Abstract
Pseudomonas extremaustralis is an Antarctic bacterium with high stress resistance, able to grow under cold conditions. It is capable to produce polyhydroxyalkanoates (PHAs) mainly as polyhydroxybutyrate (PHB) and, to a lesser extent, medium-chain length polyhydroxyalkanoates (mclPHAs). In this work, we analyzed the role of PHAs and cold adaptation in the survival of P. extremaustralis after lethal UVA exposure. P. extremaustralis presented higher radiation resistance under polymer accumulation conditions. This result was also observed in the derivative mutant strain PHA−, deficient for mclPHAs production. On the contrary, the PHB− derivative mutant, deficient for PHB production, showed high sensitivity to UVA exposure. Complementation of the PHB− strain restored the wild-type resistance level, indicating that the UVA-sensitive phenotype is due to the lack of PHB. All strains exhibited high sensitivity to radiation when cultured under PHAs non-accumulation conditions. A slight decrease in PHB content was observed after UVA exposure in association with increased survival. The scattering of UVA radiation by intracellular PHAs granules could also result in bacterial cell protection. In addition, cold conditions improved UVA tolerance, probably depending on PHB mobilization. Results showed that PHB accumulation is crucial in the resistance to UVA in P. extremaustralis. Mechanisms involved probably entail depolymerization and light scattering acting as a screen, both conferring protection against oxidative stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solar ultraviolet (UV) radiation induces deleterious effects in living organisms. For study purposes and according to its interaction with living matter, this light has been subdivided into UVA (320–400 nm), UVB (280–320 nm) and UVC (100–280 nm). Most solar UVB and UVC are absorbed by the ozone layer in the stratosphere, and does not reach the Earth’s surface; while solar UVA passes almost unaltered through the atmosphere (Santos et al. 2013). UVA is able to penetrate deeper than UVB, but is less damaging. UVA provokes indirect biological effects related to the generation of reactive oxygen species (ROS). ROS produce oxidative damage in lipids, proteins and DNA (Chamberlain and Moss 1987; Hu and Tappel 1992; Agogué et al. 2005; Pattison and Davies 2006; Zeeshan and Prasad 2009; Girard et al. 2011). Both UVB and UVA can have important ecological effects in aquatic and terrestrial ecosystems by affecting, for example, bacterial activities, phytoplankton photosynthesis, gene expression and flavonoid accumulation in plants and photochemical transformations of dissolved organic matter (Wilson et al. 2001; Tedetti and Sempéré 2006; Morales et al. 2010).
Bacteria show different susceptibilities to UV radiation that can be attributed to differences in the molecular targets to UV-induced damage and in the development of tolerance strategies and repair mechanisms that contribute to the ecological fitness (Borić et al. 2011; Santos et al. 2013). Strategies comprise the development of physiological responses, changes in gene expression and the presence of biomolecules, such as pigments or other secondary metabolites that allow bacteria to cope with UV-derived stress. Studies in different bacterial genera have demonstrated that adaptive responses to UVA exposure involve activation of genes coding for enzymes responsible for ROS detoxification and DNA repair (Kidambi et al. 1996; Qiu et al. 2005; Berney et al. 2006; Soule et al. 2013; Sassoubre et al. 2014). Additionally, lipid storage compounds, as triacylglycerols (Bequer Urbano et al. 2013) and polyhydroxyalkanoates (PHAs) have been described as involved in UV resistance (Tal and Okon 1985). PHAs are reserve polymers accumulated in intracellular granules, as dynamic reservoirs of carbon and reducing equivalents that increase survival and resistance to multiple stress factors (López et al. 2015). PHAs are classified according to their monomer composition in short-chain-length PHAs (sclPHAs), composed by C3–C5 monomers, and medium-chain-length PHAs (mclPHAs) that contain C6–C16 monomers. The most common and widely distributed PHA is polyhydroxybutyrate (PHB), composed by C4 monomers. Some studies have reported the role of PHAs in protection against UVC radiation in natural PHAs producers and recombinant Escherichia coli strains (Tal and Okon 1985; Kadouri et al. 2003; Zhao et al. 2007; Wang et al. 2009).
Pseudomonas extremaustralis is an extremophile bacterium, isolated from a temporary water pond in Antarctica (López et al. 2009). Genome analysis of this bacterium revealed a high potential for adaptability to extreme environments (Tribelli et al. 2012). P. extremaustralis is able to grow under cold conditions as well as at moderate temperatures and shows high stress resistance associated with the production of high amounts of PHAs, mainly accumulated as PHB (Ayub et al. 2004; Catone et al. 2014). PHB production is an uncommon characteristic in Pseudomonas spp. that usually synthesize mclPHAs (Kessler and Palleroni 2000). In P. extremaustralis, PHB accumulation was found to be important for oxidative stress resistance and essential for cold growth, freezing survival and for the maintenance of a planktonic life style at low temperatures (Ayub et al. 2009, Tribelli and López 2011), highlighting the relevance of this polymer for bacterial fitness.
Microorganisms that inhabit Antarctic areas are exposed to a wide range of stressful environmental conditions such as low temperatures, broad changes in luminosity and high UV radiation. It is expected that these microorganisms will display different strategies to thrive under these harsh environmental conditions. The aim of this work was to analyze the contribution of PHAs in the resistance to UVA exposure in P. extremaustralis under different growth conditions. We used genetic approaches involving mutant construction along with the determination of the polymer content, spectrophotometric analysis and the evaluation of oxidative stress indicators to assess the relevance of different kinds of PHAs on survival to UVA exposure.
