Abstract
Antigen presentation to peripheral memory T cells is a key step in the prompt elicitation of acquired immune responses. In the mucosa, specific sentinel lymphoid tissues called mucosa-associated lymphoid tissue serve as antigen presentation sites. Correspondingly, the concept of skin-associated lymphoid tissue (SALT) has been proposed in the 1980s. However, the details of SALT have not been clarified so far. Recently, the live imaging analysis using two photon microscopes are developed. Here, we have identified inducible lymphoid clusters in the skin, we called it inducible SALTs (iSALTs), using a murine contact hypersensitivity model. In the elicitation phase, dendritic cells (DCs) formed clusters and interacted for several hours with effector memory T cells in the dermis. This interaction was essential for proliferation and activation of effector memory T cells in situ in an antigen dependent manner. Interestingly, DC clusters were abrogated by depletion of skin macrophages. Furthermore, IL-1 treatment induced CXCL2 production from macrophages and DC clusters were suppressed with the blockade of IL-1R or CXCR2. Taken together, this sustained conjugation between DCs and memory T cells, iSALTs, is essential for establishment of the effector phase in acquired cutaneous immunity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skin is one of the organs constituting a border with the outside world such as lung or guts, and frequently exposed to foreign antigens. Therefore skin is the site of various immune responses as the front line of the biophylaxis.
Contact dermatitis is one of the most common skin acquired immune diseases and classical acquired immune responses, which has been extensively studied by using contact hypersensitivity (CHS) as a murine model of contact dermatitis [1, 2]. CHS responses consist of the sensitization and the elicitation phases. In the sensitization phase, after first antigen exposure to the skin, skin dendritic cells (DCs), such as langerhans cells (LCs) or dermal dendritic cells (dDCs), capture the antigen and migrate toward draining lymph nodes (LNs), and establish contact with naive T cells and activate antigen-specific population. On the other hand, in the elicitation phase, after re-exposure to foreign antigen, antigen presenting cells (APCs) capture the antigen and present it to skin-infiltrating T cells and activate antigen specific T cell population in the skin, and these a series of responses induce prompt immune responses.
Antigen presentation to memory T cells in periphery is another key step for efficient and prompt elicitation of acquired immune responses. In the submucosal areas, specific sentinel lymphoid tissues called mucosa-associated lymphoid tissues (MALTs) are organized to serve antigen presentation sites in the periphery [3]. As for the skin, the concept of skin-associated lymphoid tissue (SALT) has been proposed by Streilein in the 1980s [4], however, the details of SALT have not been clarified so far. In addition, it still remains unknown which subset of APCs presents antigens to skin T cells and how skin T cells efficiently encounter APCs. Furthermore, dermal macrophages are key modulators in CHS responses [5], however the detailed mechanisms by which macrophages are involved in the recognition of antigen in the skin have not yet been clarified. Thus, through the detailed analysis of the elicitation phase of CHS with two-photon microscopy, we clarified a part of the mechanisms of T cell activation in the skin and identified lymphoid structure composed of DCs, T cells and dermal macrophages which induce efficient T cell activation in the skin. We propose that the structures to be termed as ‘inducible SALTs (iSALTs)’ [6]. This article reviews the novel findings of the mechanisms of contact dermatitis and also iSALTs which is a new concept of acquired cutaneous immune responses.
