Abstract
Chromium is one of the most widely used metals in industry. Hexavalent form [Cr(VI)], which is found in industrial discharges, is very toxic and very soluble in water. From soil taken from an abandoned lead and iron mine, a bacterial strain capable of reducing Cr(VI) was isolated and identified as Brachybacterium paraconglomeratum ER41. Objective of this work was to evaluate the power of this bacterium to reduce Cr(VI). Results obtained showed that this bacterium is capable of eliminating 100 mg/L of Cr(VI) after 48 h (pH 8 and temperature 30 °C). For modeling biosorption kinetics, pseudo-first-order and intraparticle diffusion models gave a better fit. Furthermore, the adsorption mechanism conformed well to Langmuir’s isothermal model indicating monolayer type sorption. Biomass analysis of this bacterium before and after contact with chromium by scanning electron microscopy–energy-dispersive X-ray and by Fourier transform infrared spectroscopy showed that the surface ligands of bacterial wall are probably responsible for biosorption and bioreduction process. These results suggest a potential application of B. paraconglomeratum ER41 in bioremediation of polluted discharges.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Majority of the heavy metals are released from various anthropogenic activities, including mining, ore processing, leather tanning, fossil fuel burning, and electroplating, as well as from various products, including paints and pigments, preservatives, fertilizers, and chemicals (Kumar et al. 2017; Bharagava and Mishra 2018; Hanfi et al. 2020). These activities and products involve high amounts of toxic heavy metals, representing major sources of heavy metal contamination of the environment (Wu et al. 2016; Arora et al. 2017; Wang et al. 2021). The uncontrolled release of heavy metals into soil and water bodies results in severe adverse effects on human health through bioaccumulation in the food chain (Hou et al. 2020; Adimalla 2020; Shi et al. 2020). The accumulation of high concentrations of such toxic heavy metals into water bodies and soil is harmful to the aquatic and terrestrial flora and fauna, and these toxic metals can also negatively affect soil fertility and crop productivity (Mishra and Bharagava 2016; Rai et al. 2019). Toxic heavy metals can enter the human body through contaminated water, dust, and food, leading to severe health hazards and developmental abnormalities by disrupting metabolic activities (Ahmad 2011; Arora et al. 2017; Ali et al. 2019). Concentrations of heavy metals that exceed the limit values imposed for discharges can be a major source of soil contamination and be hazardous to the ecological balance (Ali et al. 2019; Zhang et al. 2021). They can cause several life-threatening complications in humans, such as cancer, neural disorders, genotoxic abnormalities, vital organ damage, developmental and reproductive disorders, pulmonary diseases, and biological system dysfunction (Ayangbenro and Babalola 2017; Mishra et al. 2019, 2020).
Owing to their low degradability, chemical complexity, and toxicity, heavy metal pollutants are difficult to degrade and can only be converted from their highly toxic forms to the less toxic ones or be removed via chemical or biological processes (Mishra and Bharagava 2016; Mishra et al. 2019; Dhaliwal et al. 2020). Various physicochemical approaches, such as adsorption (He et al. 2017), chemical precipitation (Chen et al. 2018), nanomaterial-based removal (Yu et al. 2021), reverse osmosis (Li 2017), ion exchange (Pan and An 2019), and membrane filtration (Peng and Guo 2020), have conventionally been employed for the decontamination and recovery of heavy metal-polluted environments.
Conventional methods have drawbacks, including the need for high-energy equipment, monitoring systems costly, incomplete removal of metals and consequently generation of secondary waste (Gu et al. 2015; Pepi et al. 2016). From where the obligation to move towards alternative processes, less expensive, effective, easy to be applied, profitable and most important eco-friendly, speaks about the process of biosorption conveyed via a microbial and particularly bacterial treatment (Javanbakht et al. 2014).
Biological removal including biosorption and bioaccumulation has been regarded as a cost-effective technique for the treatment of wastewaters containing heavy metals (Volesky 2007; Saxena et al. 2016). Biosorption is an adsorption process that involves the use of biomasses as adsorbents. They are called biosorbents, which have the advantage of not generating solid residues and do not produce toxic substances during the process (Costa et al. 2016). Biosorption performance of live biomass depends on nutrient, toxicity tolerance of microorganism and cell age, whereas use of dead biomass can eliminate these problems in addition to their ease of use, storage, easy regeneration and reusability (Yan and Viraraghavan 2000; Gadd 1992).
Hexavalent chromium [Cr(VI)] is one of the hazardous heavy metals, a most toxic form, which poses a real threat to the environment due to the improper disposal of industrial effluents loaded with chromium-based compounds, and the industrial processes particularly concerned are leather tannery, textiles, dye production, electroplating and steelmaking (Lai et al. 2016; Lyu et al. 2017). In contrast, trivalent form is 100 times and 1000 times less toxic and mutagenic, respectively, compared to the hexavalent form. Moreover, Cr(III) is an essential element playing an important role in the maintenance of human metabolism and homeostasis (Chojnacka 2010; Thatoi et al. 2014; Fernández et al. 2018). Therefore, the biosorption of Cr(VI) and its reduction to Cr(III) before releasing it into water bodies is an effective and necessary strategy for the remediation of chromium.
The potential of microbial Cr(VI) reduction has been envisaged by several authors for a long time, however, biosorption and bioaccumulation of Cr(VI) has gained attention during the recent past only (Gadd and White 1993; Sharma and Forster 1993; Fude et al. 1994). Nowadays, a large number of bacteria with high Cr(VI)-removal potential have been reported including Enterobacter and Pseudomonas (Ma et al. 2018), Penibacillus sp. (Wani et al. 2017), Exiguobacterium sp. (Batool et al. 2014), and Mesorhizobium sp. (Wani et al. 2009). Plasmids containing bacterial strains are able to degrade the toxic chemicals from contaminated soil and water (Martini et al. 2015). Plasmid-mediated heavy metal resistance is widely studied in order to understand the molecular genetics and functions of metal-resistance systems (Guo et al. 2018). In recent years, a large number of bacterial plasmids have been identified that encode specific resistance for various toxic heavy metal ions such as Cd2+, Co2+, CrO42−, Cu2+, Hg2+, AsO2−, Zn2+, and Ni2+ (Monchy et al. 2007; Bukowski et al. 2019). For a given microorganism, the efficiency of bioremoval by targeted microbial activity depends on factors such as culture age, cell form, pH, contact time, and initial metal concentrations in solution (Wang and Chen 2006).