Methodology
Strains, plasmids and culture conditions
Strains and plasmids employed in this study are described in Table 1. P. extremaustralis 14-3b (Tribelli et al. 2012), hereinafter the wild type, and its isogenic derivatives deficient for PHAs production (Ayub et al. 2009; Tribelli et al. 2010; Catone 2013) were grown in complete LB broth (10-g tryptone, 5-g yeast extract and 5-g NaCl bringing the volume up to 1000 mL in distilled water). For solid medium, 15 g/L agar was added. For PHAs accumulation, the LB medium was supplemented with 0.25% sodium octanoate (LBO) (López et al. 2009). When necessary, antibiotics were added at the following concentrations: 20 µg/mL kanamycin (Km), 10 µg/mL gentamicin (Gm) and 10 µg/mL tetracycline (Tc). Cultures were performed under aerobic conditions (200 rpm) at 30 °C for 24 h or at 10 °C for 72 h.
For complementation studies, the entire P. extremaustralis phbC gene containing its native promoter was amplified by PCR and cloned into the pGEM-T Easy Plasmid (Promega) and subsequently cloned into pBBR1MSC-3 (Kovach et al. 1995). The resulting plasmid, named pMCS3Cbsec, was introduced into the PHB− strain by conjugation according to the methodology of Friedrich et al. (1981). Cells from the transconjugant colonies were verified to analyze their ability to produced PHB as previously described (Catone et al. 2014).
Irradiation source
Cell suspensions were irradiated from above with two Philips TDL 18 W/08 tubes (more than 95% of the UVA emission at 365 nm) mounted on a bench, with aluminum-anodised reflectors, to enhance the fluence rate on the section to be irradiated. The incident fluence was measured at the surface of the suspension with a 9811.58 Cole-Parmer Radiometer (Cole-Parmer Instruments Co., Chicago, IL). The fluence rate employed (20 W/m2) may be normally encountered in the environment (Hoerter et al. 2005), and it was obtained by placing the tubes about 18 cm from the suspension surface.
Irradiation procedure
Bacteria were grown at 10 °C or 30 °C to stationary phase in LB or LBO. Cultures were centrifuged (10,000 g, 10 min, 20 °C) and the cell pellets washed once and suspended in saline solution (NaCl 0.1 M) at OD650 0.4. The suspensions were divided into two 30-mL fractions, which were each placed in a glass beaker (diameter of the exposed surface 5 cm) open to air. One of these fractions was irradiated from above at a fluence rate of 20 W/m2 at the level of the free surface of the suspension, while the other fraction remained in the dark. Samples of cell suspensions exposed to UVA radiation or maintained in the dark were taken at the indicated times and plated on LB solid medium after dilution with 0.1 M NaCl. Plates were immediately incubated at 30 °C in the dark to prevent light-induced DNA repair and the colonies were counted after 24–48 h. Survival was expressed as a fraction of the number of colony forming units (CFU) per mL at initial time (t0).
Catalase assay
To determine total catalase activity, stationary phase cultures were centrifuged at 10,000 g for 10 min at 4 °C. The cell pellet was suspended in ice-cold 50 mM sodium phosphate buffer (pH = 7), sonicated in an ice-water bath, and clarified by centrifugation at 12,000 g for 10 min at 4 °C to obtain a cell extract. Total catalase activity was monitored by following the decomposition of 10 mM H2O2 according to Aebi (1984). One unit of activity was that which decomposes 1 µmol of hydrogen peroxide per min per mg of protein. Protein content was determined by Lowry’s method (Lowry et al. 1951).
Sensitivity to H2O2
Sensitivity to H2O2 was determined as previously described (Hasset et al. 1995). Briefly, sterile Whatman No. 1 filter disks (6 mm diameter) impregnated with 5 µL of 30% H2O2 (Merck) were placed on top of LB plates seeded with cultures grown until stationary phase in LB or LBO media. Inhibition zones were measured after incubation at 30 °C for 24 h.
Cellular redox state measurement
Samples of 1 mL from dark- and UVA-exposed bacterial suspensions were centrifuged and the pellets were used to determine the NADH/NAD+ ratios. Briefly, the pellets were transferred to pre-cooled plastic tubes and the metabolic activity was quenched by immersion of the tubes in liquid N2. These pellets were further used to determine the dinucleotide content using a commercial kit for NADH/NAD+ measurement (MAK037, Sigma) following the manufacturer’s instructions. The dinucleotide content was normalized to the OD600.
Gas chromatography analysis of PHAs
Cellular PHAs quantification and monomer composition was determined from lyophilized cell pellets subjected to methanolysis (Braunegg et al. 1978), using methanol containing 15% (v/v) of H2SO4 and chloroform. This mixture was incubated at 100 °C for 140 min in an oil bath. Benzoic acid (0.5 mg/mL) was used as internal standard (Manso Cobos et al. 2015). The resulting methyl esters of monomers were analyzed using an Agilent 7820, a gas chromatographer (GC) with flame ionization detector (FID) and an automatic liquid sampler ALS 7693. The separation was conducted on a HP-5 capillary column (30 m; 0.25-µm film thickness and 0.25-mm ID). The GC oven was initially heated at 40 °C for 0.5 min, then to 65 °C at 5 °C/min. Finally, the oven was ramped to 130 °C at 15 °C/min, which was held for 3 min. The injector and FID temperatures were set at 250 °C and 300 °C, respectively. Nitrogen was used as carrier gas at a flow rate of 3 mL/min. The injection volume was 1 µL with a 45:1 split ratio. Standard solutions were prepared using poly (3-hydroxybutyric acid) (SIGMA Aldrich) and mclPHAs from Pseudomonas putida KT2440. Triplicate experiments from independent cultures were performed with three GC measurements for each sample. The PHAs content was calculated as percentage of cell dry weight (CDW). PHAs monomers composition was confirmed by GC–mass spectrometry using the service of the Department of Organic Chemistry (FCEN-UBA).