Dermal DCs are essential for T cell activation and the elicitation of CHS responses
The cutaneous APCs are classified into two subsets phenotypically, epidermal LCs and dDCs. Initially, we evaluated the antigen-presenting capacity of cutaneous APCs subsets using the subset specific depletion strategy. To deplete mice of all cutaneous DC subsets, we used mice with sequence expressing the diphtheria toxin receptor (DTR) under the control of the promoter of the gene encoding langerin as recipients (in such ‘Langerin-DTR’ mice, treatment with diphtheria toxin (DT) leads to depletion of langerin-positive cells) and mice that express a transgene encoding DTR under the control of promoter of the gene encoding CD11c as donors (in such ‘CD11c-DTR’ mice, treatment with DT leads to transient depletion of CD11c + DC populations). To selectively deplete mice of LCs or dDCs, we transferred bone marrow (BM) cells from C57BL/6 mice or CD11c-DTR mice into Langerin-DTR or C57BL/6 mice, respectively. Each APCs depleted BM-chimeric mice were sensitized with hapten DNFB (2,4-dinitrofluorobenzene) on shaved abdomen, then we injected DT into them to deplete of each DC subset before elicitation and examined the ear swelling of each chimeras. As a result, we found that ear swelling and inflammatory histological findings were significantly attenuated in the lack of dDCs but not in the lack of LCs. Furthermore, the production of interferon-γ (IFN-γ) from skin- infiltrated T cells was suppressed in mice lacking dDCs. These results suggested that dDCs were essential for T cell activation and the elicitation of CHS responses.
DCs and memory T cells form lymphoid clusters in the dermis
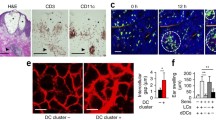
Histological examination of human contact dermatitis showed spongiosis, intercellular edema in the epidermis and co-localization of perivascular infiltrates of CD3+ T cells and CD11c (the common DC marker)+ DCs in the dermis, especially beneath the vesicles (Fig. 1a). These findings led us to hypothesize that focal accumulation of T cells and DCs in the dermis might induce dermatitis. Next, we analyzed the dynamics of dDCs and memory T cell in the elicitation phase of CHS using multi-photon microscopy. We isolated T cells from the draining LNs of mice sensitized with the DNFB, and labeled the cells with fluorescent dye. Then, we transferred them into mice that express CD11c tagged with yellow fluorescent protein (YFP). In the steady state, YFP+ dDCs distributed diffusely and migrated randomly in the dermis, as reported before [7]. After DNFB application, interestingly enough, YFP+ dDCs transiently increased their velocity and gradually formed clusters in the dermis (Fig. 1b), and transferred labeled T cells accumulated in consort with YFP+ dDCs and interacted with them for several hours in the dermis (Fig. 1c), that is, the antigen presentation sites were induced within the dermis in the elicitation phase of CHS. Additionally, we examined whether T cells proliferate via the formation of DC-T cell clusters in the dermis with cell proliferation assay. We purified CD4+ or CD8+ T cells from the draining LNs of DNFB-sensitized mice, labeled the cells with a division-tracking dye and transferred the cells into naive mice. Twenty-four hours after the application of DNFB to the recipient mice, we collected the skin to evaluate T cell proliferation by dilution of fluorescence intensity. CD8+ T cells proliferated actively in an antigen dependent manner, whereas the CD4+ T cells showed low proliferative potency. Because the integrin LFA-1 on T cells binds to cell-surface glycoproteins, such as the intercellular adhesion molecule ICAM-1, on APCs [8], which is essential for the proliferation and activation of naive T cells during antigen recognition in the LNs, we demonstrated DC-T cell interactions could be inhibited by blocking the adhesion molecule LFA-1, which resulted in reduced ear swelling and less production of IFN-γ by CD8+ T cells. These findings indicated that skin infiltrated CD8+ T cells proliferated and activated via the formation of DC-T cell clusters within the dermis in an antigen- and integrin- dependent manners.