In this study, we isolated a metal-resistant bacterium from an extreme Moroccan biotope; the soil of abandoned lead and iron mines; identified as Brachybacterium paraconglomeratum. According to the literature, this bacterium has not been studied before in bioremediation of polluted discharges by Cr(VI). In this research, this bacteria was studied for their resistance to chromium and other metals [Co(II), Cu(II), Ni(II), Zn(II), Hg(II), Pb(II), Cd(II), Fe(II)] and for ability to reduce Cr(VI). Specific objectives were: (i) optimizing the conditions for Cr(VI) biosorption and bioreduction, (ii) clarifying its mechanism and modeling the reactions developed in the process, and (iii) characterizing the bacteria biomass before and after Cr(VI) removal.
Materials and methods
Chemicals and reagents
The chemicals and reagents used in the present study were of analytical grade and were purchased from Sigma and Merck. The stock solution of hexavalent chromium was prepared in sterile distilled water and successive dilutions were made for individual working concentrations. The initial pH of the medium was adjusted using hydrochloric acid (10%) or sodium hydroxide (10%).
Isolation of chromium hexavalent-tolerant bacteria
The bacterium was isolated from the soil of an abandoned lead and iron mine in the region of Taza, located in North-eastern Morocco (34°05′55.6″ N and 4°01′52.1″ W). For bacterial isolation, about 5 g of the soil sample was transferred to an Erlenmeyer flask containing 45 mL of sterile physiological saline (0.9%). Then, a tenfold dilution series was performed using the homogenized soil samples. Aliquots of 100 μL were spread uniformly over the Luria Bertani (LB) agar medium (peptone 10 g/L, NaCl 10 g/L, yeast extract 5 g/L, agar 17 g/L), and the culture plates were incubated at 30 °C for 48 h, then bacterial isolates were purified by continuous streaking on LB agar medium. Isolated bacterial colonies of different morphologies were collected and seeded in LB agar plates containing increasing concentrations of Cr(VI) (100–1000 mg/L). The plates were incubated for 48 h at 30 °C, and growth was evaluated until the isolate was unable to produce colonies on the medium (Holt et al. 1994).
Evaluation of multi-metal resistant
The bacterial isolate showing a high tolerance to Cr(VI) on LB agar was subjected to the minimum inhibitory concentration (MIC) test against Cr(VI) and other toxic metals (Hg, Cd, Pb, Cu, Co, Ni, Zn and Fe) in LB broth using the microdilution technique as described by Güllüce et al. (2007), with some modifications. The initial metal concentration varies from 5 to 2000 mg/L, and after 24 h of incubation at 30 °C, the minimum concentration of metal which inhibits complete growth was taken as MIC.
Molecular identification of Cr(VI)-resistant bacteria
To identify the bacterial isolate, a molecular approach based on the amplification by polymerase chain reaction (PCR) and sequencing of the 16 S rRNA gene was used. PCR amplification was performed using the primer pair fD1 (5′-AGAGTTTGATCCTGGCTCAG-3′) and rP2 (5′-TACGGCTACCTTGTTACGACTT-3′) which allow to amplify a DNA fragment of approximately 1.5 kbp (Weisburg et al. 1991). A final volume of 20 μL containing: buffer (1×), 1.5 mM MgCl2, 200 μM of each dNTP, 0.5 μM of each primer, 0.2 units of Taq polymerase and 2 μL of the DNA sample. The mixture was first denatured at 94 °C for 5 min. Then, 35 cycles of PCR were performed by denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 1 min 30 s. At the end of the last cycle, the mixture was incubated at 72 °C for 10 min. The fragments obtained were sequenced at City of Innovation of Sidi Mohamed Ben Abdellah University, Fez, Morocco. These sequences were compared with database sequences. The program used was BLAST NR 2.9.0 + through the National Center for Biotechnology Information (NCBI).
Biosorption experiments
Optimization of Cr(VI) biosorption process
The Cr(VI) biosorption experiments were carried out in 200 mL of Erlenmeyer containing 50 mL of LB broth supplemented with initial concentration of Cr(VI) (50–300 mg/L) and seeded with a bacterial biomass of 0.4 g/L, with 150 rpm stirring. The pH and temperature conditions of the metal biosorption experiment were also optimized (Das et al. 2014). Elimination of Cr(VI) by bacterial adsorption was determined at different time intervals by measuring the concentration of chromium ions in a fixed volume of supernatant obtained after centrifugation at 7000 rpm for 10 min. Chromium reducing activity was estimated as the decrease in chromium concentration over time using 0.25% (w/v) of the hexavalent chromium-specific colorimetric S-diphenylcarbazide (DPC) reagent prepared in acetone to minimize deterioration, as described by Greenberg et al. (1992). The removal rate of Cr(VI) was calculated using the equation (Shafiq et al. 2021) described as follows:
where Q is the elimination rate, Ci (mg/L) is the initial concentration of Cr(VI), and Cf (mg/L) is the final concentration of Cr(VI).
Biosorption kinetic and equilibrium isotherm models
In order to study the rate and type of liquid–solid interactions (physisorption or chemisorption), the pseudo-first-order (PFO) and second-order (PSO) models were widely used, whereas the intraparticle diffusion model was applied to describe the internal diffusion of the metal in the bacterial wall. To investigate the efficiency of biosorption and the equilibrium relationship between sorbate and sorbent, the isothermal models of Langmuir and Freundlich were applied (Febrianto et al. 2009). The linear equations of biosorption kinetic and isothermal models used in this study were produced and described in Table 1.
Characterization of biomass
The characterization of B. paraconglomeratum ER41 biomass was carried out by SEM-EDX and Fourier transform infrared (FTIR) analysis.
Fourier transform infrared spectroscopy analysis (FTIR)
To identify the functional groups, present on the surface of the bacterial isolate, as well as their role in the Cr(VI) biosorption, the Fourier transform infrared spectroscopy (FTIR-VERTEX 70-BRUKER) was used. The cells cultured in LB broth supplemented with Cr(VI) (100 mg/L) and without Cr(VI) were sedimented by centrifugation at 7000 rpm for 15 min at 4 °C, the pellet was washed twice with sterile phosphate buffer, then spread on a glass slide and dried overnight at 50 °C. Infrared spectra of biomass were collected in the wave number range from 4000 to 400 cm−1 at a resolution of 4 cm−1.
