Abstract
A hyperthermophilic Thermotoga sp. strain PD524 was isolated from a hot spring in Northern Thailand. Cells were long-curved rods (0.5–0.6 × 2.5–10 μm) surrounded by a typical outer membrane toga. Strain PD524 is aero-tolerant at 4 °C but is aero-sensitive at 80 °C. A heat resistant subpopulation was observed in late-stationary phase. Cells from late-stationary phase were revealed remarkably less sensitive to 0.001 % SDS treatment than cells from exponential phase. The temperature range for growth was 70–85 °C (opt. temp. 80 °C), pH range was 6–8.5 (opt. pH 7.5–8.0), and NaCl range of 0 to <10 g/L (opt. 0.5 g/L). Glucose, sucrose, maltose, fructose, xylose, mannose, arabinose, trehalose, starch, and cellobiose were utilized as growth substrates. Growth was inhibited by So. Growth yield was stimulated by SO =4 but not by S2O =3 and NO3 −. Analysis of 16S rRNA gene sequence (KF164213) of strain PD524 revealed closest similarity (96 %) to Thermotoga maritima MSB8T, T. neapolitana NEST, T. petrophila RKU-1T, and T. naphthophila RKU-10T.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The order Thermotogales comprises many thermophilic and hyperthermophilic species that thrive in geothermal environments including continental hot springs and undersea hydrothermal vents. Members of the Thermotogales all possess a characteristic outer sheath-like membranous structure, the so-called toga. Most members have optimal temperatures ranging from 50 to 90 °C including five described hyperthermophilic species, Thermotoga maritima MSB8T, T. neapolitana NEST, T. petrophila RKU-1T, T. naphthophila RKU-10T, and Fervidobacterium changbaicum CBS-1 (Huber et al. 1986; Jannasch et al. 1988; Takahata et al. 2001; Cai et al. 2007). Currently, 12 official genera are reported, including Fervidobacterium (Patel et al. 1985), Thermotoga (Huber et al. 1986), Thermosipho (Huber et al. 1989), Geotoga (Davey et al. 1993), Petrotoga (Davey et al. 1993), Thermopallium (Duckworth et al. 1996), Marinitoga (Wery et al. 2001), Kosmotoga (DiPippo et al. 2009), Oceanotoga (Jayasinghearachchi and Lal 2011), Mesotoga (Nesbø et al. 2012), Defluviitoga (Ben Hania et al. 2012), and Mesoaciditoga (Reysenbach et al. 2013). Based on 16S rRNA phylogenetic, conserved signature indels in protein sequences and comparative genomic analyses, the members of order Thermotogales have been recently reclassified into 3 orders (Thermotogales, Kosmotogales ord. nov., and Petrotogales ord. nov.). In addition, the thermophilic combinations (a subgroup of genus Thermotoga) including Thermotoga thermarum, T. lettingae, T. elfii, T. subterranea, and T. hypogea were renamed as Pseudothermotoga gen. nov. (Bhandari and Gupta 2014). These bacteria share common morphological features of pleomorphic rod-shaped cells, except Kosmotoga shengliensis (Feng et al. 2010; Nunoura et al. 2010) which has coccoid shaped, and are either Gram-negative or Gram-nonreactive. The order is described categorically as nonsporeforming although survival strategies are expected in this taxonomic group. Species assigned to the genera Thermotoga and Pseudothermotoga are flexible rods containing a balloon-like membranous bleb at each cell pole (Huber et al. 1986; Jannasch et al. 1988; Windberger et al. 1989; Jeanthon et al. 1995; Ravot et al. 1995; Fardeau et al. 1997; Takahata et al. 2001; Balk et al. 2002). Cells of Pseudothermotoga subterranea strain SL1T growing in late exponential phase were proved sensitive to dissolved oxygen at growth temperature; however, cell viability was retained at 4 °C for 1 day, and approx. 100 cells/mL (from the initial 108 cells/mL) of oxygen-resistant cells remained after 4 weeks’ exposure to air (Jeanthon et al. 1995).
