Abstract
Hydrogen peroxide (H2O2) produces hydroxyl radicals that directly attack a variety of biomolecules and cause severe cellular dysfunction. An extremely thermophilic bacterium, Thermus thermophilus HB8, possesses at least three enzymes that can scavenge H2O2: manganese-containing catalase (TTHA0122, MnCAT), a possible peroxiredoxin homologue (TTHA1300), and a possible heme peroxidase (HPX) homologue (TTHA1714). To investigate the roles of these proteins, we attempted to disrupt each of these genes in T. thermophilus HB8. Although we were able to completely disrupt ttha1300, we were unable to completely delete ttha0122 and ttha1714 because of polyploidy. Quantitative real-time PCR showed that, compared to the wild type, 31 % of ttha0122 and 11 % of ttha1714 remained in the ∆ttha0122 and ∆ttha1714 disruption mutants, respectively. Mutants with reduced levels of ttha0122 or ttha1714 exhibited a significant increase in spontaneous mutation frequency. ∆ttha1714 grew slower than the wild type under normal conditions. ∆ttha0122 grew very poorly after exposure to H2O2. Moreover, ∆ttha0122 did not show H2O2-scavenging activity, whereas ∆ttha1300 and ∆ttha1714 scavenged H2O2, a property similar to that exhibited by the wild type. MnCAT purified from T. thermophilus HB8 cells scavenged H2O2 in vitro. The recombinant form of the possible HPX homologue, reconstituted with hemin, showed peroxidase activity with H2O2 as an oxidant substrate. Based on these results, we propose that not only MnCAT but also the possible HPX homologue is involved in protecting the cell from oxidative stress in T. thermophilus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Molecular oxygen gives rise to reactive oxygen species (ROS) as inevitable byproducts of aerobic metabolism. The initial products in ROS formation are superoxide anions, which strongly oxidize iron-sulfur clusters in proteins (Imlay 2003, 2008). In an aerobic organism, superoxide dismutase eliminates superoxide but produces another ROS, hydrogen peroxide (H2O2). H2O2 has the lowest reactivity and the highest stability among the biologically relevant ROS (Giorgio et al. 2007); however, when ferrous iron transfers an electron to H2O2 (the Fenton-reaction), it produces a hydroxyl radical. This radical is the only ROS that can directly damage most biomolecules (Imlay 2003, 2008). For example, it attacks DNA to generate oxidatively damaged bases such as 8-oxoguanine (Imlay 2008; Morita et al. 2010). These DNA lesions are toxic and mutagenic. To protect itself from oxidative stress due to ROS, an aerobic organism possesses fundamental enzymes for detoxifying ROS (Mishra and Imlay 2012) and repairing oxidized DNA (Imlay 2008; Morita et al. 2010).
There are multiple H2O2-scavenging enzymes in an aerobic organism (Mishra and Imlay 2012). Seaver and Imlay analyzed the phenotypes of E. coli mutants lacking scavenging enzymes and proposed that alkyl hydroperoxide reductase scavenges low levels of H2O2, whereas catalases scavenge high levels (Mishra and Imlay 2012; Seaver and Imlay 2001a). As is the case in E. coli, scavenging enzymes in each organism are thought to play different roles in coping with the oxidative stress caused by H2O2.
The extremely thermophilic bacterium, Thermus thermophilus HB8, is an aerobic bacterium (Oshima and Imahori 1974). This organism has a relatively small genome size (2.2 Mbp), and its biological system is thought to consist of minimum essential proteins (Yokoyama et al. 2000). T. thermophilus HB8 is thought to have at least three enzymes that can scavenge H2O2. The first protein, TTHA0122, is a manganese-containing catalase (MnCAT). MnCAT converts H2O2 into water and oxygen and is different from other heme-containing catalases in that it has a di-manganese center in the active site (Chelikani et al. 2004; Whittaker 2012). The second protein, TTHA1300, is a possible peroxiredoxin homologue. Peroxiredoxin reduces H2O2 into water through its active site cysteine (Wood et al. 2003). The resulting sulfenic acid is recycled to a thiol by a cellular reducing system such as the NADPH-dependent thioredoxin system (Wood et al. 2003). The third protein, TTHA1714, is a possible heme peroxidase (HPX) homologue (Ebihara et al. 2005). TTHA1714 was originally annotated as a conserved hypothetical protein. Based on its crystal structure (PDB ID: 1VDH), we showed that TTHA1714 has a heme-binding site with an Fe-His-Asp triad. This is a common feature of HPX proteins and, therefore, we proposed it as a possible HPX homologue (Ebihara et al. 2005). This set of homologous proteins has a highly conserved tertiary structure containing a heme-binding site and is classified in the dye-decolorizing peroxidase family (Zubieta et al. 2007a, b). Although the aforementioned three proteins in T. thermophilus HB8 are thought to be involved in protection from H2O2-induced oxidative stress, their contribution to the cellular protection system is poorly understood.
