Abstract
A gene (estA', 804 bp) from Streptomyces lividans TK24 was artificially synthesized and successfully overexpressed as a 6His-tagged fusion protein in Escherichia coli. It encoded a carboxylesterase (EstA) that composed of 267 amino acids with a predicted molecular weight of 28.56 kDa. Multiple sequence alignment indicated that EstA has typical characteristics of esterases, including a catalytic triad (Ser93-Asp194-His224) and a conserved pentapeptide motif (Gly91-Leu92-Ser93-Met94-Gly95). Simultaneously, phylogenetic analysis indicated that EstA belongs to family VI. Biochemical characterization displayed its optimum enzyme activity was at 55 ℃ and pH 8.5. Additionally, EstA exhibited higher activity towards short carbon substrates and showed the outstanding catalytic efficiency for pNPA2 with kcat/Km of 2296.14 ± 10.35 s−1 mM−1. Notably, EstA has hyper-thermostability and good alkali stability. The activity of EstA did not change obviously when incubated at 50 and 100 ℃ for 337 and 1 h, independently. Besides, by incubating at 100 ℃ for 6 h, EstA remained about half of its initial activity. Moreover, EstA showed stability at pH ranging from 8.0 to 11.0, and about 90% residual enzyme activity was reserved by being treated at pH 8.0 or 9.0 for 80 h, especially. Such multiple features prepare EstA for a potential candidate in the field of biological catalysis of some industrial applications under harsh conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esterases (EC 3.1.1.X), representing a diverse and ubiquitous group belonging to hydrolases, catalyze the formation and cleavage of ester bonds and are extensively distributed in animals, plants and microbes (Arpigny and Jaeger 1999; Bornscheuer 2002). Based on the substrate preference, they are divided into two major groups including carboxylesterases (EC 3.1.1.1) and triacylglycerol lipases (EC 3.1.1.3). Carboxylesterases prefer to accelerate the synthesis and hydrolysis of acylglycerols with relatively short-chain fatty acids (≤ 10 carbons) while lipases prefer to catalyze that with long-chain (≥ 10 carbons) fatty acids (Bornscheuer 2002). Traditionally, Arpigny and Jaeger proposed dividing esterases into eight representative families depended mainly on their fundamental biological properties and the difference among their amino acid sequences (Arpigny and Jaeger 1999). As more and more microbial esterases were identified, the classification of esterases has been extended to 18 families (Samoylova et al. 2018). Furthermore, according to our recent study, the classification of esterases has been expanded to 19 families (Wang et al. 2020). As an important group of biocatalysts, esterases display more unique characteristics: high stereo-, region-, and chemo-selectivity; Besides, there is usually no cofactor required during the reaction process (Bornscheuer 2002; Ramnath et al. 2017). These advantages make esterases are widely used in biochemical, industrial, pharmaceutical and biotechnological applications (Javed et al. 2018; Panda and Gowrishankar 2005).
Most industrial processes where enzymes are utilized undergo severe conditions, for instance, high temperature and alkaline conditions. Affected by harsh reactive situations, limited ability to remain stable of enzymes with high catalytic activity has aroused demand that searching robust enzymes possessing special traits such as good thermostability and alkali stability (Bora et al. 2013; Dumorne et al. 2017). Traditionally, new enzymes have been discovered by screening cell extracts with suitable substrates (Tesch et al. 1996; Xin and Hui-Ying 2013; Yong et al. 2016). Now, companying the progressively completed genome-sequencing database and developments of bioinformatics, searching and discovering enzymes with desired properties from the predicted gene becomes an important method in industrial biocatalysts (Brault et al. 2012; Li et al. 2018; Shestakov 2012; Soror et al. 2007).
Although existing in almost all living organisms, carboxylesterases originated from microbial sources show greater importance in industrial applications than from others. Streptomyces is a kind of spore-forming and Gram-positive bacteria in soil, which have active secondary metabolisms and are also responsible for producing numerous commercial antibiotics and biocatalysts (van Keulen and Dyson 2014). Esterases, as the representative secondary metabolites existed widely and abundantly in Streptomyces, have been researched gradually. For example, an extracellular esterase was characterized from S. diastatochromogenesa (Tesch et al. 1996), two cold-active esterases EstB and EstC were isolated and characterized from S. coelicolor A3(2) (Brault et al. 2012; Soror et al. 2007), a thermostable lipase MAS1 was screened and characterized from marine S. sp. strain W007 (Yuan et al. 2016), two thermostable esterase EstW and EstC were identified from S. lividans (Wang et al. 2016, 2020) and so forth. To our knowledge, S. lividans TK24 contained at least 59 open reading frames (ORFs) encoded for putative esterase according to the search result in the GenBank database. However, none of these putative esterase genes but EstC with hyper-thermostability and good alkali stability has been cloned and characterized so far (Wang et al. 2020). Therefore, S. lividans TK24 will be an ideal resource for esterases equipped with desirable characteristics with further researches. In this study, a carboxylesterase (EstA) from S. lividans TK24 was classified by analysis of the phylogenetic tree and constructed to a structural model based on the template found in the PDB database. In addition, our study demonstrated successful expression of EstA in E. coli, purification using Co2+ affinity chromatography, and identification for industrial applications.
Materials and methods
Strains, plasmids and chemicals
S. lividans TK24, Escherichia coli (E. coli) DH5α, Rosetta (DE3), as well as the expression vector pET-28b( +) were maintained at – 80 ℃. The recombinant plasmid, pET-estA', was synthesized artificially by Genscript (Nanjing, China). PrimeStar™ Max DNA polymerase and Co2+ metal affinity resin were obtained from TaKaRa (Dalian, China). Protein molecular weight standards and restriction enzymes were purchased from Thermo Fisher Scientific (Shanghai, China). Gel extraction kit and plasmid miniprep kit were gained from Axygen (California, USA). Kit of bacteria DNA was purchased from TIANGEN (Beijing, China). The pNP and pNP esters of different acyl chain lengths (pNPA2, pNPB4; pNPC6; pNPC8; pNPC10; pNPL12) were acquired from Sigma-Aldrich (St. Louis, MO, USA). Isopropyl-β-d-thiogalactopyranoside (IPTG) and bradford protein assay kit were bought from Sangon (Shanghai, China). All other chemicals were analysis reagents and used with no more purifying. Ultrapure water whose resistivity greater than 18.25 MΩ·cm was utilized by a Milli-Q Academic system (MA, USA) throughout this study.