In this study, a hyperthermophilic Thermotoga species isolated from a solfataric hot spring in Northern Thailand was characterized with respect to morphology, phylogenetic analysis of 16S rRNA gene, growth kinetics, and physiological properties. In addition, survival mechanisms relating to resistance to oxygen (at permissive and nonpermissive growth temperatures), SDS concentrations, and thermal sensitivity of strain PD524 in exponential and stationary phases were compared.
Materials and methods
Media
The 480GM5 medium (an isolation medium) was composed of (per L) NaCl (0.5 g), NH4Cl (0.33 g), CaCl2·2H2O (0.15 g), MgCl2·6H2O (0.35 g), KCl (0.3 g), KH2PO4 (0.3 g), pancreatic digestion of casein (5 g) (Criterion, CA, USA), yeast extract (0.5 g) (Criterion, CA, USA), A5 solution (1 mL), resazurin solution (0.5 mL of 0.2 g/L solution), and 3 mL Na2S.9H2O solution [25 % (w/v), pH 7]. The pH was adjusted to 7.2–7.5 at room temperature using 1 N NaOH or 1 N HCl before sterilization. The medium was prepared anaerobically in serum bottles under a 1 atm N2 headspace and sealed with a butyl rubber stopper. Sterilization was performed at 121 °C for 20 min. The A5 stock solution was composed of Co(NO3)2·6H2O (0.00494 g), CuSO4·5H2O (0.0079 g), H3BO3 (0.286 g), MnCl2·4H2O (0.181 g), Na2MoO4·2H2O (0.039 g), and ZnSO4·7H2O (0.0222 g) per L.
The 480G and YE5 media are as described for the 480GM5 medium except that the concentration of pancreatic digestion of casein was reduced to 1 g/L and replaced by 5 g/L of yeast extract, respectively. CT and CT5 basal media are similar to the 480GM5 medium except that 0.1 g/L of pancreatic digestion of casein and 0.05 g/L of yeast extract, and 0.5 g/L of pancreatic digestion of casein were employed as major organic nitrogen sources. The CT5 medium was used to test the utilization of carbohydrates.
Isolation of strain PD524
A sediment sample was collected anaerobically from a 95 to 98 °C thermal source at Pong Duet hot spring, in Northern Thailand (N19°13′53.23″, E98°40′9.73″). Enrichment was performed at 80 °C using 480GM5 medium. Strain PD524 was obtained in pure culture using the end-point dilution technique in Hungate tubes performed three consecutive times. Strain PD524 was deposited as DSM 28089.
Morphology
Cell morphology was examined using a phase-contrast microscope (Nikon eclipse 50i) and a Cam ScanMX 2000 scanning electron microscope. Specimen for scanning electron microscopy was prepared as follows. Strain PD524 was grown in 480GM5 and YE5 media for 24 h. Cell pellets were harvested by centrifugation. The pellets were fixed using fresh 2.5 % glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 2 h. The fixed cells were dropped on a cover slide and dried at 80 °C. The cover slide was rinsed (30 s) using 0.1 M phosphate buffer pH 7.4 for 3–5 times and air dried at room temperature. The specimen was then dehydrated using successive 20 min washes of 30, 50, 70, 75, 90, 95, and 100 % ethanol rinses. The dried specimen was sputter coated with gold and kept in a desiccator.
Growth kinetics
The effects of temperature, pH and NaCl concentration on growth were determined in 480GM5 medium modified by addition of NaCl or pH adjustment using 1 N NaOH or 1 N HCl. Triplicate serum bottles of prewarmed medium (100 mL) were inoculated to an initial density of 105 cells/mL. To measure growth rates, samples (1 mL) were taken at appropriate time intervals (2–3 h) during exponential phase. Cell numbers were enumerated using a Neubauer chamber under phase contrast. Specific growth rate constants (µs) were estimated from regression analysis of semilogarithmic plots.
Carbohydrate utilization
Glucose, maltose, sucrose, fructose, lactose, galactose, trehalose, arabinose, mannose, xylose, sorbose, xylitol, sorbitol, mannitol, cellobiose, carboxymethyl cellulose, and starch were tested at 0.1 % w/v final concentration in CT5 basal medium (triplicate tubes). Cell numbers were counted at 48 h and compared to controls (without added carbohydrate). Growth was determined by the increase of cell density. Doubling of the cell density of the control was recorded as positive growth and ≥1.5 to <2 times of the control was recorded as slight growth stimulation.