Scanning electron microscopy (SEM) and energy-dispersive X-ray analysis (EDX)
To understand the mechanism of metal–bacteria interactions, it is important to visualize on the one hand, the morphology of cells by scanning electron microscopy (SEM), and on the other hand, to detect the metal adsorbed on the bacterial surface by an energy-dispersive X-ray analysis (EDX). For this, the overnight bacterial culture was carried out in Luria broth with Cr(VI) (100 mg/L) and without Cr(VI), centrifuged at 7000 rpm for 15 min at 4 °C, and the bacterial pellet was washed twice with sterile solution of potassium nitrate. After, the samples were deposited on conductive adhesive strips of stainless steel and coated with gold for SEM-EDX analysis (JSM-IT500HR).
Statistical analysis
All experiments were performed in triplicate and the results were subjected to statistical analysis. The mean and standard error were calculated, and Fisher’s least significant difference (LSD) (with a confidence level of 95.0%) was performed with XLSTAT Version 2016.02.28451.
Results and discussion
Evaluation of multi-metal resistant
A bacterial collection composed of two hundred and one isolates were subjected to MIC screening to check their resistance against high concentration of Cr(VI) ranges from 100 to 1000 mg/L. Among these 201 Cr(VI)-resistant bacteria isolates, an isolate exhibited on LB agar medium the highest Cr(VI) tolerance at 900 mg/L. Then, it was tested for its resistance to chromium on LB broth medium to determine its MIC. Based on the results obtained, our isolate was found to have a high MIC of 700 mg/L to Cr(VI). The comparable difference in metal tolerance of the isolate on LB agar medium and on LB broth medium is explained by the formation of a complex between the agar and the metal, which will not expose the bacteria to high metal concentrations, whereas in broth, bacteria are directly exposed to metal toxicity. Similar results were found by Wani et al. (2019). In comparison with the results obtained by Sandhya, our isolate shows a higher resistance to Cr(VI) (900 mg/L) on LB agar medium compared to Microbacterium paraoxydans (SCRB 19) which tolerates 500 mg/L (Sandhya et al. 2021).
Due to the multiple heavy metals’ pollution of industrial wastewater, the isolate was also evaluated for its multi-resistance (Table 2) to other metals and it showed remarkable MICs such as Fe(III) 1700 mg/L, Pb(II) 1600 mg/L, Cu(II)=Ni(II) 500 mg/L, Zn(II)=Co(II) 300 mg/L, Cd(II) and Hg(II) 25 and 20 mg/L, respectively. Our bacterial isolate showed significantly higher resistance to Cr(VI) than previously reported bacteria resistant to this metal such as Pseudomonas sp. MH458856 and Rhizobium strain ND2 with a MIC of 450 mg/L (Wani et al. 2019; Karthik et al. 2017a). For Bacillus sp. CRB-B1 isolated by Tan et al. (2020) tolerate only 420 mg/L of Cr(VI), while, Cellulosimicrobium funkei strain AR6 showed a strong resistance to Cr(VI) which arrives to 1200 mg/L (Karthik et al. 2017b).
Molecular identification of Cr(VI)-resistant bacteria
The bacteria isolated were Gram positive, cocci, circular, whole, smooth, and yellow colored. Based on 16S rRNA gene sequencing investigation, the bacterium was recognized as Brachybacterium paraconglomeratum ER41. The nucleotide sequence encoding the 16S rRNA gene of B. paraconglomeratum ER41 has been deposited in to the GenBank database under accession number OL989236.
Optimization of Cr(VI) biosorption process
A large number of microorganisms have been reported including Pseudomonas, Enterococcus, Bacillus, and Escherichia that resist and reduce Cr(VI) by developing metal-resistance mechanisms of periplasmic biosorption, intracellular bioaccumulation, DNA methylation, metal-resistant plasmids and biotransformation to Cr(III) all the way through enzymatic reaction or in some way by making complex metabolites (Megharaj et al. 2003).
Effect of pH
The choice of pH is one of the key factors that influence the mechanism of metal ion biosorption by bacteria through interacting with functional groups on the cell surface, moreover it has been reported that pH ranging from 6 to 8 is an effective pH for Cr(VI) bioreduction (Karthik et al. 2017b; Wani et al. 2017; El-Moselhy et al. 2013). Removal of 100 mg/L Cr(VI) by B. paraconglomeratum ER41 was studied and shown in Fig. 1a. From the results, it was revealed that maximum sorption (100%) of Cr(VI) was obtained at pH 8 after 48 h of growth. Bacterial strain showed an elimination rate of 79% and 69% at pH 9 and 7, respectively, after 48 h of treatment. In contrast, at pH 5 and 6 did not absorb a considerable amount of chromium ions which were 27 and 41%, respectively, thus suggesting that B. paraconglomeratum to be best suited for alkaline and slightly acidic conditions. From this study, it was observed that the binding affinity of cationic chromium ions to negatively charged cell surface functional groups depended on the pH of the medium. Moreover, the acidity of the solution affects the ionization of the functional groups present on the bacterial wall as they are strongly associated with protons and limited the binding of chromium ions (Wu et al. 2019; Mohapatra et al. 2016; Ren et al. 2015; Wierzba and Latała 2010).
Effect of temperature
A significant correlation exists between Cr(VI) removal and temperature, as illustrated in Fig. 1b. Based on the results obtained, it was revealed that 30 °C is the optimum temperature for chromium biosorption and bioreduction by B. paraconglomeratum. However, a temperature below and above 30 °C caused a slowdown in bacterial growth and consequently a decrease in the sorption of Cr(VI). These data can probably be explained by the damage on the structure of the bacterial membrane, the inactivation of certain enzymatic activities or the synthesis of intervening proteins (Das et al. 2014; Kathiravan et al. 2011). This behavior was also well observed previously in the study by Zhu et al. (2019) on the mechanisms of Cr(VI) reduction by Bacillus sp. CRB-1, in which maximum removal was accomplished at the optimum temperature of 42 °C within 24 h.