Spectrophotometric assays of P. extremaustralis cells
P. extremaustralis was cultured until stationary growth phase at 30 °C with shaking in LB or LBO. The cultures were centrifuged (10 min, 10,000 g, 20 °C) and the cell pellets were washed with saline solution. Cell suspensions were centrifuged again under the same conditions and suspended in saline solution to achieve a cell concentration of 5 × 107 CFU/mL. The spectra of the cell suspensions were recorded against saline solution in standard 1-cm cuvettes. For turbidity assays, measurements were made with a Mecasys Optizen Alpha spectrophotometer. For absorption assays, measurements were made with a Shimadzu 3600 plus spectrophotometer equipped with an integrating sphere.
Cell dimensions (length and width) were determined in stationary phase cultures in LB and LBO by microscopy as the average of at least 30 Nile Blue-stained cells using ImageJ software.
Statistical analysis
The significance of each treatment was evaluated by Student’s t test with confidence levels at 95% (i.e., P < 0.05 was considered as significant).
Results
Role of PHAs in the response to lethal UVA exposure in P. extremaustralis
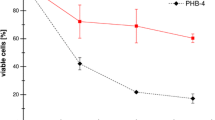
To determine the role of PHAs in the defense against lethal UVA doses in P. extremaustralis, the wild-type and its derivative mutants PHB− and PHA− (Table 1) were exposed to this radiation under PHAs accumulation and non-accumulation conditions (LBO and LB). Similar samples were kept in the dark as control. As shown in Fig. 1, all strains exhibited high sensitivity to radiation when cultured in LB, showing not significant differences between them after 300 min of UVA exposure (P > 0.05). When bacterial strains were cultured in LBO, a significant increase in cell viability (P < 0.05) was observed for the wild type and the PHA− strain compared to the PHB− mutant (Fig. 1). Complementation of the PHB− strain by introduction of plasmid pMCS3Cbsec (Table 1) restored the wild-type phenotype, demonstrating that the UVA-sensitive phenotype is due to the lack of PHB (Fig. 1). Strains kept in the dark did not show significant changes in cell survival (data not shown).
Survival curves of P. extremaustralis and PHAs mutants exposed to lethal UVA doses. Suspensions of stationary phase cells grown in LB or LBO at 30 °C were exposed to a fluence rate of 20 W/m2 for 300 min (total dose 360 kJ/m2) or kept in the dark. Samples were taken at different times and plated to determine survival. Error bars represent standard deviations of at least three independent experiments. *P < 0.05
Analysis of PHAs composition before and after UVA exposure
Monomers composition of the PHAs present at the beginning and after UVA exposure was determined by GC analysis of methanolized samples (Table 2). Controls in dark conditions were also analyzed. In LB cultures, the polymer was not detected. P. extremaustralis accumulated both PHB and mcl-PHAs when sodium octanoate was added to the culture medium (Table 2). PHB production was on average around of 47% at initial time when cultures were incubated at 30 °C and this value did not show significant differences in comparison with dark control (Table 2, P > 0.05). A 20% decrease in PHB content was observed after 300 min of UV exposure; however, this difference was not statistically significant (Table 2, P > 0.05). Accumulation of mclPHAs composed by C6 and C8 monomers was lower in comparison with PHB (Table 2) and differences between the initial time and after treatment (irradiation and dark control) either were not significant (P > 0.05).
Oxidative stress indicators
In P. aeruginosa the main catalase, KatA, is essential for the optimal response against lethal doses of UVA (Costa et al. 2010; Pezzoni et al. 2014). Catalase decomposes hydrogen peroxide, one of the ROS involved in the toxic effects of UVA radiation. To analyze the role of this enzyme in the PHB-dependent UVA response of P. extremaustralis, we analyzed catalase activity of cultures grown in LB and LBO of the wild type and its derivatives deficient for PHAs production. The initial catalase activity was similar between all the strains and independent of the presence of sodium octanoate in the culture medium (Fig. 2a). The results suggest that this enzyme is not responsible for the UVA survival difference observed in the PHB− mutant strain.
Total catalase activity (a) and sensitivity to hydrogen peroxide (b) of P. extremaustralis and PHAs mutants grown at 30 °C in LB or LBO. Fold change of NADH/NAD+ ratio of P. extremaustralis cells grown on LB or LBO after 300 min of UVA exposure; values were relativized to the initial time (c). Error bars represent standard deviations of three independent experiments. **P < 0.05 LB versus LBO for each strain; *P < 0.05 wild type versus PHAs mutants in LBO
In addition, sensitivity to H2O2 was assayed (Fig. 2b). The wild type and its PHAs mutants showed increased tolerance to H2O2 when grown in LBO compared to LB (P < 0.05). All strains showed no significant differences between them when grown in LB. However, in the presence of octanoate, both PHA− and PHB− were significantly more sensitive to H2O2 compared to the wild type (P < 0.05, Fig. 2b). These results indicate that the accumulation of both PHB and mclPHA have a role in peroxide resistance in P. extremaustralis.
After degradation, PHAs can be a source of NADH or NADPH and these molecules are frequently cofactors of antioxidant enzymes (Cabiscol et al. 2000). Because of this, we also determined the cellular redox state after UVA irradiation. At the end of the experiment, the cellular redox state relative to the initial time under PHA accumulation conditions (LBO) was more reduced in comparison with that found in cells cultivated in LB, although these differences were not significant (Fig. 2c). The results suggest that degradation of PHAs can lead to a higher NADH availability to cope with UVA stress.