Effect of inorganic compounds and elemental sulfur on growth
The effects of SO =4 , S2O =3 , and NO3 − on growth were tested in triplicate bottles of 480G medium (100 mL). Briefly, an overnight culture was inoculated to an initial density of 105 cells/mL, and the cultures were incubated at 80 °C for 24 h. Then SO =4 , S2O =3 , and NO3 − were amended into the growing cultures to a final concentration of 20 mM. Sterile distilled water was added to controls. All were further incubated for an additional 24-h period. The effect of So on growth was tested in 2 %S° containing 480G and 480G (control) media. Cell densities (at 24 and 48 h) were determined using direct count technique. Cell yields were compared with controls. Statistical analysis was performed with One-Way ANOVA and LSD with a p value <0.05 considered as significant.
Air sensitivity test
Oxygen sensitivity of strain PD524 growing at 80 °C in mid-exponential (15–16 h) and late-stationary (>60 h) phases was tested using a modification of the method described by Jeanthon et al. (1995). Briefly, two sets of cultures growing in 100 mL of YE5 medium were flushed vigorously with sterilized air by 0.45 µm membrane filters (one inlet and one outlet filters) for a few minutes until the resazurin containing medium turned pink. Immediately, one set of the aerated samples was further incubated at 80 °C, and the other was exposed to air at 4 °C (both sets with the inserted filters). Survival over time was determined using the MPN method (5 replicates) and YE5 medium as diluent. Numbers of turbid cultures were recorded after 2 days of incubation and were employed to determine MPN values.
SDS sensitivity test
Strain PD524 growing at 80 °C (480GM5 medium) was subjected to SDS treatments. Briefly, 5 mL of 16 and 64 h cultures (approx. total of 107–108 MPN) was treated for 5 min by adding an equal volume of SDS solution (to obtain final of 0.001, 0.003 and 0.005 %). The treated cells were centrifuged (5600 rpm for 15 min) and washed twice with sterile 480GM5 medium. The washed cell pellets were suspended in 1 mL and rinsed with another 0.5 mL of the medium. Both liquid fractions were injected into 100 mL of the medium (dilution factor of 102). Controls were treated with sterile N2 saturated water (5 mL) under the same conditions. Viable cells were enumerated using MPN technique and appropriate dilution. Turbid cultures were recorded after 2 days. The decrease in survival numbers relative to controls (recovery) was considered as follows: not sensitive (≤magnitude of 10), slightly sensitive (≤magnitude of 103), and sensitive (>magnitude of 103–107), respectively.
Thermal sensitivity test
Strain PD524 growing at 80 °C in mid-exponential and late-stationary phases were subjected to heat shock at 98 °C. Briefly, cells from 16 h and 66 h cultures were transferred into 100 mL of prewarmed YE medium (98 °C) to obtain approx. final concentrations of 107 and 105 MPN/mL, respectively. Bottles were immediately incubated upside down at 98 °C. For the exponential phase, three sets of experiment were conducted. For the late-stationary phase, two sets of experiment were conducted. Samples were removed after a range of heat shock exposures (0–4 min, 0–15 min, and 10–70 min). The samples were immediately diluted for MPN determination using YE5 medium as diluent at room temperature. Survival was enumerated using the MPN technique. Turbid cultures were recorded after 2 days. The decimal reduction time (D value) of cells in 16 h culture, i.e., mid-exponential phase, was determined using data collected from 0 to 4 min treatment to compare vegetative cells to dormant cultures.
Phylogenetic analysis of 16S rRNA gene sequences
A specific primer to Order Thermotogales named “THER3F” was designed for this study. Nucleotide sequence of UA1406R (5′ ACGGGCGGTGWGTRCAA 3′) was as previously described (Baker and Cowan 2004). This primer pair was employed to amplify a 16S rDNA fragment (1380 bp). The fragment was cloned into a PCR cloning vector, pTG19-T. Plasmid DNAs were sequenced using forward and reverse primers by AITbiotech (Singapore). Closest relatives were identified by homology searches using the blastN algorithm at NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Nucleotide sequences were aligned using CLUSTAL W (Thompson et al. 1994). Pairwise distances were computed using the program MEGA 5.1 (Tamura et al. 2011). A neighbor joining phylogenetic tree was constructed using a bootstrap value of 1000.