Effect of initial Cr(VI) concentration
Effect of varying initial chromium concentration (50, 100, 200 and 300 mg/L) on bacterial growth, on biosorption and bioreduction capacity of 0.4 g/L biomass of B. paraconglomeratum at optimum parametric conditions (pH 8, 30 °C temperature and 150 rpm shaking speed) were studied and presented in Fig. 2. Complete removal of metal was achieved at initial concentrations of 50, 100 and 200 mg/L after 24, 48 and 120 h, respectively, while the highest initial concentration of 300 mg/L was reduced to 90.1 ± 2.82% under 148 h. On the other hand, maximum bacterial growth was observed during treatments with low concentrations of Cr(VI) (50 and 100 mg/L). While a higher concentration of metal, a significant toxicity, considerably lengthened the adaptation and growth time of the bacteria and consequently increased the removal time of chromium ions. Shekhar et al. also studied the bacterial growth kinetics of Pseudomonas sp. strain V3 at increasing Cr(VI) concentration from 20 to 200 mg/L. This study indicated that the maximum bacterial cell growth was achieved at initial lower concentration of 20 mg/L, whereas bacterial growth was drastically reduced at higher Cr(VI) concentration of 200 mg/L. Moreover, increasing Cr(VI) concentration results in prolonged lag phase of bacterial growth. This extended lag period could be because of the adaptation of bacterial cells with the higher Cr(VI) concentration in the medium (Shekhar et al. 2014).
The results clearly confirmed that the time required for complete Cr(VI) biosorption and bioreduction by the strain gradually increased with increasing initial metal concentration. Ma et al. (2019) also attributed the decrease in the removal rate to the reduction in the number of cells and the lowering in biological activities caused by high concentration of Cr(VI), and a similar finding was reported by Wu et al. (2019) during the Cr(VI) removal by Bacillus sp. CRB-7. The viability of the cells at high Cr(VI) concentration was most likely associated with the alteration of genetic property or morphology of bacteria and altered metabolic reactions in bacteria. The evaluation of Cr(VI) toxicity on bacterial growth was also influenced by nutrient rich media. The nutrient rich media with higher peptides, amino acids and vitamins support good bacterial growth at higher concentration (Banerjee et al. 2019).
Biosorption kinetics
The results of experimental Cr(VI) removal capacity by B. paraconglomeratum ER41, shown in Fig. 3a, showed that the absorption process is largely dependent on time, during which the greatest amount of absorbed metal ions occurs. In the first 48 h of contact time, the involvement in addition to the active sorption process of a passive process such as physical adsorption or ion exchange interaction at the cell surface (Daneshvar et al. 2019). Over time, Cr(VI) biosorption and bioreduction continue to increase for longer periods with a slower rate, suggesting saturation of active function groups present on the bacterial surface. To simulate the data of the adsorption kinetics of chromium, the pseudo-first-order, pseudo-second-order and intraparticle diffusion models were used, and the fitted parameters as well as the values of the coefficient of determination are provided in Table 3. The pseudo-first-order and pseudo-second-order kinetic regression values established that for the Cr(VI) biosorption by B. paraconglomeratum, the PFO kinetics provided a better fit, since the coefficient of determination was the higher R2 = 0.97 as illustrated in Fig. 3b, suggesting that the rate of metal sorption by our strain was proportional to the concentration. This, therefore, confirms that B. paraconglomeratum had rapid adsorption kinetics. Similarly, to this work, chromium biosorption using mixed bacterial consortium showed that pseudo-first-order model was a better kinetic expression (Ma et al. 2019). In addition to the adsorption of metal ions on the bacterial wall of the biomass, there can be diffusion of the ion through the cell membrane to the intracellular part. The experiments were well described by the intraparticle diffusion model (Fig. 3d) and the results gave a high coefficient of determination R2 = 0.98, proving the importance of the internal surface for the biosorption properties. These findings suggest that ID was the main factor in the limited rate of Cr(VI) sorption. Same results were obtained by Mohapatra et al. (2019) during the biosorption study of Pb(II) using the biomass of Bacillus xiamenensis PbRPSD202, justifying that the biosorption of the metal by bacteria may be due to the active transport of metal ions via the intervention of enzymatic and metabolic activities.
Equilibrium isotherm models
To determine the mechanism involved in the chromium ions biosorption through the surface of bacterial biomass, the surface properties, as well as the affinity of the adsorbent in a liquid medium, two isotherm models such as Langmuir and Freundlich are used (Ren et al. 2015; Aly et al. 2014; Bueno et al. 2008). Langmuir’s model predicts that biosorption is based on the attachment of a monolayer with homogeneous forces and that there is equal energy for all sites and once these sites are fully saturated, no further adsorption can take place (Daneshvar et al. 2019; Behera et al. 2017); whereas the Freundlich model was developed for the heterogeneous adsorption process, explaining the biosorption of the multilayer metal on the sorbent surface or at surface support sites of different affinities (Batool et al. 2018; Bulgariu et al. 2013). From the graphical representations of the two models (Fig. 4), their constants and coefficients of determination listed in Table 4. It turned out that the Langmuir model describes an appropriate adjustment of the Cr(VI) biosorption by B. paraconglomeratum ER41 due to the high value of R2 (0.99) compared to the Freundlich model. Therefore, we can conclude that the adsorption followed monolayer biosorption mechanism. Studies in this context supporting the Langmuir isotherm model are well confirmed by several observations in which the biomass of strains such as Aeromonas caviae (Loukidou et al. 2004) and Rhizopus sp. (Espinoza-Sánchez et al. 2019) have been able to successfully remove Cr(VI) following a homogeneous surface adsorption mechanism.
Characterization of biomass
FTIR analysis
Peptidoglycan component of bacterial cell wall constituted to be potent binder of Cr(III) complexes. Binding of such reduced Cr(III) is mainly dependent of the distribution of reactive functional groups present on the cell surface. FTIR analysis was performed to identify these functional groups. The FTIR spectra obtained before and after treatment of B. paraconglomeratum by chromium are shown in Fig. 5. The FTIR spectrum of the control cells shows bands corresponding to hydroxyl (–OH), alkynes (C≡C), ketones (C=O), and alkanes (CH3) groups which are from 3250 to 3450, 2100, 1600 and 1400 cm−1, respectively. While the Cr(VI)-treated cells showed the same peaks with slight changes such as disruption of (C≡C) 2100 cm−1 shifted to 1550 cm−1 aromatic cycles (C=C) and the appearance of primary alcohol (–OH) 1050 cm−1. It is worth noting that hydroxyl, alkane and ketones groups play a role in the binding of chromium to the cell wall. Similar results were found by Karthik et al. (2017b) in the study of the biosorption and biotransformation of Cr(VI) by Cellulosimicrobium funkei strain AR6. In conclusion, the variation of the spectra following the adsorption of chromium ions on the bacterial wall may be due to mechanisms of expression or suppression of the functional groups, in order to ensure tolerance to the toxicity of the metal (Arivalagan et al. 2014; Kamnev 2008).