Interaction of PHAs granules with UVA radiation
A preventive mechanism to face UVA radiation is related to the presence of metabolites that act as shields. These compounds absorb or deviate the UV radiation preventing photons from reaching cellular targets, ensuring the maintenance of cell viability (Gao and Garcia-Pichel 2011). Two mechanisms have been described to explain the interaction of these metabolites with UV radiation: light scattering and light absorption. Scattering consists in the deviation of photons from its trajectory by heterogeneities in the cells or the medium. Absorption is the capture of photons by photoactive molecules, causing photochemical and, eventually, photobiological effects. To deepen in the mechanism related to the role of PHAs in the defense against UVA in P. extremaustralis, we investigated the possibility that granules act as a protective shield. First, OD or turbidity (which represents the sum of light scattering and absorption) between 190 and 800 nm was measured with a standard spectrophotometer. This range involved part of the UVC and the complete UVB–UVA–Vis regions (Fig. 3a). As OD measurements depends (among other parameters) on cell size, analyses of cell dimensions of P. extremaustralis under accumulation and non-accumulation conditions were performed. Microscopy analysis revealed that when bacteria were grown in LBO, cell dimensions were on average 1.85 ± 0.087 in length and 0.83 ± 0.083 width; whereas, when PHAs were not accumulated (LB), cells showed a slight decrease being 1.73 ± 0.084 in length and 0.66 ± 0.075 in width (P < 0.05). As shown in Fig. 3a, when cells were grown in LBO, the OD was higher throughout the whole spectrum compared to the same number of control cells. At 365 nm, the predominant UVA wavelength employed in this study, the OD of the PHAs-accumulating cells duplicated that of the control culture. To analyze a possible photoactive effect of PHAs, a similar analysis was performed but using a UV–Vis spectrophotometer integrating sphere system. Using this approach, the effect of light scattering was reduced and absorbance instead of OD was indicated in the Y axis (Fig. 3b). Figure 3b shows that beyond 300 nm, the region comprising part of the UVB and the whole UVA and visible regions, light was absorbed by neither of two suspensions. Below 300 nm (UVC and part of UVB region), a marked absorption was observed by both suspensions. A peak around 260 nm could be differentiated by this methodology, about 1.6-fold higher in the PHA-accumulating condition compared to the control (Fig. 3b), perhaps due to absorption by granules. These results suggest that light scattering could be another mechanism contributing to the protective effect of PHB against UVA radiation.
UV–Vis spectra of P. extremaustralis wild-type cells. Suspensions of stationary phase cells (5 × 107/mL) grown in LB or LBO were assayed for OD (a) and absorbance (b) in the wavelength range between 190 and 800 nm (190–280, UVC; 280–320, UVB; 320–400, UVA; 400–800, Visible). Representative spectra are shown
Effect of cold growth in the response to lethal UVA exposure in P. extremaustralis
Results showed that several mechanisms involving PHA accumulation could act in concert explaining increased UVA resistance in P. extremaustralis. This bacterium is capable to grow well under cold conditions (Ayub et al. 2009). As low temperature causes cellular stress including the production of ROS with the concomitant biomolecules damage (Chattopadhyay et al. 2011), we evaluated the tolerance to UVA exposure under these harsh conditions. We hypothesized that since bacteria grown under low temperature display a wide battery of resistance mechanisms, they will be more resistant to UVA exposure. Survival after UVA exposure was higher when bacteria were grown at 10 °C in comparison with 30 °C (Fig. 4) (P < 0.05). These differences were higher under non-accumulation conditions. Under cold conditions, increased survival was observed when bacteria were grown in LBO in comparison with LB (Fig. 4), showing significant differences (P < 0.05). Accumulation of both kinds of PHAs was lower under low temperature cultivation compared to 30 °C, mainly in the case of PHB (Table 2); however, a significant decrease in PHB content was observed, reaching undetectable values by GC measurements after UVA exposure (Table 2). In P. extremaustralis PHA accumulation is necessary to grow under cold conditions, as impaired grow in nutrient broth was observed (Ayub et al. 2009). When cultured in LB under cold conditions slight growth was observed, but cells in that conditions showed higher survival to UVA exposure, suggesting a different physiological state of cell conferred by cold growth. Additionally, the initial catalase activity under these conditions was analyzed. The wild type grown both in LB or LBO showed similar values (LB 37 ± 7 U/mg; LBO 35 ± 7.5 U/mg). Similar results were obtained when this strain was grown at 30 °C; then no significant differences were observed between both temperatures (P > 0.05). Taking as a whole, these results showed that cold growth is a factor capable to increase UVA survival in P. extremaustralis by mechanisms dependent on PHB under accumulation conditions. On the contrary, mclPHAs and catalase activity seem not to be involved in this response.
Effect of cold growth on survival to UVA exposure of P. extremaustralis. Suspensions of stationary phase cells grown in LB or LBO at 10 °C or 30 °C were exposed to a fluence rate of 20 W/m2 for 300 min (total dose 360 kJ/m2) or kept in the dark. Samples were taken at different times and plated to determine survival. Error bars represent standard deviations of at least three independent experiments. *P < 0.05
Discussion
UV radiation is one of the most important environmental stressors for life. Microorganisms exposed to UV light have developed diverse defensive systems to allow them to survive exposure. For example, extremophiles microorganisms can thrive under radiation due to mechanisms provided by metabolites (extremolytes) able to absorb a wide spectrum of radiation (Gabani and Singh, 2013). In this work, we selected the extremophile bacterium P. extremaustralis to analyze the contribution of PHAs and cold adaptation on the survival to UVA exposure. PHAs have been recognized as having a relevant role in resistance to multiple stress factors (López et al. 2015). Regarding their role in the protection against UV, most studies focused in short-wave radiation (UVC). Experiments performed with PHB-rich and PHB-poor cells of Azospirillum brasilense Cd exposed to UVC showed that when contained low quantities of PHB, cells died rapidly while polymer-rich bacteria remained live (Tal and Okon 1985). In line with this finding, wild-type cells of A. brasilense strain Sp7 exhibited greater tolerance to UVC in comparison with PHB-deficient mutants (Kadouri et al. 2003). In addition, a PHBHHx mutant strain of Aeromonas hydrophila was more sensitive to UVC exposure than its isogenic wild-type strain (Zhao et al. 2007). The protection conferred for PHB against UVC radiation was also demonstrated in recombinant E. coli strains expressing PHB biosynthesis and PHB depolymerase genes of Cupriavidus necator in which increased tolerance compared to wild-type E. coli was observed (Wang et al. 2009). Recently, the protective role of PHB against UVA radiation was demonstrated in C. necator using genetic approaches involving mutant construction (Slaninova et al., 2018). It was determined that the wild-type strain, able to accumulate the polymer, showed higher UVA radiation resistance than a PHB-synthesis-deficient mutant (Slaninova et al. 2018).