G+C content of DNA
The G+C content of DNA was determined using thermal denaturation (Marmur and Doty 1962). Increased absorbance at 260 nm was measured using a model T70 UV–VIS Spectrophotometer (PG Instruments Ltd) and a micro quartz cuvette. The genomic DNA of Pyrococcus furiosus DSM 3638 (40.8 mol %) was employed as a reference.
Results
DNA properties and phylogeny
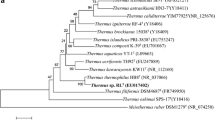
The G+C content of genomic DNA of strain PD524 was 45 mol % (Table 1). A 1380 bp stretch of 16S rRNA gene (KF164213) was sequenced. BlastN alignment accounted for 100 % of KF164213 confirming the lack of chimeras in this sequence. BlastN analysis revealed 96 % identity to Thermotoga maritima MSB8T, T. neapolitana NEST, T. petrophila RKU-1T, and T. naphthophila RKU-10T; 93 % identity to Pseudothermotoga thermarum DSM 5069T; and 90-91 % identity to Thermotoga caldifontis AZM44c09T, T. profunda AZM34c06T, Pseudothermotoga elfii G1T, Pst. subterranea SL1T, and Pst. lettingae TMOT (Bhandari and Gupta 2014). The results suggest that Thermotoga caldifontis AZM44c09T and T. profunda AZM34c06T (Mori et al. 2014) are the closest relatives of Pseudothermotoga rather than the hyperthermophilic Thermotoga. In addition, the hyperthermophilic Thermotoga and thermophilic Pseudothermotoga were phylogenetic placed as two distinctive clades (Fig. 1). Strain PD524 formed the deepest branch of the clade shared with the four hyperthermophilic species (Thermotoga maritima MSB8T, T. neapolitana NEST, T. petrophila RKU-1T, and T. naphthophila RKU-10T).
A neighbor joining tree of 16S rRNA gene sequences of species belonging to genera Thermotoga and Pseudothermotoga (fam. Thermotogaceae, ord. Thermotogales). The 16S rRNA gene sequences of Fervidobacterium nodosum Rt17-B1T (NR_074093.1) (fam. Fervidobacteriaceae, ord. Thermotogales), Petrotoga mexicana (AY125964) (fam. Petrotogaceae, ord. Petrotogales), Kosmotoga olearia TBF 19.5.1T (NR_044583.1) (fam. Kosmotogaceae, ord. Kosmotogales), and Mesoaciditoga lauensis DSM 25116T (JQ347593.1) were employed as representatives for the other families and orders belonging to phylum Thermotogae. A bootstrap value of 1000 is presented as percentage
Morphology
Morphology was examined after growth in 480GM5 and YE5 media. Typical cells of strain PD524 are encapsulated by a “toga,” a characteristic membranous structure ballooning over the ends. Endospores characterized by a light refractile appearance under phase contrast, typical of the genus Clostridium were not observed. Cells appeared as long-curved rods with a small terminal bleb at both termini (Fig. 2) and were stained Gram-negative. A size range of 0.5–0.6 × 2.5–10 μm was determined. Filamentous cells sized of up to 40 μm were frequently detected (Fig. 2a, e). Spheroid bodies (diameter range of 2–4 µm) were commonly observed (Fig. 2b). A novel spherical protuberance at a subterminal position, as named in this study a “golf club structure” (Fig. 2c–e), was rarely detected in both 480GM5 and YE5 media.
SEM (a, d, e) and phase-contrast (b, c) images of strain PD524. a Typical rod-shaped and filamentous cells. b A spheroid. c A golf club-shaped cell. d Typical rod-shaped and golf club-shaped cells. e Cluster of typical cells and a cell harboring, a subterminally dark spherical form or a club head. Scale bars are indicated
Growth kinetics
A temperature range of 70 to <90 °C (opt. temp. 80 °C), a pH range of >5.5 to 8.5 (opt. pH 7.5–8.0), and a NaCl concentration range of 0 to <10 g/L (opt. 0.5 g/L) were determined (Fig. 3a–c). The optimal growth condition (80 °C, pH 7.5, 0.5 g/L NaCl) was identified. Under optimal conditions, viable cell densities of 4.6 × 107 ± 1.5 × 107 and 1.4 × 105 ± 3.6 × 104 MPN/mL were obtained from mid-exponential (16 h) and late-stationary (64 h) phases, respectively. The results reveal that cultivatable cell numbers in the late-stationary phase decreased remarkably compared to the mid-exponential phase.