SEM-EDX analysis
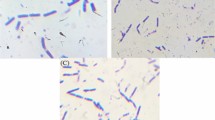
In order to better understand the mechanism of Cr(VI) biosorption and bioreduction and to visually analyze the morphology of B. paraconglomeratum ER41 under metallic stress, scanning electron microscopy analysis was used and the results are illustrated in Fig. 6. In the absence of the metal, cells exhibited a regular cocci shape with a smooth surface (Fig. 6a). However, the bacterial cells exposed to the metal presented an irregular, rough and fissured morphology with some perforations (Fig. 6b).
The EDX analysis results are shown in Fig. 7. Cr(VI) did not detect in untreated cells (Fig. 7a). However, EDX analysis of chromium-treated cells showed prominent peak of chromium at binding energy of 5.4 keV, which attributed to chromium for the intracellular absorption to the bacterial strain those treated with Cr(VI) (Fig. 7b).
Therefore, the EDX analysis of Cr-treated cells confirms the presence of Cr on bacterial cell surface, whereas non-treated cells confirms the absence of Cr. The similar results of SEM-EDX analysis were also demonstrated by Elahi et al. 2019 who investigated the interaction of Cr(VI) on the surface of Microbacterium testaceum B-SH2 and its intracellular accumulation during the bioremediation process by the EDX and SEM analysis. Therefore, these changes in the surface of bacteria are strictly related to the adsorption and reduction of Cr(VI). The results obtained can be explained by the reduced mechanical force under the effect of the interaction between the surfactant functional groups and the chromium ions (Huang et al. 2013). Identical results were found during the treatment of Bacillus sp. CRB-B1 (Tan et al. 2020) and Bacillus sp. CRB-7 (Wu et al. 2019) with Cr(VI), where cell deformations were probably caused by the metal toxicity.
Conclusion
Extreme environments are very attractive biotopes for screening microorganisms of biotechnological interest. This research work focused on isolation and screening of metallo-resistant bacteria from the soil of abandoned lead and iron mines in the Taza region of Morocco. This is the first study to show the power of B. paraconglomeratum ER41 to reduce hexavalent chromium. It showed high resistance to Cr(VI) (700 mg/L) and could grow and completely biosorb and bioreduce 100 mg/L of Cr(VI) in 48 h at pH 8 and 30 °C. The process of Cr(VI) reduction is highly dependent on pH, temperature, chromium concentration and contact time. Moreover, the modeling of the biosorption kinetics followed both the pseudo-first-order model as well as the intraparticle diffusion model, with monolayer adsorption of chromium ions on homogeneous sites on the bacterial surface, indicating that the Langmuir model describes a suitable adjustment of chromium ion biosorption by B. paraconglomeratum. In addition, the characterization of the bacterial biomass before and after contact with Cr(VI), by SEM-EDX and FTIR, indicated that the biosorption and the biotransformation of Cr(VI) were attributed to the presence of various active groups at the surface of the cell wall. Thus, the obtained results explored that B. paraconglomeratum ER41 with significant Cr(VI) reduction potential and can be a promising bio-agent for eco-friendly clean up strategies of toxic Cr(VI). This study explores the new sources of bioremediation of toxic chromium contaminated environment. Hence, further more detailed studies on the molecular characterization in this bacterium could be a vital step to exploit the key insights the reduction mechanism of Cr(VI) by B. paraconglomeratum ER41.
References
Adimalla N (2020) Heavy metals pollution assessment and its associated human health risk evaluation of urban soils from Indian cities: a review. Environ Geochem Health 42:173–190. https://doi.org/10.1007/s10653-019-00324-4
Ahmad MSA (2011) Essential roles and hazardous effects of nickel in plants. Rev Environ Contam Toxicol 214:125–167. https://doi.org/10.1007/978-1-4614-0668-6_6
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity and bioaccumulation. J Chem 4:1–14. https://doi.org/10.1155/2019/6730305
Aly Z, Graulet A, Scales N, Hanley T (2014) Removal of aluminium from aqueous solutions using PAN-based adsorbents: characterisation, kinetics, equilibrium and thermodynamic studies. Environ Sci Pollut Res 21(5):3972–3986. https://doi.org/10.1007/s11356-013-2305-6
Arivalagan P, Singaraj D, Haridass V, Kaliannan T (2014) Removal of cadmium from aqueous solution by batch studies using Bacillus cereus. Ecol Eng 71:728–735. https://doi.org/10.1016/j.ecoleng.2014.08.005
Arora S, Jain CK, Lokhande RS (2017) Review of heavy metal contamination. Int J Environ Sci Nat Res 3:139–144. https://doi.org/10.19080/IJESNR.2017.03.555625
Ayangbenro AS, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Public Health 14(94):1–16. https://doi.org/10.3390/ijerph14010094
Banerjee S, Misra A, Chaudhury S, Dam B (2019) A Bacillus strain TCL isolated from Jharia coalmine with remarkable stress responses, chromium reduction capability and bioremediation potential. J Hazard Mater 367:215–223. https://doi.org/10.1016/j.jhazmat.2018.12.038
Batool R, Qurrat-ul-ain K, Naeem A (2014) Comparative study of Cr(VI) removal by Exiguobacterium sp. in free and immobilized forms. Bioremediat J 18(4):317–327. https://doi.org/10.1080/10889868.2014.938722
Batool F, Akbar J, Iqbal S, Noreen S, Bukhari SNA (2018) Study of isothermal, kinetic, and thermodynamic parameters for adsorption of cadmium: an overview of linear and nonlinear approach and error analysis. Bioinorg Chem Appl. https://doi.org/10.1155/2018/3463724
Behera SS, Sourav D, Parhi PK, Tripathy SK, Mohapatra RK, Mayadhar D (2017) Kinetics, thermodynamics and isotherm studies on adsorption of methyl orange from aqueous solution using ion exchange resin Amberlite IRA-400. Desalin Water Treat 60:249–260. https://doi.org/10.5004/dwt.2017.0171