In this work, we reported the protective effect of PHB against UVA radiation in P. extremaustralis. The results are in agreement with previous reports highlighting the relevance of PHB to thrive under cold conditions (Ayub et al. 2009, Tribelli and López, 2011). This bacterium accumulates mainly PHB and minor amounts of mclPHAs when grown with octanoate or glucose as carbon sources (Catone et al. 2014). PHB production is an uncommon characteristic of Pseudomonas species that usually accumulate mclPHAs (Kessler and Palleroni, 2000). PHB genes of P. extremaustralis are located in a genomic island suggesting that they were acquired by horizontal transfer events (Ayub et al. 2007). As the maintenance in the genome of horizontally transferred genes suggests the existence of an adaptive advantage conferred to the recipient host, then our results regarding UVA tolerance also highlight the importance of the acquisition of foreign PHB genes for P. extremaustralis fitness.
As mentioned, the main effect of UVA radiation comprises the formation of chemical intermediates such as ROS that generate damage to macromolecules. The contribution of PHAs to cope with oxidative stress has been demonstrated in different bacterial species including A. brasilense, A. hydrophila, and Pseudomonas spp. (Kadouri et al. 2003, Zhao et al. 2007, Ayub et al. 2004, Ruiz et al. 2004). Previous work of our laboratory showed that PHB was relevant to cope with oxidative stress in P. extremaustralis (Ayub et al. 2004, 2009). In this work, we found that both PHB and mclPHAs, although produced in low amounts, are relevant in the tolerance to hydrogen peroxide.
Mechanisms related to increased stress tolerance conferred by PHAs were mostly associated with polymer mobilization as PHAs are highly reduced carbon and energy storage compounds involved in cellular redox balance (López et al. 2015). Regarding UVA protection, we found that PHB is the main kind of polymer implied in this process in P. extremaustralis. Quantification of PHAs under UVA exposure at 30 °C showed that PHB content exhibits a tendency to decrease. By contrast, under cold conditions, a low accumulation of PHB was achieved. During low temperature growth, a dynamic cycle of synthesis and degradation enables to reach an antioxidative physiological state for coping with cold-derived oxidative stress. This physiological state led to a higher UVA tolerance that was probably focused in a fast mobilization of PHB. We have also verified the contribution of PHAs in the tolerance to oxidative stress in P. extremaustralis strains.
In addition, the role of PHAs on bacterial UVA resistance could involve global genetic regulatory mechanisms. In Pseudomonas, master regulators as the alternative sigma factor RpoS and those involved in the Stringent Response and Quorum Sensing, have been related to the UV response by their control on the antioxidative system (Miller et al. 2001; Costa et al. 2010; Pezzoni et al. 2012). Interestingly, a relationship between stress resistance, PHAs metabolism and RpoS was reported in P. putida, where the degradation of PHA was related with the accumulation of the alarmone (p)ppGpp and the increase of RpoS intracellular levels (Ruiz et al. 2001, 2004). RpoS was also involved in the transcription of PHB genes in A. vinelandii (Peralta-Gil et al. 2002) and in polymer mobilization in P. putida (Raiger Iustman and Ruiz, 2008). Moreover, a protective function has been attributed to the monomer 3-hydroxybutyrate (3HB) that can act as chaperone capable of defending enzymes against adverse effects of oxidative damage (Obruca et al. 2016), which could also contribute in the whole UVA tolerance.
In addition to stress resistance mechanisms dependent of PHAs mobilization, several studies reported that a higher survival to stress factors could not encompass polymer mobilization. It was observed that C. necator was able to survive when cells containing PHB were cultured in the absence of carbon and nitrogen sources and could not be efficiently mobilized (Handrick et al. 2000), but the mechanism was not clarified. Similarly, when C. necator was grown autotrophically, the polymer was not degraded completely during the PHB utilization phase, and it was proposed that carbon starvation would constitute a stress condition, leading to regulation of stress response genes (Volova et al. 2013). Enhancement of stress tolerance without PHAs mobilization was also investigated by Goh et al. (2014) by analyzing the relevance of PHB and the copolymer poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (P(3HB-co-3HV) on cell survival during exogenous carbon starvation in Delftia acidovorans. They showed that mobilization was a mechanism to survive starvation at low 3HV content (11–40 mol%), whereas cells containing higher content (P (3HB-co-94 mol% 3HV)) were able to survive without polymer mobilization (Goh et al. 2014). This study also analyzed bacterial survival of a recombinant E. coli strain harboring C. necator PHB genes after exposure to oxidative stress by UVA/fluorescent light-activated titanium dioxide. Results showed that cells containing PHB had higher survival compared to cells without PHB without mobilization when exposed to photo-activated titanium dioxide (Goh et al. 2014).
Recently, light scattering by PHA granules has been proposed as the mechanism involved in the protection against the harmful effects of UVA radiation in C. necator (Slaninova et al. 2018). This study points out the biophysical properties of the polymer as the main responsible of the enhanced UV survival by reduction of intracellular ROS levels (Slaninova et al. 2018). In that report, as no considerable changes in cell dimensions between the wild type and a PHB mutant strain of C. necator were found (Mravec et al. 2016), the increase in light scattering of the PHB producing strain was attributed to the fraction of light scattered on PHA granules. In P. extremaustralis, some changes in cell dimensions were observed; then, light scattering could reflect superimposed effects. However, light scattering by PHB granules cannot be rule out, and then it appears as another possible mechanism to explain the protective effect of PHB against UVA radiation.