Carbohydrate utilization
No growth of strain PD524 was observed in CT basal medium containing glucose or other test carbohydrates (0.1 % w/v ea.). Slight growth was observed on CT5 basal medium, and thus, the medium was employed to test carbohydrate utilization. Glucose, sucrose, maltose, fructose, xylose, mannose, arabinose, trehalose, starch, and cellobiose were utilized as growth substrates. However, lactose, galactose, sorbose, xylitol, sorbitol, mannitol, starch, and carboxymethyl cellulose were not utilized (Table 1).
Effect of inorganic compounds and elemental sulfur on growth
Growth in 480G medium (3.0 × 107 ± 2.1 × 106 cells/mL) was inhibited by S°, and no growth was detected in S° containing 480G medium. Growth yield after addition of SO =4 (3.5 × 107 ± 2.5 × 106 cells/mL) was enhanced significantly (p value <0.05). However, growth was not stimulated by addition of S2O =3 (2.9 × 107 ± 3.1 × 105 cells/mL) and NO3 − (2.6 × 107 ± 1.2 × 106 cells/mL).
Air sensitivity test
Strain PD524 tested negative for catalase and did not grow in aerobic medium, as indicated by a pink color of the redox indicator resazurin. Aerobic conditions at 80 °C were lethal. Initial active cell density of the 15 h culture age (1.7 × 108 MPN/mL) declined sharply within 3 h, and no survival was detected after 6 h aeration at 80 °C. A specific death rate constant (a negative µ of −1.56 h−1) was determined. Under these conditions, an initial cultivatable cell density (4.0 × 106 MPN/mL) of the 66 h culture age appeared to be more resistant (a negative µ of −1.08 h−1), and about 20 MPN/mL survival was detected at 6 h after the air treatment. In contrast, both active and dormant populations survived aerobic conditions at 4 °C for >221 h and 96 h, respectively (Supplementary Fig. S1).
SDS sensitivity
Cells of strain PD524 from mid-exponential (16 h) and late-stationary phases (64 h) were revealed sensitive to SDS in a dose-dependent manner compared with their controls (Supplementary Fig. S2). Approx. 3.1 × 107 ± 1.3 × 107 (16 % of the initial load) and 6.5 × 105 ± 5.2 × 105 (6 % of the initial load) MPN of controls were recovered, respectively. Survival of 0–1000 MPN/mL were obtained after the 0.003 and 0.005 % SDS treatments. However, cells from both phases were slightly sensitive to 0.001 % SDS treatment (Supplementary Table S1). To compare resistant properties of the actively growing and dormant cells, the experiment was repeated at 0.001 % SDS. The results confirmed that actively growing cells (survival of 2.2 × 104 ± 7.9 × 103 MPN/mL) were substantially more sensitive to the membrane active detergent than those dormant cells (survival of 2.1 × 105 ± 2.7 × 104 MPN/mL), respectively (Supplementary Table S2 and Fig. S3).
Thermal sensitivity of cells in exponential and late-stationary phases
Cells in exponential phase (16 h) proved to be extremely sensitive to lethal heat shock at 98 °C, and decimation of viable cells was detected with the decimal reduction time (D value) value of 3.9 min (Fig. 4a). However, approx. 10 % of the population was detected and survived after 60 min treatment. A D value at 98 °C (60 min) was predicted for this subpopulation (Fig. 4b). In contrast, cells in late-stationary phase were more resistant to heat. A longer D value at 98 °C (100 min) was calculated, and no substantial reduction of the culture viability was observed after 70 min treatment (Fig. 4c).