Sequence comparison and phylogenetic analysis
Amino acid sequences of EstA with other esterases were downloaded from the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/gorf/gorf.html). Similarity searches of amino acid sequences were completed by protein BLAST (http://blast.ncbi.nlm.nih.gov/) against the public GenBank database and the SignalP 4.0 server (http://www.cbs.dtu.dk/services/SignalP/) was used to predict whether EstA had a signal peptide. Multiple alignments based on secondary structure were performed by Clustal W within BioEdit and further depicted by ESPript 3.0 (http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi). Mega 7.0 constructed the phylogenetic tree with 1000 replicates’ bootstrap test by the Neighbor-Joining (NJ) method. The protein structure of EstA was predicted and simulated with the SWISS-MODEL (http://swissmodel.expasy.org/). Subsequently, PyMOL was used to analyze the generated three-dimensional structural model.
Construction of plasmid and strain
Containing two restriction sites: NdeI (forward primer underlined) and XhoI (reverse primer underlined), a pair of homologous primers: EstA-F' (5′- GGAATTCCATATGATGTCAGTGTTACCGGGTGC -3′) and EstA-R' (5′- CCGCTCGAGAACCGCCGCCGGCGG -3′), was designed to amplify estA (Locus SLIV_RS27090) by PCR based on the S. lividans TK24 genomic DNA. PCR-amplification was as follows: 94 °C for 5 min, 35 cycles (94 °C for 30 s, 56 °C for 30 s, 72 °C for 1 min), 72 °C for 10 min. To construct pET-estA, the PCR purification digested by Nde I and Xho I was inserted into pET-28b( +). Then, pET-estA which verified using DNA sequencing was transformed into E. coli Rosetta for the heterologous expressing.
In addition, the gene named estA', encoding the same amino acid sequence as estA, was synthesized by artificial optimization and was constructed to the plasmid pET-estA'. Further, pET-estA' was transformed into E. coli Rosetta and was expressed as well.
Protein expression and purification
For heterologous expression, E. coli Rosetta (DE3) cells encompassing recombinant plasmids (pET-estA or pET-estA') were inoculated in the 100 mL (LB) with chloromycetin (25 μg mL−1) and kanamycin (30 μg mL−1) added. The culture was spun at 225 rpm and 37 ℃ until OD600 reached 0.5. Then, 0.1 mM IPTG was put into the culture following further culturing for 20–24 h at 20 ℃ and 180 rpm. Next, cells harvest lasted 5 min by centrifugation method at 4 ℃ and 5000 rpm. Harvested cells were broken with a Xingzhi SM-1000D ultrasonic wave cell pulverizer (Ningbo, China) on ice. In the process of cells breaking, the buffer that we selected is the mixture of 300 mM of NaCl and 50 mM of NaH2PO4 (pH 8.0). Subsequently, the crude enzyme liquid was centrifugated at 10,000 rpm for 20 min at 4 ℃ followed by the obtaining of the target protein (EstA) by purification of supernatant with Co2+ affinity chromatography. Finally, the purified protein was analyzed by 12% SDS-PAGE and determined on concentration using the Bradford method with BSA as the standard.
Determination of EstA's activities
To study EstA's activities, monitoring quantities of p-nitrophenol (pNP) discharged through esterase-catalyzed hydrolysis in 5 min at the OD410 using spectrophotometry. The assay of standard enzymatic activity was proceeded under the following conditions: at 25 ℃, within 1 mL volume including 10 μL diluted enzyme (0.02 mg mL−1), 980 μL 50 mM Tris–HCl buffer (pH 8.5), and 10 μL 50 mM pNPB4. Correspondingly, the mixture contained identical composition except for the enzyme was considered as a control to determine the spontaneous hydrolysis of the substrates. It is described as one enzyme unit (U) to require a certain quantity of enzymes for producing 1 μmol of pNP per minute at pH 8.5 and 25 ℃. All measurements were independently performed in triplicate.
Specificities of substrates and kinetics measurements
EstA's specificities of substrates were measured at standard reaction conditions with different pNP esters (C2–C12) as substrates. The kinetic parameters of EstA towards pNPA2, pNPB4 and pNPC6 were analyzed by measuring the pNP release rate using different final concentrations of substrates within a range of 0.01–1 mM under the condition of standard assays. The standard curve of pNP release was generated by the change of absorption value with different concentrations of pNP at 410 nm. On this basis, the value of Vmax and the value of Km were calculated with the employment of the Lineweaver–Burk plots by a fitted non-linear regression transform of the initial velocity of pNP release versus substrate concentration. Equation kcat = Vmax/[E], where [E] stands for the molar concentration of EstA, was employed to determine the catalytic rate constant kcat (s−1).
Influences of pH and temperature on EstA
To identify the optimal pH of EstA, the study monitored enzymatic activities at a range of pH 6.0–10.5 within the following conditions: 50 mM sodium acetate, pH 6.0–7.0; 50 mM Tris–HCl, pH 7.0–9.0; and 50 mM KH2PO4-KOH, pH 9.0–10.5. An optimal temperature was acquired within the range of 10–65 °C using pNPB4 as the substrate for every interval of 5 ℃.
Thermostability and pH stability of EstA
The thermostability of EstA was detected after independent incubations of EstA at various temperatures of 10–100 ℃ for 1 h. What's more, residual activities were determined after the pre-incubation of EstA at 50 and 100 °C for various periods, respectively, under standard conditions. Subsequently, to examine the pH stability under standard assay conditions, the residual activity of EstA was measured after incubation for a while at the pH range from 8.0 to 11.0 and 4 ℃. In all calculations, the activity of EstA without pre-incubation was 100% by default.