Bharagava RN, Mishra S (2018) Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicol Environ Saf 147:102–109. https://doi.org/10.1016/J.ECOENV.2017.08.040
Bueno BYM, Torem ML, Molina FALMS, De Mesquita LMS (2008) Biosorption of lead(II), chromium(III) and copper(II) by R. opacus: equilibrium and kinetic studies. Miner Eng 21(1):65–75. https://doi.org/10.1016/j.mineng.2007.08.013
Bukowski M, Piwowarczyk R, Madry A, Zagorski-Przybylo R, Hydzik M, Wladyka B (2019) Prevalence of antibiotic and heavy metal resistance determinants and virulence-related genetic elements in plasmids of Staphylococcus aureus. Front Microbiol 10:805. https://doi.org/10.3389/fmicb.2019.00805
Bulgariu L, Lupea M, Bulgariu D, Rusu C, Macoveanu M (2013) Equilibrium study of Pb(II) and Cd(II) biosorption from aqueous solution on marine green algae biomass. Environ Eng Manage J. https://doi.org/10.30638/EEMJ.2013.021
Chen Q, Yao Y, Li X, Lu J, Zhou J, Huang Z (2018) Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J Water Process Eng 26:289–300. https://doi.org/10.1016/j.jwpe.2018.11.003
Chojnacka K (2010) Biosorption and bioaccumulation—the prospects for practical applications. Environ Int 36(3):299–307. https://doi.org/10.1016/j.envint.2009.12.001
Costa F, Tavares T (2016) Biosorption of nickel and cadmium in the presence of diethylketone by a Streptococcus equisimilis biofilm supported on vermiculite. Int Biodeterior Biodegrad 115:119–132. https://doi.org/10.1016/j.ibiod.2016.08.004
Daneshvar E, Vazirzadeh A, Bhatnagar A (2019) Biosorption of methylene blue dye onto three different marine macroalgae: effects of different parameters on isotherm, kinetic and thermodynamic. Iran J Sci Technol Trans A Sci 43(6):2743–2754. https://doi.org/10.1007/s40995-019-00764-8
Das S, Mishra J, Das SK, Pandey S, Rao DS, Chakraborty A, Thatoi H (2014) Investigation on mechanism of Cr(VI) reduction and removal by Bacillus amyloliquefaciens, a novel chromate tolerant bacterium isolated from chromite mine soil. Chemosphere 96:112–121. https://doi.org/10.1016/j.chemosphere.2013.08.080
Dhaliwal SS, Singh J, Taneja PK, Mandal A (2020) Remediation techniques for removal of heavy metals from soil contaminated through different sources: a review. Environ Sci Pollut Res 27(2):1319–1333. https://doi.org/10.1007/S11356-019-06967-1
Elahi A, Ajaz M, Rehman A, Vuilleumier S, Khan Z, Hussain SZ (2019) Isolation, characterization and multiple heavy metal resistant and hexavalent chromium-reducing Microbacterium testaceum B-HS2 from tannery effluent. J King Saud Univ Sci 31(4):1437–1444. https://doi.org/10.1016/j.jksus.2019.02.007
El-Moselhy KM, Shaaban MT, Ibrahim HA, Abdel-Mongy AS (2013) Biosorption of cadmium by the multiple-metal resistant marine bacterium Alteromonas macleodii ASC1 isolated from Hurghada harbor, Red Sea. Arch Sci 66(2):259–272
Espinoza-Sánchez MA, Arévalo-Niño K, Quintero-Zapata I, Castro-González I, Almaguer-Cantú V (2019) Cr(VI) adsorption from aqueous solution by fungal bioremediation based using Rhizopus sp. J Environ Manag 251:109595. https://doi.org/10.1016/j.jenvman.2019.109595
Febrianto J, Kosasih AN, Sunarso J, Ju YH, Indraswati N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater 162(2–3):616–645. https://doi.org/10.1016/j.jhazmat.2008.06.042
Fernández PM, Viñarta SC, Bernal AR, Cruz EL, Figueroa LI (2018) Bioremediation strategies for chromium removal: current research, scale-up approach and future perspectives. Chemosphere 208:139–148. https://doi.org/10.1016/j.chemosphere.2018.05.166
Fude L, Harris B, Urrutia MM, Beveridge TJ (1994) Reduction of Cr(VI) by a consortia of sulfate-reducing bacteria (SRB-III). Appl Environ Microbiol 60(5):1525–1531. https://doi.org/10.1128/aem.60.5.1525-1531.1994
Gadd GM, White C (1992) Removal of thorium from simulated acid process streams by fungal biomass: potential for thorium desorption and reuse of biomass and desorbent. J Chem Technol Biotechnol 55(1):39–44. https://doi.org/10.1002/jctb.280550107
Gadd GM, White C (1993) Microbial treatment of metal pollution––a working biotechnology? Trends Biotechnol 11(8):353–359. https://doi.org/10.1016/0167-7799(93)90158-6
Greenberg AE, Clesceri LS, Eaton AD (1992) Standard methods for examination of water and wastewater, 18th edn. American Public Health Association, Washington, pp 451–454
Gu Y, Xu W, Liu Y, Zeng G, Huang J, Tan X (2015) Mechanism of Cr(VI) reduction by Aspergillus niger: enzymatic characteristic, oxidative stress response, and reduction product. Environ Sci Pollut Res Int 22:6271–6279. https://doi.org/10.1007/s11356-014-3856-x
Güllüce M, Sahin F, Sokmen M, Ozer H, Daferera D, Sokmen A, Polissiou M, Adiguzel A, Ozkan H (2007) Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. longifolia. Food Chem 103(4):1449–1456. https://doi.org/10.1016/j.foodchem.2006.10.061
Guo X, Peng Z, Huang D, Xu P, Zeng G, Zhou S, Gong X, Cheng M, Deng R, Yi H, Luo H, Yan X, Li T (2018) Biotransformation of cadmium-sulfamethazine combined pollutant in aqueous environments: Phanerochaete chrysosporium bring cautious optimism. Chem Eng J 347:74–83. https://doi.org/10.1016/j.cej.2018.04.089
Hanfi MY, Mostafa MYA, Zhukovsky MV (2020) Heavy metal contamination in urban surface sediments: sources, distribution, contamination control, and remediation. Environ Monit Assess 192(1):32–32. https://doi.org/10.1007/S10661-019-7947-5
He X, Qiu X, Chen J (2017) Preparation of Fe(II)-Al layered double hydroxide: application to the adsorption/reduction of chromium. Colloids Surf A Physicochem Eng Asp 516:362–374. https://doi.org/10.1016/j.colsurfa.2016.12.053