The formation of DC-T cell clusters contribute to vesicle formation in contact dermatitis (modified from Ref. [6]). a Pathological findings of a skin biopsy of a human eczematous legion, stained with hematoxylin and eosin (HE) or immunohistochemistry with antibody to anti-CD3 (CD3) or anti-CD11c (CD11c). Asterisk epidermal vesicles, arrowheads indicate DC-T cell clusters. Scale bars 250 μm. b Sequential images of DC-T cell clusters in the elicitation phase of CHS. White outlined areas indicate dermal accumulation of DCs (green) and T cells (red). Scale bar 100 µm. c Magnification of DC-T cell cluster in b. DCs (green and white dashed line), T cells (red and white line). Scale bar 10 µm (color figure online)
Perivascular macrophages induce DC-T cell clustering
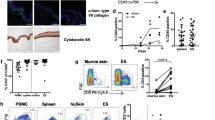
We next examined the initiation factors of formation of DC-T cell clusters. We evaluated the abundance of DC clusters in response to the application of hapten using mice that lack various immune cells, such as LysM- DTR mice (macrophages-depleted mice), RAG-2-deficient mice (T cells and B cells-lacking mice), Mas-TRECK mice (mast cells-depleted mice) [9], Bas-TRECK mice (basophils-depleted mice) [10], ALY/NscJcl- aly/aly mice (alymphoblastic mice) [11]. As a result, DC clustering was abrogated in LysM-DTR chimeric mice (with sequence encoding a DTR cassette inserted into the gene encoding lysozyme M) followed by treatment of the recipients with DT to deplete both macrophages and neutrophils, which resulted in reduced ear-swelling and less production of IFN-γ by T cells. On the other hand, depletion of neutrophils alone, by administration of antibody 1A8 to Ly6G, did not interfere with the formation of DC clusters.
Next, to examine the migratory kinetics of dermal macrophages and DCs in vivo, we observed them by two-photon microscopy. After application of DNFB to the ears of mice that previously sensitized with DNFB, dDCs accumulated mainly around blood vessels. Time-lapse live imaging revealed that some dDCs showed directional migration toward perivascular macrophages that were labeled by incorporation of extravasated TRITC-dextran (Fig. 2). These results suggested that the formation of DC clusters played a crucial role in T cell activation and required dermal perivascular macrophages.
Macrophages mediate the perivascular formation of DC clusters (modified from Ref. [6]). In the elicitation phase of CHS. Arrows indicate sebaceous glands of hair follicles (green and white line), blood vessels (yellow and red: tubular structures), macrophages (red and white dashed line). Scale bar 100 µm (color figure online)
Molecular mechanism of formation of DCs clusters
We attempted to clarify the mechanism of formation of DC clusters. We observed that DC accumulation occurred during the sensitization phase, so we hypothesized an antigen-nonspecific mechanism activated perivascular macrophages. Because interleukin (IL)-1 is associated with induction of inflammation as a kind of danger signals to external stimuli, we focused our attention on IL-1 [12, 13]. IL-1 is associated with induction of inflammation or the formation of DC clusters was not suppressed in mice deficient in NLRP3 or deficient in caspase-1 and caspase-11, but it was significantly lower in IL-1R1-deficient mice [which lack the receptor for IL-1α and IL-1β and for the IL-1 receptor antagonist (IL-1ra)], as well as after the subcutaneous administration of IL-1ra than before treatment with the antagonist.
In addition, the ear thickness and IFN-γ production by skin T cells in the elicitation phase of CHS were significantly attenuated in mice that lacked both IL-1α and IL-1β. These observations suggest that IL-1α activate perivascular macrophages after hapten application as the initial trigger of elicitation of CHS. However, it still remains unknown which cells produce IL-1α. In a previous paper, epidermal keratinocytes store a large quantity of IL-1α in themselves in steady state [14], and produce IL-1α by external stimuli without the cell death [15]. Because keratinocytes are known to produce IL-1α upon application of a hapten [16], we supposed that a major source of IL-1α were keratinocyte.
To further investigate how macrophages attract dDCs, we examined expression of the gene encoding IL-1Rα (Il1r1) in BM-derived activated M1 and activated M2 macrophages.