Impacts of metal ions and various chemical reagents on EstA
Impacts of metal ions (K+, Fe2+, Mn2+, Ca2+, Na+, Zn2+, Mg2+, Al3+, Cu2+, Co2+, Cr3+, Hg2+, Ni2+) and inhibitors (PMSF; EDTA) on EstA were analyzed with different ultimate concentrations which are 1 and 10 mM. Further, the influences of organic solvents (DMSO, methanol, ethanol, isopropanol, DMF, acetonitrile, acetone) and detergents (Triton X-100, SDS, CTAB, Tween-80, Tween-20) were determined at a volume percentage (v/v) of various final concentrations of 0.1 or 1.0%, and 15 or 30%, separately. Each of reactions was performed three times independently under the optimal pH at 25 °C and the activity of EstA without treatments was 100% by default.
The circular dichroism (CD), fluorescence and UV–Vis absorption spectroscopies
To identify the effects of temperature on enzyme structure, three states of EstA, including EstA without any treatment and EstA treated, respectively, at 100 ℃ for 2 and 6 h, were analyzed by CD, fluorescence and UV–Vis spectra. Far-UV CD spectra of EstA were recorded by the Jasco-810 Circular dichroism spectrometer (Tokyo, Japan). All protein concentrations were adjusted to 0.2 mg mL−1 in the buffer which is a mixture of 20 mM NaH2PO4 and 75 mM Na2SO4 (pH 7.5). At 50 nm/min scan speed and a 1 nm bandwidth, the data were collected at room temperature from 190 to 280 nm using the quartz cuvette with 1 mm path length. Each sample was measured in triplicates and the mean residue ellipticity ([θ], deg cm2 dmol−1) was calculated by the equation as follow:
Where mdeg represents for the measured CD data, l is the path length of quartz cuvette (1 mm), c stands for the protein molar concentration (mM), N represents the number of amino acid residues and the constant number 1000 stems from the conversion of the unit.
Fluorescence analysis was measured by a Shimadzu RF-5301PC spectrofluorometer (Tokyo, Japan). For monitoring changes of fluorescence, EstA was scanned at the excitation wavelength of 280 nm. The spectra were recorded in a range of 290–450 nm, excited at a slit width of 5 nm and emitted at that of 10 nm.
A UV–Vis spectrophotometer, Shimadzu UV-1700 (Tokyo, Japan), was the facility for acquiring the UV–Vis spectra of EstA. The absorbance of EstA was recorded between 200 and 800 nm according to the reading of 50 mM Tris–HCl buffer as the baseline.
Results
Sequences analysis of estA and estA'
The estA from S. lividans TK24 is an open reading frame (ORF) in a length of 804 bp. It exhibits a relatively high GC content (72.5%) which agrees with the former study that the genomic DNA of Streptomyces had a high GC content (Ruckert et al. 2015). Although the gene was cloned and the recombinant engineering bacteria Rosetta (DE3) pLysS/pET-estA was constructed successfully, the soluble expression of the estA in E. coli had not been completed due to the high GC content. For more conducive to the realization of the expression of the gene in E. coli, a gene named estA', was synthesized by artificial optimization and had been registered in the GenBank with an accession number: MN194168. Under the premise that the total length of the optimized gene sequence and the sequence of encoded amino acid remained consistent with estA, the artificial optimization of estA' was carried out by replacing the rare codons with the preferred codons of E. coli and decreasing the GC content from 72.5 to 54.0% (Supplementary Fig. S1).
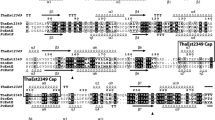
The protein EstA which was encoded by estA or estA' has 267 amino acids. Calculated molecular weight and predicted isoelectric point of EstA was 28.56 kDa and 6.71, respectively (http://isoelectric.org/calculate.php). The result of signal peptide prediction showed that the sequence did not have any kind of signal peptide. The BioEdit analysis revealed that EstA shared sequence identities of 82.3, 76.8, 62.6 and 47.6% with esterases from S. olindensis (KDN75141), S. filamentosus NRRL 15998 (EFE73899), S. sp. DJ (PLW72091) and Blastococcus sp. CCUG 61487 (TKJ30472) in the GenBank database, respectively (Supplementary Table S1). Hence, the multiple sequence alignment analysis based on the secondary structure was performed with the above esterases from other bacteria (Fig. 1). The result showed that EstA contained a conserved catalytic triad consisted of Ser93, Asp194 and His224, and the active site Ser93 residue was located within the conserved pentapeptide motif (Gly91-Leu92-Ser93-Met94-Gly95), which is a typical characteristic commonly found in esterases (Jaeger et al. 1999).
Multiple sequence alignment of EstA and other related proteins. GenBank accession numbers represent as follows: 4KEA, lipase from Bacillus sp. H-257; KDN75141, esterase from Streptomyces olindensis; EFE73899, esterase from Streptomyces filamentosus NRRL 15,998; PLW72091, esterase from Streptomyces sp. DJ; TKJ30472, carboxylesterase from Blastococcus sp. CCUG 61,487. The alignment was carried out with Clustal W and rendered by ESPript 3.0. Red triangles indicate amino acids forming the catalytic triad and the conserved pentapeptide motif was boxed by a black rectangle. The alpha helix, beta sheet, random coil, and beta turn are represented by “α”, “β”, “η”, and “T”, respectively
To explore the evolutionary relationship between EstA and other known esterases, the phylogenetic analysis was carried out according to Arpigny and Jaeger's classical classification (Arpigny and Jaeger 1999). After alignments of EstA with the five enzymes mentioned above and other family members, the constructed phylogenetic tree demonstrated that EstA was classified as family VI (Supplementary Fig. S2). Esterases, belonging to family VI, are one of the smallest ones known and possess molecular mass ranging from 23 to 26 kDa in most cases according to Arpigny (Arpigny and Jaeger 1999). Additionally, to further clarify the relationship between EstC and family VI enzymes, another neighbor-joining phylogenetic tree was constructed using five of family VI bacterial esterase amino acid sequences proposed in the original Jaeger’s paper and seven of that which had been identified and grouped in family VI to date. As depicted in Fig. 2, EstA and the five enzymes mentioned above were clustered with MHlip from Antarctic soil metagenome, once again demonstrating that EstA was a new number of this family. As shown in Table S2 in the supplementary, in terms of biochemical properties, except for R-est6, EstA and other identified family VI bacterial esterases displayed substrate specificity towards that with short carbon chains, which was consistent with the fact that all family VI esterases have the maximum activities with the small fatty acid chain esters and do not hydrolyze the long fatty acid chain esters (Soni et al. 2016). Besides, all of them severally exhibited maximum activity at a pH range of 6.5–9.0 and a temperature range 30–60 ℃. Unlike others, however, EstA showed superior thermostability and pH stability as it remained about half of its initial activity after incubation at 100 ℃ for 6 h and reserved about 90% residual enzyme activity by being treated at pH 8.0 or 9.0 for 80 h, respectively.