Here, we also demonstrated the protective effect of low temperatures against UVA radiation in this bacterium originally isolated from Antarctica. Exposure of bacteria to cold induces several adjustments of the cellular machinery such as the expression of genes encoding antioxidative enzymes and the downregulation of ROS-producing pathways (De Maayer et al. 2014). In P. extremaustralis, capability to accumulate PHB constitutes a key feature under cold conditions as a PHB-deficient mutant is unable to grow at 10 °C in nutrient broth medium and to develop a planktonic life style (Ayub et al. 2009, Tribelli and López 2011). The PHB mutant also has increased lipid peroxidation, an indicator of oxidative damage, and a noticeable decrease in NADH/NAD+ ratio and NADPH content, in comparison with the wild-type strain (Ayub et al. 2009). These findings indicate a crucial function of PHB in the antioxidative defenses in P. extremaustralis and are in line with observations reporting the induction of antioxidative enzymes under cold conditions (De Maayer et al. 2014), some of which use nicotinamide dinucleotide cofactors (Cabiscol et al. 2000).
In summary, overall results indicate that in P. extremaustralis, the protective role of PHAs on UVA radiation lies in several factors such as the increased tolerance to oxidative stress, probably related to polymer mobilization along with light scattering that would act as protective shield against radiation. Exposure to cold conferred higher protection against UVA effects. PHB production, a metabolic capability acquired by horizontal transfer mechanisms, reaches relevance in this process in comparison with production of mclPHA. These findings have ecological interest giving evidences to understand mechanisms that could allow the colonization of harsh environments and promote the development of biotechnological applications in natural environments subjected to high UV radiation.
Abbreviations
- UV:
-
Ultraviolet
- ROS:
-
Reactive oxygen species
- PHAs:
-
Polyhydroxyalkanoates
- PHB:
-
Polyhydroxybutyrate
- mclPHAs:
-
Medium-chain-length PHAs
- CFU:
-
Colony forming units
- GC:
-
Gas chromatography
- OD:
-
Optical density
References
Aebi H (1984) Catalase in vitro. In: Parker L (ed) Methods in enzymology. Academic Press, London, pp 121–126
Agogué H, Joux F, Obernosterer I, Lebaron P (2005) Resistance of marine bacterioneuston to solar radiation. Appl Environ Microbiol 71:5282–5289. https://doi.org/10.1128/AEM.71.9.5282-5289.2005
Ayub ND, Pettinari MJ, Ruiz JA, López NI (2004) A polyhydroxybutyrate-producing Pseudomonas sp. isolated from Antarctic environments with high stress resistance. Curr Microbiol 3:170–174. https://doi.org/10.1007/s00284-004-4254-2
Ayub ND, Pettinari MJ, Méndez BS, López NI (2007) The polyhydroxyalkanoate genes of a stress resistant Antarctic Pseudomonas are situated within a genomic island. Plasmid 58:240–248. https://doi.org/10.1016/j.plasmid.2007.05.003
Ayub ND, Tribelli PM, López NI (2009) Polyhydroxyalkanoates are essential for maintenance of redox state in the Antarctic bacterium Pseudomonas sp. 14-3 during low temperature adaptation. Extremophiles 13:59–66. https://doi.org/10.1007/s00792-008-0197-z
Bequer Urbano S, Albarracín VH, Ordoñez OF, Farías ME, Alvarez HM (2013) Lipid storage in high-altitude Andean Lakes extremophiles and its mobilization under stress conditions in Rhodococcus sp. A5, a UV-resistant actinobacterium. Extremophiles 2:217–227. https://doi.org/10.1007/s00792-012-0508-2
Berney M, Weilenmann H-U, Egli T (2006) Gene expression of Escherichia coli in continuous culture during adaptation to artificial sunlight. Environ Microbiol 8:1635–1647. https://doi.org/10.1111/j.1462-2920.2006.01057.x
Borić M, Danevčič T, Stopar D (2011) Prodigiosin from Vibrio sp. DSM 14379; a new UV-protective pigment. Microb Ecol 3:528–536. https://doi.org/10.1007/s00248-011-9857-0
Braunegg G, Sonnleitner B, Lafferty RM (1978) A rapid gas chromatographic method for the determination of poly-b-hydroxybutyric acid in microbial biomass. Eur J Appl Microbiol Biotechnol 6:29–37
Cabiscol E, Tamarit J, Ros J (2000) Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol 3:3–8
Catone MV (2013) Identificación y análisis de los genes asociados al metabolismo de polihidroxialcanoatos en Pseudomonas extremaustralis. (Tesis Doctoral. Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires). http://hdl.handle.net/20.500.12110/tesis_n5288_. Accessed 12 Sept 2019
Catone MV, Ruiz JA, Castellanos M, Segura D, Espin G, López NI (2014) High polyhydroxybutyrate production in Pseudomonas extremaustralis is associated with differential expression of horizontally acquired and core genome polyhydroxyalkanoate synthase genes. PLoS ONE 9:1–8. https://doi.org/10.1371/journal.pone.0098873
Chamberlain J, Moss SH (1987) Lipid peroxidation and other membrane damage produced in Escherichia coli K1060 by near-UV radiation and deuterium oxide. Photochem Photobiol 45:625–630
Chattopadhyay MK, Raghu G, Sharma YVRK, Biju AR, Rajasekharan MV, Shivaj S (2011) Increase in oxidative stress at low temperature in an Antarctic bacterium. Curr Microbiol 62:544–546. https://doi.org/10.1007/s00284-010-9742-y
Costa CS, Pezzoni M, Fernández RO, Pizarro RA (2010) Role of the quorum sensing mechanism in the response of Pseudomonas aeruginosa to lethal and sublethal UVA irradiation. Photochem Photobiol 86:1334–1342. https://doi.org/10.1111/j.1751-1097.2010.00800.x