Thermal sensitivity of strain PD524 tested at 98 °C on cells from exponential (16 h) and late-stationary phases (66 h). a Two series of experiment were conducted on 16 h culture age, 0–4 and 0–15 min treatments. Sampling intervals (dosed) were as indicated. Symbols: White diamond represents survivors during the first 4 min treatment. Black circle and line represents survivor during the first 15 min treatment. Dotted line represents exponential trend line (μ = −0.59 min−1). b A 60 min treatment was conducted on resistant subpopulation of the 16 h culture age. Dotted line represents exponential trend (μ = −0.04 min−1). c A 70 min treatment experiment was conducted on dormant population (66 h). Dotted line represents exponential trend line (μ = −0.02 min−1)
Discussion
A hyperthermophilic strain PD524 was isolated from a fresh water hot spring in Thailand. Based on 16S rRNA gene sequence (KF164213), strain PD524 was identified as belonging to the hyperthermophilic clade of the genus Thermotoga (Fig. 1). The G+C content of strain PD524 (45 mol %) was determined. Typical cells appeared as flexible rods (0.5–0.6 × 2.5–10 μm), completely surrounded by an outer membrane, with extrusions at both ends, a structural characteristic shared with most bacterial lineages belonging to the deep branching order Thermotogales. Similar to Pseudothermotoga subterranea strain SL1T, cells of strain PD524 were observed pleomorphic in a stationary phase. Strain PD524 developed a novel protuberance as named in this study a “golf club structure” in both 480GM5 and YE5 media (Fig. 2). An optimal growth condition (80 °C, pH 7.5, 0.5 g/l NaCl) was identified (Fig. 3). Differential characteristics of strain PD524 and other Thermotoga species are shown in Table 1. In Table 1, strain PD524 is sensitive to the lowest NaCl concentration (max. conc. <10 g/L), unlike the marine species T. maritima MSB8T (max. conc. <37.5 g/L), T. petrophila RKU-1T (max. conc. 55 g/L), and T. naphthophila RKU-10T (max. conc. 60 g/L). Similar to T. petrophila RKU-1T and T. naphthophila RKU-10T, growth of strain PD524 was inhibited by S°. Growth yield of strain PD524 was stimulated by adding SO =4 , but not by adding S2O =3 and NO3 −.
In general, most microorganisms developed resistant cell stage(s) to cope with unfavorable environmental conditions (Roszak and Colwell 1987; González et al. 1996). In order to understand survival mechanism of this obligately anaerobic, hyperthermophilic strain PD524, its sensitivities to air at 4 and 80 °C, SDS concentrations and thermal sensitivity of cells from the exponential and stationary phases were compared. In this study, viable cells of strain PD524 growing in the late-stationary phase sharply decreased and developed a dormant subpopulation (approx. 0.1–10 %) relative to cells in the exponential phase (4.6 × 107 ± 1.5 × 107 MPN/mL). The dormant subpopulation of strain PD524 was substantially more resistant to air at optimal growth temperature than actively growing cells. However, both cell types were resistant to air at the nonpermissive growth temperature (Supplementary Fig. S1). It is likely that this is a survival strategy of this strictly anaerobic, hyperthermophilic bacterium in coping with aerobic conditions downstream of its anaerobic geothermal isolation locale. Although active growing and dormant cells were very sensitive to high concentrations of SDS (≥0.003 %), strong detergent, dormant cells in late-stationary phase were remarkably more resistant to 0.001 % SDS than exponential phase (Supplementary Fig. S3), suggesting differential cell surface compositions of both subpopulations belonging to this membrane encapsulated bacteria. Heat shock experiments revealed that approx. 90 % of active cells were highly sensitive to heat (D value of 3.9 min at 98 °C). However, a dormant subpopulation survived heat treatment at 98 °C for >60 min (Fig. 4b, c) indicating heat resistant variants in late-stationary phase. Although no known morphologically distinguishable resistant stage could be demonstrated using microscopic methods, an unknown functional golf club structure mentioned above as well as other related unusually shaped cells was observed in the early stationary phase. Whether or not they play a role in survival mechanism remains to be investigated.
In conclusion, this study presents a hyperthermophilic bacterium, Thermotoga sp. strain PD524, isolated from an unexplored hot spring in Thailand and its characterization including morphological, physiological, and phylogenetical perspectives. It highlights the survival strategies of this microorganism against potential environmental changing conditions of oxygenation, denaturing agents, and temperature pointing to the presence of differential survival in distinctive subpopulations within this hyperthermophile. The results suggest that strain PD524 employed a number of strategies to thrive within an unstable range of conditions, at or around their typical hot spring environments.