Phylogenetic relationship of EstA (closed triangle) and other proteins of family VI. "*" means the experimentally characterized enzymes. The tree based on the Neighbor-Joining method was constructed by Mega 7.0 with a bootstrap test of 1000 replicates. Except for EstA, other protein sequences were retrieved from GenBank and the accession numbers of the sequences are stated in brackets after each enzyme. The number near every branch indicates the reliability percentage in the bootstrap test of this branch
Structural model of EstA
A lipase (PDB code: 4KEA) used as a template was selected to create a homology model of EstA. As the Fig. 3 showed, the deduced structure adopted an α/β folding core and a partly cap region. In this whole 3D-structure of EstA, the central β-sheet composed of seven β-strands was surrounded by seven α-helices to form a spherical protein, and Ser93, Asp194, His224 gathering spatially to develop a catalytic triad positioned in a groove on the surface of the model. The other striking feature in the structure of EstA was a small ‘α-helix cap’, formed only four amino acid residues (His123, Gly124, Val125 and Ala126), located above the catalytic triad and covered the active site (Fig. 3a). Additionally, the electrostatic potential surface of EstA was shown (Fig. 3b). The blue areas representing the positive charges and the red areas representing the negative potential almost cover equally the surface of the protein.
The predicted structure and surface electrostatic potential analysis of EstA. a The EstA 3D model predicted by SWISS-MODEL with 4KEA as the template. The overall structure was composed of two main domains: an α/β hydrolase folding core and an α-helix cap. The alpha helix, beta sheet and random coil were colored in red, yellow and green, respectively. The cap region was represented in orange. The catalytic triad consisting of Ser93, Asp194 and His224 was indicated in stick representation. b The surface electrostatic potential of EstA analyzed by PyMOL. The cool color areas represented the positive charges and the warm color represented the negative charges
Expression and purification of EstA
Soluble expression of estA from S. lividans TK24 in E. coli Rosetta (DE3) had not been completed and the estA' gene synthesized artificially was successively heterogeneous expressed as a 6His-tagged fusion protein. Moreover, the homogeneity of protein was purified using Co2+ affinity chromatography from the crude enzyme liquid. The purifying on cell lysate using His-tag produced a purification of 4.11-fold and a 92.78% yield of activity (Supplementary Table S3). Subsequently, the purified protein exhibited a single band between 25 and 35 kDa on coomassie brilliant blue G-250 stained SDS-PAGE (12%) (Fig. 4). That proves the molecular mass of purified protein was close to the predicted one which is 28.56 kDa.
Determination of substrate specificities and kinetic parameters
The substrate specificity and kinetic parameters of EstA were measured using pNP esters with various lengths of acyl chain (C2–C12) (Table 1). The results showed that EstA displayed higher activity towards substrates with short carbon chains, such as pNPA2 (47.9 ± 0.7 U mg−1), pNPB4 (31.7 ± 0.8 U mg−1) and pNPC6 (16.1 ± 0.3 U mg−1). Meanwhile, Km values of EstA were decreased with acyl chain length (C2–C6) increasing, which corresponded with the results of substrate specificities. In addition, EstA displayed the outstanding catalytic efficiency for pNPA2 and pNPB4. Kinetic parameters (Km, Vmax, kcat/Km) for pNPA2 were 0.34 ± 0.01 mM, 16.4 ± 0.5 μM min−1 and 2296.1 ± 10.4 s−1 mM−1, respectively.
Impacts of pH and temperature on the EstA
Effects of pH on EstA were studied at 25 °C with the substrate of pNPB4. EstA displayed a maximum of its activity at pH 8.5 while maintained greater than 50% compared with its original enzyme activity within a range of pH 7.5–9.5 (Fig. 5a). Influences of temperature on EstA were performed by detecting the hydrolysis of pNPB at 10–65 °C. When conducting reactions at 45–65 °C, EstA showed more than 70% of its highest activity while the highest activity was observed at 55 °C. Additionally, under the temperature of 10 ℃, EstA maintained more than 20% of its highest activity (Fig. 5b).
Effects of pH and temperature on enzyme activity. a Effects of pH on EstA. The enzyme activity was measured in the following buffers at different values of pH from 6.0 to 10.5: 50 mM sodium acetate buffer (pH 6.0–7.0, black square), 50 mM Tris–HCl buffer (pH 7.0–9.0, red circle) and 50 mM KH2PO4-KOH buffer (pH 9.0–10.5, blue triangle). b Effects of temperature on EstA. The optimum temperature was measured from 10 to 60 °C in 50 mM Tris–HCl buffer (pH 8.5) and the maximum activity was defined as 100%. c Thermostability of EstA at 100 ℃ for 8 h. The enzyme was incubated at 100 ℃ for the indicated time and the residual activity was measured with the activity before incubation as 100%. d pH stability of EstA. The enzyme was incubated in various buffers with pH range from 8.0 to 11.0 (pH 8.0, black square; pH 9.0, red circle; pH 10.0, blue triangle; pH 11.0, green star) for 110 h, the activity of enzyme without any treatment was defined as 100%
The thermostability and pH stability of EstA were also determined. Surprisingly, EstA displayed hyper-thermostability and good alkali stability. Incubation at the temperature ranging from 10 to 100 °C for 1 h and 50 °C for 337 h hardly altered the activity of EstA (Supplementary Fig. S3). Furthermore, after incubation at 100 ℃, EstA maintained over 90% of the original activity for 2 h and it reached a half-life for about 6 h (Fig. 5c). Moreover, EstA kept good stability at a wide range of pH (from 8.0 to 11.0). This indicated that it was an alkali-stable enzyme. The enzyme activity of EstA was not changed with pre-incubated at pH 8.0–11.0 for 12 h, and it retained over 70% residual activity after 24 h. Furthermore, after incubation for 110 h at pH 9.0 (Tris–HCl buffer), it still held almost 50% of its initial activity (Fig. 5d).