M2 macrophages had higher expression of Il1r1 mRNA than did M1 macrophages. Next, we used microarray analysis to examine the effect of IL-1α on the expression of chemokine-encoding genes in M2 macrophages. Treatment with IL-1α increased the expression of Ccl5, Ccl17, Ccl22 and especially Cxcl2 mRNA in M2 macrophages. Furthermore, we indicated that blockage of CXCL2 by the CXCR2 inhibitor SB265610 [17] suppressed the formation of DC clusters and elicitation of CHS. We found that perivascular M2 macrophages were essential for initiating the formation of DC clusters through the production of CXCL2.
A concept of inducible lymphoid clusters, iSALTs, as the site of antigen presentation in the skin
In humans, lymphoid structures are observed in mucosal areas, which are called as mucosa-associated lymphoid tissue (MALT) [3] or bronchus-associated lymphoid tissue (BALT) [18] and organized to serve antigen presentation sites in the periphery. As for the skin, the concept of skin-associated lymphoid tissue (SALT) has been proposed previously [4, 19], however, the organogenesis of SALT has not been detected yet. Here we have identified an inducible structure formed by perivascular M2 macrophages, dDCs and effector T cells. Because formation of this structure was essential for efficient activation of memory T cells, these inducible leukocyte clusters may function as SALTs. These immune cells clusters were not detected in the steady state but were induced during the elicitation phase of CHS. Therefore, these clusters might be more appropriately called ‘inducible SALTs’, similar to inducible BALTs in the lung [20].
Conclusion
Herein, we showed the review of the antigen-presenting mechanism in the skin. Together our results indicated that, in the elicitation phase of CHS, DC-T cell clustering were formed by antigen application. In addition, perivascular M2 macrophages were essential for initiating the formation of DC clusters through the production of CXCL2, and DC clustering had a crucial role in the efficient activation of memory T cells and prompt elicitation of acquired immune responses (Fig. 3). Therefore, we proposed that these perivascular dDC clusters might provide antigen-presentation sites for efficient activation of memory T cells. However, perivascular DC-T cell clusters have been observed in several inflammatory skin disorders [21, 22]. Therefore, it should be investigated whether iSALT or iSALT-like structures are established in other conditions, such as atopic dermatitis, psoriasis vulgaris, and so on. Finally, our findings suggest that approaches for the selective inhibition of this structure or may have novel therapeutic benefit in inflammatory disorders of the skin.
A schema of the mechanism of immunological event via iSALT formation in the elicitation phase of CHS (modified from Ref. [6]). After antigen application, keratinocytes release IL-1, which activates M2 macrophages that subsequently attract dDCs to perivascular area via CXCL2 to form clusters. The antigen is recognized efficiently in the DC clusters by antigen-specific memory T cells to form clusters, and inflammation is induced promptly via activation and proliferation of antigen-specific memory T cells
References
Kaplan DH, Igyarto BZ, Gaspari AA (2012) Early immune events in the induction of allergic contact dermatitis. Nat Rev Immunol 12:114–124
Honda T, Egawa G, Grabbe S, Kabashima K (2013) Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J Invest Dermatol 133:303–315
Brandtzaeg P, Kiyono H, Pabst R, Russell MW (2008) Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol 1:31–37
Streilein JW (1983) Skin-associated lymphoid tissues (SALT): origins and functions. J Invest Dermatol 80(Suppl):12s–16s
