trigger GLP-1 secretion in primary murine L-cells. Peptides 77:16–20
Yoshida S, Tanaka H, Oshima H, Yamazaki T, Yonetoku Y, Ohishi T, Matsui T, Shibasaki M (2010) AS1907417, a novel GPR119 agonist, as an insulinotropic and β-cell preservative agent for the treatment of type 2 diabetes. Biochem Biophys Res Commun 400:745–751
Kim SR, Kim D, Park SH, Kim YS, Kim CH, Ha TY, Yang J, Rhee J (2013) In vivo efficacy of HD0471953: a novel GPR119 agonist for the treatment of type 2 diabetes mellitus. J Diabetes Res 269569:1–7
Nunez DJ, Bush MA, Collins DA, Mcmullen SL, Gillmore D, Poss G, Schott R, Feldman PL (2012) Novel and profound lipid effects of GSK1292263, a potent and selective GPR119 agonist in dyslipidemic subjects. Circulation 126:A9918
Kim M, Kim TH, Cheyong Y, Chae YA, Jung IH, Lee K, Choi SM, Yang JS, Son M, Kang KK (2015) Long-term treatment of DA-1241, a novel GPR119 agonist, improved glucose control via preserved beta cell mass in a progressive diabetic mice model. In: 75th American Diabetes Association, 280–LB
Kang KW, Lee K, Yang JW (2017) Pharmaceutical composition containing GPR119 ligand as active ingredient for preventing or treating non-alcoholic fatty liver disease. Pub. no. US 2017/0049773 A1, 1–25
Yang JW, Kim HS, Choi YW, Kim YM, Kang KW (2018) Therapeutic application of GPR119 ligands in metabolic disorders. Diabetes Obes Metab 20:257–269
Kern M, Klöting N, Niessen HG, Thomas L, Stiller D, Mark M, Klein T, Blüher M (2012) Linagliptin improves insulin sensitivity and hepatic steatosis in diet-induced obesity. PLoS One 7:e38744
Shirakawa J, Fujii H, Ohnuma K, Sato K, Ito Y, Kaji M, Sakamoto E, Koganei M, Sasaki H, Nagashima Y, Amo K, Aoki K, Morimoto C, Takeda E, Terauchi Y (2011) Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes 60:1246–1257
Klein T, Fujii M, Sandel J, Shibazaki Y, Wakamatsu K, Michael M, Yoneyama H (2013) Linagliptin alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis. Med Mol Morphol 47:137–149
Jojima T, Tomotsune T, Iijima T, Akimoto K, Suzuki K, Aso Y (2016) Empagliflozin (an SGLT2 inhibitor), alone or in combination with linagliptin (a DPP–4 inhibitor), prevents steatohepatitis in a novel mouse model of non–alcoholic steatohepatitis and diabetes. Diabetol Metab Syndr 8:1–11
Balaban YH, Korkusuz P, Simsek H, Gokcan H, Gedikoglu G, Pinar A, Hascelik G, Asan E, Hamaloglu E, Tatar G (2007) Dipeptidyl peptidase IV (DDP IV) in NASH patients. Ann Hepatol 6:242–250
Yilmaz Y, Atug O, Yonal O, Duman D, Ozdogan O, Imeryuz N, Kalayci C (2009) Dipeptidyl peptidase IV inhibitors: therapeutic potential in nonalcoholic fatty liver disease. Med Sci Monit 15:HY1–HY5
Bahirat UA, Shenoy RR, Talwar R, Goel RN, Nemmani KVS (2017) Co-administration of APD668, a G protein-coupled receptor 119 agonist and linagliptin, a DPPIV inhibitor, prevents progression of steatohepatitis in mice fed on a high trans-fat diet. Biochem Biophys Res Commun 495:1608–1613
Fujii M, Shibazaki Y, Wakamatsu K, Honda Y, Kawauchi Y, Suzuki K, Arumugam S, Watanabe K, Ichida T, Asakura H, Yoneyama H (2013) A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med Mol Morphol 46:141–152
Edvardsson U, Ljungberg A, Lindén D, William-Olsson L, Peilot-Sjögren H, Ahnmark A, Oscarsson J (2006) PPARα activation increases triglyceride mass and adipose differentiation-related protein in hepatocytes. J Lipid Res 47:329–340
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321
Clapper JR, Hendricks MD, Gu G, Wittmer C, Dolman CS, Herich J, Athanacio J, Villescaz C, Ghosh SS, Heilig JS, Lowe C, Roth JD (2013) Diet-induced mouse model of fatty liver disease and nonalcoholic steatohepatitis reflecting clinical disease progression and methods of assessment. Am J Physiol Gastrointest Liver Physiol 305:G483–G495
Takahashi Y, Soejima Y, Fukusato F (2012) Animal models of nonalcoholic fatty liver disease nonalcoholic steatohepatitis. World J Gastroenterol 18:2300–2308
Kim M, Kim TH, Lee S, Jung I, Chae YN, Yang JS (2017) Effects of DA-1241, a novel GPR119 agonist, on lipid control in disease models mediated by regulating an AMPK/SREBP1c signaling path. In: 77th American Diabetes Association 161–LB
Ding X, Saxena SK, Lin S, Gupta N, Anania FA (2006) Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 43:173–181
Ben-Shlomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, Barzilai N, Oren R, Fishman S (2011) Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol 54:1214–1223
Ip E, Farrell G, Hall P, Robertson G, Leclercq I (2004) Administration of the potent PPARα agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology 39:1286–1296
Acknowledgements
This work was supported and funded by Lupin Limited (Research Park), India. We express our sincere thanks to Mr. Vikram Jadhav, Mr. Shyam Sundar for technical assistance, Mr. Kiran Powale, Mr. Gururaj Vishwase and Dr. Sharad Sharma for their contribution in histopathology analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bahirat, U.A., Talwar, R., Shenoy, R.R. et al. Combination of APD668, a G protein-coupled receptor 119 agonist with linagliptin, a DPPIV inhibitor, prevents progression of steatohepatitis in a murine model of non-alcoholic steatohepatitis with diabetes. Med Mol Morphol 52, 36–43 (2019). https://doi.org/10.1007/s00795-018-0200-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-018-0200-4