Impacts of various additives on EstA
Impacts of different metal ions and inhibitors on EstA were determined under standard assay conditions (Table 2). EstA's activity was improved in the presence of 1 mM K+ (153.3%), Fe2+ (148.6%), Mn2+ (144.2%), Na+ (121.8%), Ca2+ (109.9%) and inhibited by 1 mM Hg2+ (73.4%) or Ni2+ (70.2%). When the concentration of ions was 10 mM, the activity of EstA was still stimulated by K+ (111.5%), Fe2+ (115.2%), Mn2+ (118.4%), Ca2+ (129.8%) or Mg2+ (109.2%) and was dramatically inhibited by Zn2+ (35.2%), Cu2+ (34.4%) or Ni2+ (10.2%). The activity of EstA had little effect by EDTA (1 and 10 mM) or PMSF (1 mM), but it was inhibited 27.4% by PMSF at 10 mM.
Additionally, EstA was slightly affected by all detected detergents at 0.1% concentration and strongly inhibited at 1.0% concentration by Span-20 (30.8%), SDS (35.6%) and CTAB (44.2%) (Table 3). Furthermore, the reaction mixture was added with various organic solvents to assess their influence on enzyme activity with a final concentration of 15 or 30% (Table 4). EstA was relatively stable in DMSO, ethanol, methanol, DMF, and was inhibited obviously in the presence of 30% acetone (48.3%) or acetonitrile (29.6%).
The analyses of CD, fluorescent changes and variations of UV–Vis spectra
Effects of temperature on EstA's structure were examined by measuring the changes of CD, fluorescence and UV–Vis spectroscopies. As for CD spectrums of untreated EstA, there was a positive peak at 193 nm and two negative spikes at 208 and 220 nm separately, which signifies a typical mixed α/β folding structure (Batumalaie et al. 2018). After incubation at 100 ℃ for 2 h, there was no significant change in the direction of the curve and peak positions, indicating that the native structural conformation and functionality were retained. However, when incubation time increased to 6 h, an obvious reduction of signal intensity occurred at 193 nm. In addition, two spikes of negative ellipticity were observed to shift to 200 and 215 nm, respectively (Fig. 6a). These results indicated that there was an apparent alternation of the secondary structure of EstA after heat treatment at 100 ℃ for 6 h compared to the intrinsic CD spectra, which was consistent with the conclusions of thermostability measurements.
For fluorescence and UV–Vis spectra, the absorption peaks are caused by individual amino acids. The maximum intensity on fluorescence and UV–Vis spectroscopies were detected at 340 and 280 nm when EstA in the native state, respectively. After incubation at 100 ℃ for 2 or 6 h, little shift of λmax and a decline of intensity on fluorescence spectra were observed (Fig. 6b), on the other hand, the UV–Vis spectra also showed no changes in intensity and λmax (Fig. 6c). Hence, as the heating time increased, the structure of EstA may be damaged to some extent, but the amino acid sequence as the primary structure remained stable.
Discussion
In our research, estA from S. lividans TK24 was successfully optimized and overexpressed in E. coli. The encoded protein (EstA) was characterized on substrates specificity, thermostability, alkali stability and influences of various additives, respectively. On the performance of substrates, EstA showed the highest affinity towards pNPA2 with a Km of 0.34 mM and the affinity for substrates declined along with the increase of carbon chain length by degrees. Beyond that, there was almost no enzyme activity detected when the length of carbon chains was greater than 10. So, we defined EstA as a carboxylesterase instead of a lipase.
The effects of various additives on EstA may be due to the fact of their binding to the certain binding sites which are on the surface of the molecular and changing catalytic property of the enzyme itself (Guncheva and Zhiryakova 2011). As for metal ions, EstA was activated by several metal cations such as K+, Fe2+, Mn2+ or Ca2+, in which K+, Fe2+ and Mn2+ exhibited higher increases. Similar enhancement of K+ and Mn2+ were observed in the Est903 located in a metagenomic library of paper mill sludge (Jia et al. 2019) and the lipase from Bacillus sp. VITL8 (Balaji and Jayaraman 2014), respectively. In most studies, Fe2+ was found to inhibit enzyme activities. Nevertheless, there are also a few esterases that can be activated in the presence of Fe2+. For instance, the enzyme activities of EstAS from activated sludge metagenome (Zhang and Han 2010) and EstUT1 from Ureibacillus thermosphaericus (Samoylova et al. 2018) were increased up to 104% by 1 mM Fe2+ and 152% by 10 mM Fe2+, respectively. Generally, Ca2+ was reported to be critical for enzyme activity. For example, the activities of lipase from Bacillus sp. strain L2 (Sabri et al. 2009) and LipS2 from Chromohalobacter canadensis (Ai et al. 2018) were increased fivefold and 57% in the presence of Ca2+, independently. For metal chelator, EstA is not a metal ion-dependent enzyme, as proven by that EDTA had few inhibitions on enzyme activity, corresponding with the fact that cofactors are not required in catalysis of EstC and EstW (Wang et al. 2016, 2020). Organic solvents and detergents which were detected in this study almost inhibited the activity of EstA in different degrees. This feature varies from that of lipase, which is usually switched on by detergents or organic solvents through enhancing access between the substrate and the hydrophobic binding site (Priyanka et al. 2019).