Tuckermann JP, Kleiman A, Moriggl R, Spanbroek R, Neumann A, Illing A, Clausen BE, Stride B, Forster I, Habenicht AJ, Reichardt HM, Tronche F, Schmid W, Schutz G (2007) Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J Clin Invest 117:1381–1390
Natsuaki Y, Egawa G, Nakamizo S, Ono S, Hanakawa S, Okada T, Kusuba N, Otsuka A, Kitoh A, Honda T, Nakajima S, Tsuchiya S, Sugimoto Y, Ishii KJ, Tsutsui H, Yagita H, Iwakura Y, Kubo M, Ng L, Hashimoto T, Fuentes J, Guttman-Yassky E, Miyachi Y, Kabashima K (2014) Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin. Nat Immunol 15:1064–1069
Ng LG, Hsu A, Mandell MA, Roediger B, Hoeller C, Mrass P, Iparraguirre A, Cavanagh LL, Triccas JA, Beverley SM, Scott P, Weninger W (2008) Migratory dermal dendritic cells act as rapid sensors of protozoan parasites. PLoS Pathog 4:e1000222
Springer TA, Dustin ML (2012) Integrin inside-out signaling and the immunological synapse. Curr Opin Cell Biol 24:107–115
Otsuka A, Kubo M, Honda T, Egawa G, Nakajima S, Tanizaki H, Kim B, Matsuoka S, Watanabe T, Nakae S, Miyachi Y, Kabashima K (2011) Requirement of interaction between mast cells and skin dendritic cells to establish contact hypersensitivity. PLoS ONE 6:e25538
Sawaguchi M, Tanaka S, Nakatani Y, Harada Y, Mukai K, Matsunaga Y, Ishiwata K, Oboki K, Kambayashi T, Watanabe N, Karasuyama H, Nakae S, Inoue H, Kubo M (2012) Role of mast cells and basophils in IgE responses and in allergic airway hyperresponsiveness. J Immunol 188:1809–1818
Miyawaki S, Nakamura Y, Suzuka H, Koba M, Yasumizu R, Ikehara S, Shibata Y (1994) A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur J Immunol 24:429–434
Dinarello CA (1984) Interleukin-1. Rev Infect Dis 6:51–95
Murphy JE, Robert C, Kupper TS (2000) Interleukin-1 and cutaneous inflammation: a crucial link between innate and acquired immunity. J Invest Dermatol 114:602–608
Kupper TS (1990) Immune and inflammatory processes in cutaneous tissues. Mechanisms and speculations. J Clin Invest 86:1783–1789
Lee RT, Briggs WH, Cheng GC, Rossiter HB, Libby P, Kupper T (1997) Mechanical deformation promotes secretion of IL-1 alpha and IL-1 receptor antagonist. J Immunol 159:5084–5088
Enk AH, Katz SI (1992) Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci USA 89:1398–1402
Liao L, Ning Q, Li Y, Wang W, Wang A, Wei W, Liu X, Auten RL, Tanswell AK, Luo X (2006) CXCR2 blockade reduces radical formation in hyperoxia-exposed newborn rat lung. Pediatr Res 60:299–303
Bienenstock J, Rudzik O, Clancy RL, Perey DY (1974) Bronchial lymphoid tissue. Adv Exp Med Biol 45:47–56
Egawa G, Kabashima K (2011) Skin as a peripheral lymphoid organ: revisiting the concept of skin-associated lymphoid tissues. J Invest Dermatol 131:2178–2185
Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD (2004) Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med 10:927–934
Katou F, Ohtani H, Nakayama T, Ono K, Matsushima K, Saaristo A, Nagura H, Yoshie O, Motegi K (2001) Macrophage-derived chemokine (MDC/CCL22) and CCR4 are involved in the formation of T lymphocyte-dendritic cell clusters in human inflamed skin and secondary lymphoid tissue. Am J Pathol 158:1263–1270
Kim TG, Jee H, Fuentes-Duculan J, Wu WH, Byamba D, Kim DS, Kim DY, Lew DH, Yang WI, Krueger JG, Lee MG (2014) Dermal clusters of mature dendritic cells and T cells are associated with the CCL20/CCR6 chemokine system in chronic psoriasis. J Invest Dermatol 134:1462–1465
Acknowledgments
We thank all members of the Kenji Kabashima’s Laboratory for their help to prepare this manuscript, and H. Yagita (Juntendo University) for the KBA neutralizing antibody to LFA-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Natsuaki, Y., Kabashima, K. Inducible lymphoid clusters, iSALTs, in contact dermatitis: a new concept of acquired cutaneous immune responses. Med Mol Morphol 49, 127–132 (2016). https://doi.org/10.1007/s00795-016-0137-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-016-0137-4