De Maayer P, Anderson D, Cary C, Cowan DA (2014) Some like it cold: understanding the survival strategies of psychrophiles. EMBO Rep 15:508–517
Friedrich B, Hogrefe C, Schlegel HG (1981) Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J Bacteriol 174:198–205
Gabani P, Singh OV (2013) Radiation-resistant extremophiles and their potential in biotechnology and therapeutics. Appl Microbiol Biotechnol 97:993–1004
Gao Q, Garcia-Pichel F (2011) Microbial ultraviolet sunscreens. Nat Rev Microbiol 11:791–802. https://doi.org/10.1038/nrmicro2649
Girard PM, Francesconi S, Pozzebon M, Graindorge D, Rochette P, Drouin R, Sage E (2011) UVA-induced damage to DNA and proteins: direct versus indirect photochemical processes. J Phys Conf Ser 261:012002
Goh LK, Purama RK, Sudesh K (2014) Enhancement of stress tolerance in the polyhydroxyalkanoate producers without mobilization of the accumulated granules. Appl Biochem Biotech 172:1585–1598
Handrick R, Reinhard S, Jendrossek D (2000) Mobilization of poly (3-hydroxybutyrate) in Ralstonia eutropha. J Bacteriol 182:5916–5918
Hasset DJ, Schweizer HP, Ohman DE (1995) Pseudomonas aeruginosa sodA and sodB mutants defective in manganese- and ion-cofactored superoxide dismutase activity demonstrate the importance of the iron-cofactored form in aerobic metabolism. J Bacteriol 177:6330–6337
Hoerter JD, Arnold AA, Kucczynska DA, Shibuya A, Ward CS, Sauer MG, Gizachew A, Hotchkiss TM, Fleming TJ, Johnson S (2005) Effects of sublethal UVA irradiation on activity levels of oxidative defense enzymes and proteins oxidation in Escherichia coli. J Photochem Photobiol B 81:171–180. https://doi.org/10.1016/j.jphotobiol.2005.07.005
Hu ML, Tappel AL (1992) Potentiation of oxidative damage to proteins by ultraviolet-A and protection by antioxidants. Photochem Photobiol 56:357–363. https://doi.org/10.1111/j.1751-1097.1992.tb02171.x
Kadouri D, Jurkevitch E, Okon Y (2003) Involvement of the reserve material poly-hydroxybutyrate in Azospirillum brasilense stress endurance and root colonization. Appl Environ Microbiol 69:3244–3250. https://doi.org/10.1128/AEM.69.6.3244-3250.2003
Kessler B, Palleroni NJ (2000) Taxonomic implications of synthesis of poly-beta-hydroxybutyrate and other poly-beta-hydroxyalkanoates by aerobic pseudomonads. Int J Syst Evol Microbiol 2:711–713. https://doi.org/10.1099/00207713-50-2-711
Kidambi SP, Booth MG, Kokjohn TA, Miller RV (1996) recA-dependence of the response of Pseudomonas aeruginosa to UVA and UVB irradiation. Microbiology 142:1033–1040. https://doi.org/10.1099/00221287-142-4-1033
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM II, Peterson KM (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176
López NI, Pettinari MJ, Stackebrandt E, Tribelli PM, Põtter M, Steinbüchel A, Méndez BS (2009) Pseudomonas extremaustralis sp. nov., a poly (3-hydroxybutyrate) producer isolated from an antarctic environment. Curr Microbiol 5:514–519. https://doi.org/10.1007/s00284-009-9469-9
López NI, Pettinari MJ, Nikel PI, Méndez BS (2015) Polyhydroxyalkanoates: much more than biodegradable plastics. Adv Appl Microbiol 93:73–106. https://doi.org/10.1016/bs.aambs.2015.06.001
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mravec F, Obruca S, Krzyzanek V, Sedlacek P, Hrubanova K, Samek O, Kucera D, Benesova P, Nebesarova J (2016) Accumulation of PHA granules in Cupriavidus necator as seen by confocal fluorescence microscopy. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnw094
Manso Cobos I, Ibáñez García MI, de la Peña Moreno F, Sáez Melero LP, Luque-Almagro VM, Castillo Rodríguez F, RoldánRuiz MD, Prieto Jiménez MA, Moreno Vivián C (2015) Pseudomonas pseudoalcaligenes CECT5344, a cyanide-degrading bacterium with by-product (polyhydroxyalkanoates) formation capacity. Microb Cell Fact 14:712–771. https://doi.org/10.1186/s12934-015-0267-8
Miller CD, Mortensen WS, Braga GU, Anderson AJ (2001) The rpoS gene in Pseudomonas syringae is important in surviving exposure to the near-UV in sunlight. Curr Microbiol 43:374–377
Morales LO, Tegelberg R, Brosché M, Keinänen M, Lindfors A, Aphalo PJ (2010) Effects of solar UV-A and UV-B radiation on gene expression and phenolic accumulation in Betula pendula leaves. Tree Physiol 30:923–934. https://doi.org/10.1093/treephys/tpq051
Obruca S, Sedlacek P, Mravec F, Samek O, Marova I (2016) Evaluation of 3-hydroxybutyrate as an enzyme-protective agent against heating and oxidative damage and its potential role in stress response of poly (3-hydroxybutyrate) accumulating cells. Appl Microbiol Biotech 100:1365–1376
Pattison DI, Davies MJ (2006) Actions of ultraviolet light on cellular structures. EXS 96:131–157
Peralta-Gil M, Segura D, Guzman J, Servín-González L, Espín G (2002) Expression of the Azotobacter vinelandii poly-ß-hydroxybutyrate (PHB) biosynthetic phbBAC operon is driven by two overlapping promoters and is dependent on the transcriptional activator PhbR. J Bacteriol 184:5672–5677
Pezzoni M, Pizarro RA, Costa CS (2012) Protective effect of low UVA irradiation against the action of lethal UVA on Pseudomonas aeruginosa: role of the relA gene. J Photochem Photobiol B Biol 116:95–104