In terms of the predicted structure, the protein core of EstA employed a representative α/β hydrolase fold. Unlike a canonical fold composed of eight parallel β-strands, the central β-sheet of EstA only consisted of seven β-strands. There are analogous deviations that come from the canonical α/β hydrolase fold existing in LipA from Bacillus subtilis (van Pouderoyen et al. 2001) and MAS1 from S. sp. strain W007 (Yuan et al. 2016). A serine residue as a common feature of most esterases usually locates at the enzyme’s binding site, which causes enzymatic activities can be affected by serine inhibitors such as PMSF. Nonetheless, in this study, due to the presence of the ‘α-helix cap’, EstA was only slightly inhibited by PMSF even at a concentration of 10 mM. In fact, the cap region may keep the inhibitor from the enzyme’s active site, and this mechanism has been reported from a Pseudomonas aeruginosa lipase (Gaur et al. 2008) and a marine sediment esterase Lip3 (De Santi et al. 2016).
Generally, thermostability esterases have a broad prospect on applications with the basis of longer working life and better tolerance even in an extreme environment. In this study, the optimal temperature of EstA was 55 ℃ and the enzyme activity not only had no significant change after incubated at 50 ℃ for 337 h, but about 80% relative activity can also be maintained after the incubation at 100 ℃ for 4 h. Compared with some recently reported thermostability esterases (Table 5), the performance of EstA was better than E34Tt from Thermus thermophilus HB27 (Fuciños et al. 2011), EstDZ2 from a hot spring (Zarafeta et al. 2016), two lipases (ThLip1 and ThLip2) from Thermoanaerobacterium thermosaccharolyticum (Li et al. 2018), Est5250 from Bacillus thermocloaceae (Yang et al. 2019), EstW from S. lividans TK64 (Wang et al. 2016) and EstC from S. lividans TK24 (Wang et al. 2020). Additionally, many industrial applications are also performed under alkaline conditions. The most commercially important application of esterases is their addition to detergents due to their ability to remove fat stains and oil or fatty deposits from clothes in alkaline conditions. Simultaneously, esterases employed in the leather industry are mainly attributed to their ability to remove fats and grease from skins and hides in alkaline conditions. Consequently, for esterases, the alkali-stability is an especially important feature for their applications in the detergent and leather industry. In this study, EstA was highly active at pH 8.5 and showed good tolerance against alkaline conditions. The enzyme remained steady at the pH from 8.0 to 11.0. Furthermore, it even maintained over 90% activity compared with the original one after incubating for 80 h at pH 9.0, which was superior to most reported alkali-stable esterases, such as Est700 from Bacillus licheniformis (Zhang et al. 2018) and AMS8 from Antarctic Pseudomonas (Ganasen et al. 2016). Notably, compared with other esterases that are both thermostable and alkali-stable, EstA also had an advantage over MAS1 from Streptomyces (Yuan et al. 2016), an extracellular esterase from Bacillus cereus strain AGP-03 (Ghati and Paul 2015), Est903 from paper mill sludge (Jia et al. 2019) and EstC from S. lividans TK24 (Wang et al. 2020).
Furthermore, high catalytic efficiency is also essential for esterases on industrial applications. The specific activity of EstA towards short-chain esters (C2, 47.9 U mg−1; C4, 31.7 U mg−1; C6, 16.1 U mg−1) was greater than that of most thermostable esterases, such as Est-XG2 from the metagenomic library (Shao et al. 2013) (C2, 39.6 U mg−1; C4, 17.7 U mg−1; C6, 12.2 U mg−1) and EstW from S. lividans TK64 (Wang et al. 2016) (C2, 7.6 U mg−1; C4, 4.7 U mg−1; C6, 1.1 U mg−1). Additionally, kcat/Km value represents the catalytic efficiency of an enzyme at low substrate concentrations, it is generally used to objectively compare the catalytic efficiency of different enzymes. In this study, kinetic parameters of EstA were performed using the following substrates: pNPA2, pNPB4 and pNPC6, and the kcat/Km of EstA showed the highest value towards pNPA2 (2296.1 s−1 mM−1), which was consistent with the substrate specificity results. The value was also higher than most of thermostable esterases, such as EstC (1923.4 s−1 mM−1 for pNPA2) from S. lividans TK24 (Wang et al. 2020), Est5250 (1004.3 s−1 mM−1 for pNPA2) from Bacillus thermocloaceae (Yang et al. 2019) and Est903 (914.6 s−1 mM−1 for pNPB4) from a metagenomic library of paper mill sludge (Jia et al. 2019).
In conclusion, a carboxylesterase EstA from S. lividans TK24 being a member of family VI was successfully expressed and characterized. Under the condition of 55 °C and pH 8.5, EstA displayed maximum activity. On performances of substrates, EstA displayed outstanding catalytic efficiency for short-chain pNP esters (C2–C6), especially for pNPA2. Notably, EstA showed some attractive properties including hyper-thermostability and good alkali stability. The enzyme activity of EstA did not significantly fail after being incubated at 50 ℃ and pH 9.0 for 337 and 80 h, respectively. Such characteristics make EstA potential for various basic researches as well as industrial applications.