References
Baker GC, Cowan DA (2004) 16S rDNA primers and the unbiased assessment of thermophile diversity. Biochem Soc Trans 32:218–221
Balk M, Weijma J, Stams AJ (2002) Thermotoga lettingae sp. nov., a novel thermophilic, methanol-degrading bacterium isolated from a thermophilic anaerobic reactor. Int J Syst Evol Microbiol 52:1361–1368
Ben Hania W, Godbane R, Postec A, Hamdi M, Ollivier B, Fardeau ML (2012) Defluviitoga tunisiensis gen. nov., sp. nov., a thermophilic bacterium isolated from a mesothermic and anaerobic whey digester. Int J Syst Evol Microbiol 62:1377–1382
Bhandari V, Gupta RS (2014) Molecular signatures for the phylum (class) Thermotogae and a proposal for its division into three orders (Thermotogales, Kosmotogales ord. nov. and Petrotogales ord. nov.) containing four families (Thermotogaceae, Fervidobacteriaceae fam. nov., Kosmotogaceae fam. nov. and Petrotogaceae fam. nov.) and a new genus Pseudothermotoga gen. nov. with five new combinations. Antonie Van Leeuwenhoek 105:143–168
Cai J, Wang Y, Liu D, Zeng Y, Xue Y (2007) Fervidobacterium changbaicum sp. nov., a novel thermophilic anaerobic bacterium isolated from a hot spring of the Changbai Mountains. China. Int J Syst Evol Microbiol 57:2333–2336
Davey ME, Wood WA, Key R, Nakamura K, Stahl DA (1993) Isolation of three species of Geotoga and Petrotoga: two new genera, representing a new lineage in the bacterial line of descent distantly related to the “Thermotogales”. Syst Appl Microbiol 16:191–200
Dipippo JL, Nesbø CL, Dahle H, Doolittle WF, Birkland NK, Noll KM (2009) Kosmotoga olearia gen. nov., sp. nov., a thermophilic, anaerobic heterotroph isolated from an oil production fluid. Int J Syst Evol Microbiol 59:2991–3000
Duckworth AW, Grant WD, Jones BE, Van Steenbergen RP (1996) Phylogenetic diversity of soda lake alkaliphiles. FEMS Microbiol Ecol 19:181–191
Fardeau ML, Ollivier B, Patel BKC, Magot M, Thomas P, Rimbault A, Rocchiccioli F, Garcia JL (1997) Thermotoga hypogea sp. nov., a xylanolytic, thermophilic bacterium from an oil-producing well. Int J Syst Bacteriol 47:1013–1019
Feng Y, Cheng L, Zhang X, Li X, Deng Y, Zhang H (2010) Thermococcoides shengliensis gen. nov., sp. nov., a new member of the order Thermotogales isolated from oil-production fluid. Int J Syst Evol Microbiol 60:932–937
González JM, Kato C, Horikoshi K (1996) Culturability and survival of an extreme thermophile isolated from deep-sea hydrothermal vents. Arch Microbiol 166:64–67
Huber R, Langworthy TA, König H, Thomm M, Woese CR, Sleytr UB, Stetter KO (1986) Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90 & #xB0;C. Arch Microbiol 144:324–333
Huber R, Woese CR, Langworthy TA, Fricke H, Stetter KO (1989) Thermosipho africanus gen. nov., represents a new genus of thermophilic eubacteria within the “Thermotogales”. Syst Appl Microbiol 12:32–37
Jannasch HW, Huber R, Belkin S, Stetter KO (1988) Thermotoga neapolitana sp. nov. of the extremely thermophilic, eubacterial genus Thermotoga. Arch Microbiol 150:103–104
Jayasinghearachchi HS, Lal B (2011) Oceanotoga teriensis gen. nov., sp. nov., a thermophilic bacterium isolated from offshore oil-producing wells. Int J Syst Evol Microbiol 61:554–560
Jeanthon C, Reysenbach AL, L’Haridon S, Gambacorta A, Pace NR, Glénat P, Prieur D (1995) Thermotoga subterranea sp. nov., a new thermophilic bacterium isolated from a continental oil reservoir. Arch Microbiol 164:91–97
Marmur J, Doty P (1962) Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol 5:109–118
Mori K, Yamazoe A, Hosoyama A, Ohji S, Fujita N, Ishibashi J, Kimura H, Suzuki K (2014) Thermotoga profunda sp. nov. and Thermotoga caldifontis sp. nov., anaerobic thermophilic bacteria isolated from terrestrial hot springs. Int J Syst Evol Microbiol 64:2128–2136