Holt JG, Krieg NR, Sneath PHA, Staley JT, Willams ST (1994) Bergey’s manual of determinative bacteriology, 9th edn. Williams and Wilkins, Baltimore, pp 786–788
Hou D, O’Connor D, Igalavithana AD, Alessi DS, Luo J, Tsang DCW, Sparks DL, Yamauchi Y, Rinklebe J, Ok YS (2020) Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat Rev Earth Environ 1(7):366–381. https://doi.org/10.1038/S43017-020-0061-Y
Huang F, Dang Z, Guo CL, Lu GN, Gu RR, Liu HJ, Zhang H (2013) Biosorption of Cd(II) by live and dead cells of Bacillus cereus RC-1 isolated from cadmium-contaminated soil. Colloids Surf B 107:11–18. https://doi.org/10.1016/j.colsurfb.2013.01.062
Javanbakht V, Alavi SA, Zilouei H (2014) Mechanisms of heavy metal removal using microorganisms as biosorbent. Water Sci Technol 69(9):1775–1787. https://doi.org/10.2166/wst.2013.718
Kamnev AA (2008) FTIR spectroscopic studies of bacterial cellular responses to environmental factors, plant–bacterial interactions and signalling. J Spectrosc 22(2–3):83–95. https://doi.org/10.3233/SPE-2008-0329
Karthik C, Oves M, Sathya K, Ramkumar VS, Arulselvi PI (2017a) Isolation and characterization of multi-potential Rhizobium strain ND2 and its plant growth-promoting activities under Cr(VI) stress. Arch Agron Soil Sci 63(8):1058–1069. https://doi.org/10.1080/03650340.2016.1261116
Karthik C, Ramkumar VS, Pugazhendhi A, Gopalakrishnan K, Arulselvi PI (2017b) Biosorption and biotransformation of Cr(VI) by novel Cellulosimicrobium funkei strain AR6. J Taiwan Inst Chem Eng 70:282–290. https://doi.org/10.1016/j.jtice.2016.11.006
Kathiravan MN, Karthick R, Muthukumar K (2011) Ex situ bioremediation of Cr(VI) contaminated soil by Bacillus sp.: batch and continuous studies. Chem Eng J 169(1–3):107–115. https://doi.org/10.1016/j.cej.2011.02.060
Kumar M, Gogoi A, Kumari D, Borah R (2017) Review of perspective, problem, challenges and future scenario of metal contamination in the urban environment. J Hazard Toxic Radioact Waste 21(4):04017007. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000351
Lai CY, Zhong L, Zhang Y, Chen JX, Wen LL, Shi LD, Zhao HP (2016) Bioreduction of chromate in a methane-based membrane biofilm reactor. Environ Sci Technol 50(11):5832–5839. https://doi.org/10.1021/acs.est.5b06177
Li Q (2017) Rhamnolipid synthesis and production with diverse resources. Front Chem Sci Eng 11:27–37. https://doi.org/10.1007/s11705-016-1607-x
Loukidou MX, Zouboulis AI, Karapantsios TD, Matis KA (2004) Equilibrium and kinetic modeling of chromium(VI) biosorption by Aeromonas caviae. Colloids Surf A Physicochem Eng Asp 242:93–104. https://doi.org/10.1016/j.colsurfa.2004.03.030
Lyu H, Tang J, Huang Y, Gai L, Zeng EY, Liber K, Gong Y (2017) Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem Eng J 322:516–524. https://doi.org/10.1016/j.cej.2017.04.058
Ma S, Song CS, Chen Y, Wang F, Chen HL (2018) Hematite enhances the removal of Cr(VI) by Bacillus subtilis BSn5 from aquatic environment. Chemosphere 208:579–585. https://doi.org/10.1016/j.chemosphere.2018.06.037
Ma L, Xu J, Chen N, Li M, Feng C (2019) Microbial reduction fate of chromium (Cr) in aqueous solution by mixed bacterial consortium. Ecotoxicol Environ Saf 170:763–770. https://doi.org/10.1016/j.ecoenv.2018.12.041
Martini MC, Albicoro FJ, Nour E, Schlüter A, van Elsas JD, Springael D, Smalla K, Pistorio M, Lagares A, Del Papa MF (2015) Characterization of a collection of plasmid-containing bacteria isolated from an on-farm biopurification system used for pesticide removal. Plasmid 80:16–23. https://doi.org/10.1016/j.plasmid.2015.05.001
Megharaj M, Avudinayagam S, Naidu R (2003) Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Curr Microbiol 47:51–54. https://doi.org/10.1007/s00284-002-3889-0
Mishra S, Bharagava RN (2016) Toxic and genotoxic effect of hexavalent chromium in environment and its bioremediation strategies. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 34(1):1–32. https://doi.org/10.1080/10590501.2015.1096883
Mishra S, Bharagava RN, More N, Yadav S, Zainith S, Mani S, Chowdhary P (2019) Heavy metal contamination: an alarming threat to environment and human health. Environmental biotechnology for sustainable future. Springer Nature, Singapore, pp 103–125
Mishra S, Saratele SD, Ferreira FR, Bharagava RN (2020) Plant–microbe interaction: an ecofriendly approach for the remediation of toxic metal contaminated environments. Reference module in material science and materials engineering. Encyclopedia of renewable and sustainable materials. Elsevier, Amsterdam, pp 444–450
Mohapatra RK, Pandey S, Thatoi HN, Panda CR (2016) Screening and evaluation of multi-metal tolerance of chromate resistant marine bacteria isolated from water and sediment samples of Paradip port, Odisha coast. J Appl Microbiol 2(3):135–147
Mohapatra RK, Parhi PK, Pandey S, Bindhani BK, Thatoi H, Panda CR (2019) Active and passive biosorption of Pb(II) using live and dead biomass of marine bacterium Bacillus xiamenensis PbRPSD202: kinetics and isotherm studies. J Environ Manag 247:121–134. https://doi.org/10.1016/j.jenvman.2019.06.073
Monchy S, Benotmane MA, Janssen P, Vallaeys T, Taghavi S, Van Der Lelie D, Mergeay M (2007) Plasmids pMOL28 and pMOL30 of Cupriavidus metallidurans are specialized in the maximal viable response to heavy metals. J Bacteriol 189:7417–7425. https://doi.org/10.1128/JB.00375-07
Pan ZF, An L (2019) Removal of heavy metal from wastewater using ion exchange membranes. In: Ahamed MI, Asiri AM (eds) Application of ion exchange materials in the environment. Springer, pp 25–46
Peng H, Guo J (2020) Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption, electrochemical reduction, electrodialysis, electrodeionization photocatalysis and nanotechnology: a review. Environ Chem Lett 18:2055–2068. https://doi.org/10.1007/s10311-020-01058-x