Abbreviations
- pNP:
-
p-Nitrophenol
- pNPA2:
-
p-Nitrophenyl acetate
- pNPB4:
-
p-Nitrophenyl butyrate
- pNPC6:
-
p-Nitrophenyl hexanoate
- pNPC8:
-
p-Nitrophenyl caprylate
- pNPC10:
-
p-Nitrophenyl decanoate
- pNPL12:
-
p-Nitrophenyl laurate
- IPTG:
-
Isopropyl-β-D-thiogalactopyranoside
- SDS-PAGE:
-
Sodium Dodecyl Sulphate–PolyAcrylamide Gel Electrophoresis
- BSA:
-
Bovine Serum Albumin
- EDTA:
-
Ethylenediaminetetraacetic acid
- PMSF:
-
Phenylmethylsulfonyl fluoride
- DMSO:
-
Dimethyl sulfoxide
- DMF:
-
Dimethylformamide
- SDS:
-
Sodium Dodecyl Sulphate
- CTAB:
-
Cetyltrimethylammonium bromide
References
Ai L, Huang Y, Wang C (2018) Purification and characterization of halophilic lipase of Chromohalobacter sp. from ancient salt well. J Basic Microbiol 58:647–657. https://doi.org/10.1002/jobm.201800116
Arpigny JL, Jaeger KE (1999) Bacterial lipolytic enzymes: classification and properties. Biochem J 343:177–183
Balaji L, Jayaraman G (2014) Metal ion activated lipase from halotolerant Bacillus sp. VITL8 displays broader operational range. Int J Biol Macromol 67:380–386. https://doi.org/10.1016/j.ijbiomac.2014.03.050
Batumalaie K, Khalili E, Mahat NA, Huyop F, Wahab RA (2018) Biophysical characterization of a recombinant lipase KV1 from Acinetobacter haemolyticus in relation to pH and temperature. Biochimie 152:198–210. https://doi.org/10.1016/j.biochi.2018.07.011
Bora L, Gohain D, Das R (2013) Recent advances in production and biotechnological applications of thermostable and alkaline bacterial lipases. J Chem Technol Biotechnol 88:1959–1970. https://doi.org/10.1002/jctb.4170
Bornscheuer UT (2002) Microbial carboxyl esterases: classification, properties and application in biocatalysis. FEMS Microbiol Rev 26:73–81. https://doi.org/10.1111/j.1574-6976.2002.tb00599.x
Brault G, Shareck F, Hurtubise Y, Lepine F, Doucet N (2012) Isolation and characterization of EstC, a new cold-active esterase from Streptomyces coelicolor A3(2). PLoS ONE 7:e32041. https://doi.org/10.1371/journal.pone.0032041
De Santi C, Altermark B, Pierechod MM, Ambrosino L, de Pascale D, Willassen NP (2016) Characterization of a cold-active and salt tolerant esterase identified by functional screening of Arctic metagenomic libraries. BMC Biochem 17:1–13. https://doi.org/10.1186/s12858-016-0057-x
Dumorne K, Cordova DC, Astorga-Elo M, Renganathan P (2017) Extremozymes: a potential source for industrial applications. J Microbiol Biotechnol 27:649–659. https://doi.org/10.4014/jmb.1611.11006
Fuciños P, Pastrana L, Sanromán A, Longo MA, Hermoso JA, Rúa ML (2011) An esterase from Thermus thermophilus HB27 with hyper-thermoalkalophilic properties: purification, characterisation and structural modelling. J Mol Catal B Enzym 70:127–137. https://doi.org/10.1016/j.molcatb.2011.02.017
Ganasen M, Yaacob N, Rahman RN, Leow AT, Basri M, Salleh AB, Ali MS (2016) Cold-adapted organic solvent tolerant alkalophilic family I.3 lipase from an Antarctic Pseudomonas. Int J Biol Macromol 92:1266–1276. https://doi.org/10.1016/j.ijbiomac.2016.06.095
Gaur R, Gupta A, Khare SK (2008) Purification and characterization of lipase from solvent tolerant Pseudomonas aeruginosa PseA. Process Biochem 43:1040–1046. https://doi.org/10.1016/j.procbio.2008.05.007
Ghati A, Paul G (2015) Purification and characterization of a thermo-halophilic, alkali-stable and extremely benzene tolerant esterase from a thermo-halo tolerant Bacillus cereus strain AGP-03, isolated from ‘Bakreshwar’ hot spring, India. Process Biochem 50:771–781. https://doi.org/10.1016/j.procbio.2015.01.026
Guncheva M, Zhiryakova D (2011) Catalytic properties and potential applications of Bacillus lipases. J Mol Catal B Enzym 68:1–21. https://doi.org/10.1016/j.molcatb.2010.09.002
Jaeger KE, Dijkstra BW, Reetz MT (1999) Bacterial biocatalysts: molecular biology, three-dimensional structures, and biotechnological applications of lipases. Annu Rev Microbiol 53:315–351. https://doi.org/10.1146/annurev.micro.53.1.315
Javed S, Azeem F, Hussain S, Rasul I, Siddique MH, Riaz M, Afzal M, Kouser A, Nadeem H (2018) Bacterial lipases: a review on purification and characterization. Prog Biophys Mol Biol 132:23–34. https://doi.org/10.1016/j.pbiomolbio.2017.07.014
Jia ML, Zhong XL, Lin ZW, Dong BX, Li G (2019) Expression and characterization of an esterase belonging to a new family via isolation from a metagenomic library of paper mill sludge. Int J Biol Macromol 126:1192–1200. https://doi.org/10.1016/j.ijbiomac.2019.01.025
Li W, Shi H, Ding H, Wang L, Zhang Y, Li X, Wang F (2018) Characterization of two novel thermostable esterases from Thermoanaerobacterium thermosaccharolyticum. Protein Expr Purif 152:64–70. https://doi.org/10.1016/j.pep.2018.04.010
Panda T, Gowrishankar BS (2005) Production and applications of esterases. Appl Microbiol Biotechnol 67:160–169. https://doi.org/10.1007/s00253-004-1840-y
Priyanka P, Tan Y, Kinsella GK, Henehan GT, Ryan BJ (2019) Solvent stable microbial lipases: current understanding and biotechnological applications. Biotechnol Lett 41:203–220. https://doi.org/10.1007/s10529-018-02633-7
Ramnath L, Sithole B, Govinden R (2017) Classification of lipolytic enzymes and their biotechnological applications in the pulping industry. Can J Microbiol 63:179–192. https://doi.org/10.1139/cjm-2016-0447
Ruckert C, Albersmeier A, Busche T, Jaenicke S, Winkler A, Friethjonsson OH, Hreggviethsson GO, Lambert C, Badcock D, Bernaerts K, Anne J, Economou A, Kalinowski J (2015) Complete genome sequence of Streptomyces lividans TK24. J Biotechnol 199:21–22. https://doi.org/10.1016/j.jbiotec.2015.02.004