Nesbø CL, Bradnan DM, Adebusuyi A, Dlutek M, Petrus AK, Foght J, Doolittle WF, Noll KM (2012) Mesotoga prima gen. nov., sp. nov., the first described mesophilic species of the Thermotogales. Extremophiles 16:387–393
Nunoura T, Hirai M, Imachi H, Miyazaki M, Makita H, Hirayama H, Furushima Y, Yamamoto H, Takai K (2010) Kosmotoga arenicorallina sp. nov. a thermophilic and obligately anaerobic heterotroph isolated from a shallow hydrothermal system occurring within a coral reef, southern part of the Yaeyama Archipelago, Japan, reclassification of Thermococcoides shengliensis as Kosmotoga shengliensis comb. nov., and emended description of the genus Kosmotoga. Arch Microbiol 192:811–819
Patel BKC, Morgan HW, Daniel RM (1985) Fervidobacterium nodosum gen. nov. and spec. nov., a new chemoorganotrophic, caldoactive, anaerobic bacterium. Arch Microbiol 141:63–69
Ravot G, Magot M, Fardeau ML, Patel BKC, Prensier G, Egan A, Garcia JL, Ollivier B (1995) Thermotoga elfii sp. nov., a novel thermophilic bacterium from an African oil-producing well. Int J Syst Bacteriol 45:308–314
Reysenbach AL, Liu Y, Lindgren AR, Wagner ID, Sislak CD, Mets A, Schouten S (2013) Mesoaciditoga lauensis gen. nov., sp. nov., a moderately thermoacidophilic member of the order Thermotogales from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol 63:4724–4729
Roszak DB, Colwell RR (1987) Survival strategies of bacteria in natural environment. Microbiol Rev 51:365–379
Takahata Y, Nishijima M, Hoaki T, Maruyama T (2001) Thermotoga petrophila sp. nov. and Thermotoga naphthophila sp. nov., two hyperthermophilic bacteria from the Kubiki oil reservoir in Niigata. Japan. Int J Syst Evol Microbiol 51:1901–1909
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thompson JD, Higgins DG, Gibson TJ (1994) The CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Wery N, Lesongeur F, Pignet P, Derennes V, Cambon-Bonavita MA, Godfroy A, Barbier G (2001) Marinitoga camini gen. nov., sp. nov., a rod-shaped bacterium belonging to the order Thermotogales, isolated from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol 51:495–504
Windberger E, Huber R, Trincone A, Fricke H, Stetter KO (1989) Thermotoga thermarum sp. nov. and Thermotoga neapolitana occurring in African continental solfataric springs. Arch Microbiol 151:506–512
Acknowledgments
This work was supported by the following grants: the Scientific Promotion and Development Fund, Faculty of Science, Silpakorn University (SFR-SRG-2558-01) and the Silpakorn University Research and Development Institution from Thailand; and the Ministry of Economy and Productivity (Consolider CSD2009-00006) and the Andalusian Government (BIO288) from Spain with participation of FEDER funds. FTR acknowledges support from the NASA Exobiology Program, the US National Science Foundation and the US Air Force Office of Scientific Research. Special thanks to Witoon Wattananit, Scientific and Technological Equipment Centre, Silpakorn University for the SEM work and to Juergen Wiegel for helpful comments and discussion. This paper is IMET Contribution No 15-157.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by A. Driessen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kanoksilapatham, W., Keawram, P., Gonzalez, J.M. et al. Isolation, characterization, and survival strategies of Thermotoga sp. strain PD524, a hyperthermophile from a hot spring in Northern Thailand. Extremophiles 19, 853–861 (2015). https://doi.org/10.1007/s00792-015-0761-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-015-0761-2