Pepi M, Borra M, Tamburrino S, Saggiomo M, Viola A, Biffali E, Casotti R (2016) A Bacillus sp. isolated from sediments of the Sarno River mouth, Gulf of Naples (Italy) produces a biofilm biosorbing Pb(II). Sci Total Environ 562:588–595. https://doi.org/10.1016/j.scitotenv.2016.04.097
Rai PK, Lee SS, Zhang M, Tsang YF, Kim KH (2019) Heavy metals in food crops: health risks, fate, mechanisms and management. Environ Int 125:365–385. https://doi.org/10.1016/j.envint.2019.01.067
Ren G, Jin Y, Zhang C, Gu H, Qu J (2015) Characteristics of Bacillus sp. PZ-1 and its biosorption to Pb(II). Ecotoxicol Environ Saf 117:141–148. https://doi.org/10.1016/j.ecoenv.2015.03.033
Sandhya M, Shaohua C, Ganesh D, Rijuta GS, Luiz FRF, Muhammad B, Ram NB (2021) Reduction of hexavalent chromium by Microbacterium paraoxydans isolated from tannery wastewater and characterization of its reduced products. J Water Process Eng 39:101748. https://doi.org/10.1016/j.jwpe.2020.101748
Saxena G, Chandra R, Bharagava RN (2016) Environmental pollution, toxicity profile and treatment approaches for tannery wastewater and its chemical pollutants. Rev Environ Contam Toxicol 240:31–69. https://doi.org/10.1007/398_2015_5009
Shafiq M, Ali Alazba A, Tahir Amin M (2021) Kinetic and isotherm studies of Ni2+ and Pb2+ adsorption from synthetic wastewater using Eucalyptus camdulensis—derived biochar. Sustainability 13:3785. https://doi.org/10.3390/su13073785
Sharma DC, Forster CF (1993) Removal of hexavalent chromium using sphagnum moss peat. Water Res 27(7):1201–1208. https://doi.org/10.1016/0043-1354(93)90012-7
Shekhar S, Sundaramanickam A, Vijayanasiva G (2014) Detoxification of hexavalent chromium by potential chromate reducing bacteria isolated from turnery effluent. Am J Res Commun 2(2):205–216
Shi J, Zhang B, Cheng Y, Peng K (2020) Microbial vandate reduction coupled to co-metabolic phenanthrene biodegradation in ground water. Water Res 186:116354. https://doi.org/10.1016/j.watres.2020.116354
Tan H, Wang C, Zeng G, Luo Y, Li H, Xu H (2020) Bioreduction and biosorption of Cr(VI) by a novel Bacillus sp. CRB-B1 strain. J Hazard Mater 386:121628. https://doi.org/10.1016/j.jhazmat.2019.121628
Thatoi H, Das S, Mishra J, Rath BP, Das N (2014) Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: a review. J Environ Manage 146:383–399. https://doi.org/10.1016/j.jenvman.2014.07.014
Volesky B (2007) Biosorption and me. Water Res 41:4017–4029. https://doi.org/10.1016/j.watres.2007.05.062
Wang J, Chen C (2006) Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnol Adv 24:427–451. https://doi.org/10.1016/j.biotechadv.2006.03.001
Wang Z, Zhang B, He C, Shi J, Wu M, Guo J (2021) Sulfur-based micro trophic vanadium (V) bioreduction towards lower organic requirement and sulfate accumulation. Water Res 189:116655. https://doi.org/10.1016/j.watres.2020.116655
Wani PA, Zaidi A, Khan MS (2009) Chromium reducing and plant growth promoting potential of Mesorhizobium species under chromium stress. Bioremediat J 13(3):121–129. https://doi.org/10.1080/10889860903124289
Wani PA, Sunday OO, Kehinde AM, Oluwaseyi LA, Wasiu IA, Wahid S (2017) Antioxidants and chromium reductases by Penibacillus species enhance the growth of soybean under chromium stress. Int J Environ Sci Technol 15(7):1531–1542. https://doi.org/10.1007/s13762-017-1533-6
Wani PA, Wahid S, Khan MSA, Rafi N, Wahid N (2019) Investigation of the role of chromium reductase for Cr(VI) reduction by Pseudomonas species isolated from Cr(VI) contaminated effluent. Biotechnol Res Innov 3(1):38–46. https://doi.org/10.1016/j.biori.2019.04.001
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173(2):697–703. https://doi.org/10.1128/jb.173.2.697-703.1991
Wierzba S, Latała A (2010) Biosorption lead(II) and nickel(II) from an aqueous solution by bacterial biomass. Pol J Chem Technol 12(3):72–78. https://doi.org/10.2478/v10026-010-0038-6
Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L (2016) A review on toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res 23(9):8244–8259. https://doi.org/10.1007/S11356-016-6333-X
Wu M, Li Y, Li J, Wang Y, Xu H, Zhao Y (2019) Bioreduction of hexavalent chromium using a novel strain CRB-7 immobilized on multiple materials. J Hazard Mater 368:412–420. https://doi.org/10.1016/j.jhazmat.2019.01.059
Yan G, Viraraghavan T (2000) Effect of pretreatment on the bioadsorption of heavy metals on Mucor rouxii. Water SA 26(1):119–123
Yu G, Wang X, Liu J, Jiang P, You S, Ding N, Guo Q, Lin F (2021) Applications of nanomaterials for heavy metal removal from water and soil: a review. Sustainability 13:713. https://doi.org/10.3390/su13020713
Zhang W, Pang S, Lin Z, Mishra S, Bhatt P, Chen S (2021) Biotransformation of perfluoroalkyl acid precursors from various environmental systems: advances and perspectives. Environ Pollut. https://doi.org/10.1016/j.envpol.2020.115908
Zhu Y, Yan J, Xia L, Zhang X, Luo L (2019) Mechanisms of Cr(VI) reduction by Bacillus sp. CRB-1, a novel Cr(VI)-reducing bacterium isolated from tannery activated sludge. Ecotoxicol Environ Saf 186:109792. https://doi.org/10.1016/j.ecoenv.2019.109792
Acknowledgements
This research was internally funded by Sidi Mohamed Ben Abdellah University, Fez, Morocco. The authors thankfully acknowledge the scientific support of the City of Innovation of Sidi Mohamed Ben Abdellah University, Fez, Morocco.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Communicated by Atomi .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Harboul, K., Alouiz, I., Hammani, K. et al. Isotherm and kinetics modeling of biosorption and bioreduction of the Cr(VI) by Brachybacterium paraconglomeratum ER41. Extremophiles 26, 30 (2022). https://doi.org/10.1007/s00792-022-01278-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00792-022-01278-9