Pezzoni M, Pizarro RA, Costa CS (2014) Protective role of extracellular catalase (KatA) against UVA radiation Pseudomonas aeruginosa biofilms. J Photochem Photobiol B Biol 131:53–64. https://doi.org/10.1016/j.jphotobiol.2014.01.005
Qiu X, Sundin GW, Wu L, Zhou J, Tiedje JM (2005) Comparative analysis of differentially expressed genes in Shewanella oneidensis MR-1 following exposure to UVC, UVB and UVA radiation. J Bacteriol 187:3556–3564. https://doi.org/10.1128/JB.187.10.3556-3564.2005
Raiger Iustman LJ, Ruiz JA (2008) The alternative sigma factor, σS, affects polyhydroxyalkanoate metabolism in Pseudomonas putida. FEMS Microbiol Lett 284:218–224
Ruiz JA, López N, Fernández RO, Méndez BS (2001) Polyhydroxyalkanoate degradation is associated with nucleotide accumulation and enhances stress resistance and survival of Pseudomonas oleovorans in natural water microcosms. Appl Environ Microbiol 67:225–230
Ruiz JA, López NI, Méndez BS (2004) rpoS gene expression in carbon-starved cultures of the polyhydroxyalkanoate-accumulating species Pseudomonas oleovorans. Curr Microbiol 48:396–400
Santos AL, Oliveira V, Baptista I, Henriques I, Gomes NC, Almeida A, Correia A, Cunha  (2013) Wavelength dependence of biological damage induced by UV radiation on bacteria. Arch Microbiol 195:63–74. https://doi.org/10.1007/s00203-012-0847-5
Sassoubre LM, Ramsey MM, Gilmore MS, Boehm AB (2014) Transcriptional response of Enterococcus faecalis to sunlight. J Photochem Photobiol B Biol 130:349–356. https://doi.org/10.3389/fmicb.2018.00249
Slaninova E, Sedlacek P, Mravec F, Mullerova L, Samek O, Koller M, Hesko O, Kucera D, Marova I, Obruca S (2018) Light scattering on PHA granules protects bacterial cells against the harmful effects of UV radiation. Appl Microbiol Biotechnol 102:1923–1931. https://doi.org/10.1007/s00253-018-8760-8
Soule T, Gao Q, Stout V, Garcia-Pichel F (2013) The global response of Nostoc punctiforme ATCC 29133 to UVA stress, assessed in a temporal DNA microarray study. Photochem Photobiol 89:415–423. https://doi.org/10.1111/php.12014
Tal S, Okon Y (1985) Production of the reserve material poly-β-hydroxybutyrate and its function in Azospirillum brasilense Cd. Can J Microbiol 31:608–613. https://doi.org/10.1139/m85-115
Tedetti M, Sempéré R (2006) Penetration of ultraviolet radiation in the marine environment. A review. Photochem Photobiol 82:389–397. https://doi.org/10.1562/2005-11-09-IR-733
Tribelli PM, López NI (2011) Poly (3-hydroxybutyrate) influences biofilm formation and motility in the novel Antarctic species Pseudomonas extremaustralis under cold conditions. Extremophiles 15:541–547. https://doi.org/10.1007/s00792-011-0384-1
Tribelli PM, Méndez BS, López NI (2010) The oxygen sensitive global regulator, Anr, is involved in the biosynthesis of poly (3-hydroxybutyrate) in Pseudomonas extremaustralis. J Mol Microbiol Biotechnol 19:180–188. https://doi.org/10.1159/000320261
Tribelli PM, Raiger-Iustman L, Catone MV, Di Martino C, Revale S, Méndez BS, López NI (2012) Genome sequence of the polyhydroxybutyrate producer Pseudomonas extremaustralis a highly stress resistant Antarctic bacterium. J Bacteriol 194:2381–2382. https://doi.org/10.1128/JB.00172-12
Volova TG, Zhila NO, Kalacheva GS, Brigham CJ, Sinskey AJ (2013) Effects of intracellular poly(3-hydroxybutyrate) reserves on physiological-biochemical properties and growth of Ralstonia eutropha. Res Microbiol 164:164–171
Wang Q, Yu H, Xia Y, Kang Z, Qi Q (2009) Complete PHB mobilization in Escherichia coli enhances the stress tolerance: a potential biotechnological application. Microb Cell Fact 8:47. https://doi.org/10.1186/1475-2859-8-47
Wilson KE, Thompson JE, Huner NP, Greenberg BM (2001) Effects of ultraviolet-A exposure on ultraviolet-B-induced accumulation of specific flavonoids in Brassica napus. Photochem Photobiol 73:678–684
Zeeshan M, Prasad SM (2009) Differential response of growth, photosynthesis, antioxidant enzymes and lipid peroxidation to UV-B radiation in three cyanobacteria. S Afr J Bot 75:466–474. https://doi.org/10.1016/j.sajb.2009.03.003
Zhao YH, Li HM, Qin LF, Wang HH, Chen GQ (2007) Disruption of the polyhydroxyalkanoate synthase gene in Aeromonas hydrophila reduces its survival ability under stress conditions. FEMS Microbiol Lett 1:34–41. https://doi.org/10.1111/j.1574-6968.2007.00904.x
Acknowledgements
This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) [Grant Number PIP N° 11220130100450CO], the Universidad de Buenos Aires [Grant Number 20020170100310BA] and the Comisión Nacional de Energía Atómica (Argentina). We want to thank Dr. Mariela V. Catone for providing some genetic constructions. PMT, MP, NVM and NIL are career investigators from CONICET (Argentina). MGB has a graduate student fellowship from CONICET.
Funding
This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) [grant number PIP N° 11220130100450CO]; and the Universidad de Buenos Aires [Grant Number 20020170100310BA].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by S. Albers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tribelli, P.M., Pezzoni, M., Brito, M.G. et al. Response to lethal UVA radiation in the Antarctic bacterium Pseudomonas extremaustralis: polyhydroxybutyrate and cold adaptation as protective factors. Extremophiles 24, 265–275 (2020). https://doi.org/10.1007/s00792-019-01152-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-019-01152-1