Sabri S, Rahman RN, Leow TC, Basri M, Salleh AB (2009) Secretory expression and characterization of a highly Ca2+-activated thermostable L2 lipase. Protein Expr Purif 68:161–166. https://doi.org/10.1016/j.pep.2009.08.002
Samoylova YV, Sorokina KN, Romanenko MV, Parmon VN (2018) Cloning, expression and characterization of the esterase estUT1 from Ureibacillus thermosphaericus which belongs to a new lipase family XVIII. Extremophiles 22:271–285. https://doi.org/10.1007/s00792-018-0996-9
Shao H, Xu L, Yan Y (2013) Isolation and characterization of a thermostable esterase from a metagenomic library. J Ind Microbiol Biotechnol 40:1211–1222. https://doi.org/10.1007/s10295-013-1317-z
Shestakov SV (2012) Impact of metagenomics on biotechnological development. Appl Biochem Microl 48:705–715. https://doi.org/10.1134/s0003683812090050
Soni S, Odaneth AA, Lali AM, Chandrayan SK (2016) Expression, purification and biochemical characterization of a family 6 carboxylesterase from Methylococcus capsulatus (bath). Protein Expres Purif 122:31–37. https://doi.org/10.1016/j.pep.2016.02.007
Soror SH, Verma V, Rao R, Rasool S, Koul S, Qazi GN, Cullum J (2007) A cold-active esterase of Streptomyces coelicolor A3(2): from genome sequence to enzyme activity. J Ind Microbiol Biotechnol 34:525–531. https://doi.org/10.1007/s10295-007-0224-6
Tesch C, Nikoleit K, Gnau V, Gotz F, Bormann C (1996) Biochemical and molecular characterization of the extracellular esterase from Streptomyces diastatochromogenes. J Bacteriol 178:1858–1865. https://doi.org/10.1128/jb.178.7.1858-1865.1996
Keulen GV, Dyson PJ (2014) Production of specialized metabolites by Streptomyces coelicolor A3(2). Adv Appl Microbiol 89:217–266. https://doi.org/10.1016/B978-0-12-800259-9.00006-8
Pouderoyen GV, Eggert T, Jaeger KE, Dijkstra BW (2001) The crystal structure of Bacillus subtilis lipase: a minimal alpha/beta hydrolase fold enzyme. J Mol Biol 309:215–226. https://doi.org/10.1006/jmbi.2001.4659
Wang B, Wang A, Cao Z, Zhu G (2016) Characterization of a novel highly thermostable esterase from the Gram-positive soil bacterium Streptomyces lividans TK64. Biotechnol Appl Biochem 63:334–343. https://doi.org/10.1002/bab.1465
Wang B, Wu S, Chang X, Chen J, Ma J, Wang P, Zhu G (2020) Characterization of a novel hyper-thermostable and chlorpyrifos-hydrolyzing carboxylesterase EstC: a representative of the new esterase family XIX. Pestic Biochem Phys 170:104704. https://doi.org/10.1016/j.pestbp.2020.104704
Xin L, Yu H (2013) Purification and characterization of an extracellular esterase with organic solvent tolerance from a halotolerant isolate, Salimicrobium sp. LY19. BMC Biotechnol 13:108. https://doi.org/10.1186/1472-6750-13-108
Yang Y, Ghatge S, Hur HG (2019) Characterization of a novel thermostable carboxylesterase from thermoalkaliphilic bacterium Bacillus thermocloaceae. Biosci Biotechnol Biochem 83:882–891. https://doi.org/10.1080/09168451.2019.1574555
Yong SK, Lim BH, Saleh S, Tey L-H (2016) Optimisation, purification and characterisation of extracellular lipase from Botryococcus sudeticus (UTEX 2629). J Mol Catal B Enzym 126:99–105. https://doi.org/10.1016/j.molcatb.2016.02.004
Yuan D, Lan D, Xin R, Yang B, Wang Y (2016) Screening and characterization of a thermostable lipase from marine Streptomyces sp. strain W007. Biotechnol Appl Biochem 63:41–50. https://doi.org/10.1002/bab.1338
Zarafeta D, Moschidi D, Ladoukakis E, Gavrilov S, Chrysina ED, Chatziioannou A, Kublanov I, Skretas G, Kolisis FN (2016) Metagenomic mining for thermostable esterolytic enzymes uncovers a new family of bacterial esterases. Sci Rep 6:38886. https://doi.org/10.1038/srep38886
Zhang T, Han W (2010) Gene cloning and characterization of a novel esterase from activated sludge metagenome. Microb Cell Fact 8:67. https://doi.org/10.1186/1475-2859-9-25
Zhang W, Xu H, Wu Y, Zeng J, Guo Z, Wang L, Shen C, Qiao D, Cao Y (2018) A new cold-adapted, alkali-stable and highly salt-tolerant esterase from Bacillus licheniformis. Int J Biol Macromol 111:1183–1193. https://doi.org/10.1016/j.ijbiomac.2018.01.152
Acknowledgments
To start with, we would like to admit that, without the support of the National Natural Science Foundation of China (31400003, 31600167), this work could not have been accomplished. Next, Thanks also go to the Nature Science Foundation of Anhui Province (1608085MC67), the Provincial Project of Natural Science Research for Colleges and Universities of Anhui Province of China (KJ2016A274, KJ2019A0498), and the Postgraduate Research Innovation Program of Anhui Normal University (2019kycx055). In addition, we would like to keep a special appreciated note to the Innovation Team of Scientific Research Platform owned by Anhui Universities. Finally, I was deeply honored by getting hold of the supporting from Anhui Provincial Key Laboratory of the Conservation and Exploitation of Biological Resources.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and experiments were performed by XC, SW, AW, JC and SX. The data collection and analysis were performed by BW, AW, XC, SW, PW, and XS. The first draft of the manuscript was written by BW, AW, XC and SW, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by H. Atomi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, X., Wu, S., Chen, J. et al. Characterization of a carboxylesterase with hyper-thermostability and alkali-stability from Streptomyces lividans TK24. Extremophiles 25, 115–128 (2021). https://doi.org/10.1007/s00792-021-01215-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-021-01215-2