In this study, we attempted to disrupt ttha0122, ttha1300, or ttha1714 in T. thermophilus HB8 to investigate the effect on spontaneous mutation frequency and sensitivity to oxidative stress caused by H2O2. We showed that not only MnCAT but also the possible HPX homologue, TTHA1714, has a protective role against oxidative stress in T. thermophilus.
Materials and methods
Disruption of the ttha0122, ttha1300, and ttha1714 genes
The ttha0122, ttha1300, and ttha1714 genes were disrupted in T. thermophilus HB8 by substituting the target gene with the thermostable kanamycin resistance gene, HTK (Hoseki et al. 1999), through homologous recombination, as previously described (Hashimoto et al. 2001). The plasmids for homologous recombination were constructed by inserting HTK, flanked by approximately 500 bp upstream and downstream of each gene, into the pGEM-T Easy Vector (Promega, Madison, WI, USA). The 500-bp DNA fragments from upstream of each gene were amplified by PCR using the primer sets, 5ʹ-CCGGGGTGAGCTCCTCCCGCACCGAGAGGG-3ʹ and 5ʹ-CGCCGTCAACGGGTACCGCGGTCTATCCTC-3ʹ, 5ʹ-GCCACTACACCCCCATGCTCAAGTTCGCCC-3ʹ and 5ʹ-CGCCGTCAACGTCTAGAGTGCCCACTTCCA-3ʹ, and 5ʹ-GTTGAGGCCGAGGTTGGAGAGGAAGAGGAG-3ʹ and 5ʹ-CGCCGTCAACGGGTACCTTCGGGAACGTGC-3ʹ (BEX, Tokyo, Japan) for ttha0122, ttha1300, and ttha1714, respectively. The 500-bp DNA fragments from downstream of each gene were amplified by PCR using primer sets, 5ʹ-TGTTGGTTACGCTGCATCTCTACGAGAAGG-3ʹ and 5ʹ-ACGGATGGACCTCCTCTCCGAGTTCCCCTT-3ʹ, 5ʹ-TGTTGGTTACGCTGCAGTGAAGGCGCTCAG-3ʹ and 5ʹ-GTCCCCGTCCATGCGGAGCTCGGGCTCCCC-3ʹ, and 5ʹ-CATGTTGGTTACGCTGCAGCTGCGGGCCTT-3ʹ and 5ʹ-GCCAAAGAAAAGGACCAGCCAGCGCACCAG-3ʹ (BEX) for ttha0122, ttha1300, and ttha1714, respectively. To produce the ttha0122, ttha1300, and ttha1714 mutants (∆ttha0122, ∆ttha1300, and ∆ttha1714), T. thermophilus HB8 cells were transformed with the above plasmids, as previously described (Hashimoto et al. 2001). Gene disruptions were confirmed by PCR amplification, using isolated genomic DNA as the template.
Quantitative real-time PCR
Approximately 25 µL of SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA) was mixed with an equal volume of a solution containing 5, 2.5, 0.25, 0.05, or 0.01 ng/µL template of genomic DNA and 200 nM forward and reverse primers. It was then subjected to real-time PCR using a 7300 Real-Time PCR system (Applied Biosystems). The forward and reverse primer sets used for ttha0122 and ttha1714 amplification were 5ʹ-ATGTTCCTGAGGATAGACCGCC-3ʹ and 5ʹ-ATCTCCACCCCGGTGAGGC-3ʹ, and 5ʹ-ATGGAGCGGCACGTTCCC-3ʹ and 5ʹ-GGGAACGTGCCGCTCCAT-3ʹ (BEX), respectively. Melting curve analysis confirmed specific amplification from the genomic DNA with each primer set (data not shown). Data were analyzed as previously described (Cao et al. 2010; Pfaffl 2001). The ttha1934 gene, which is present in equal amounts in the wild type and mutants, was used as a reference gene. The efficiency (E) is given by:
where slope is the slope of the plot of log(dilution) vs. threshold cycle number (C t). The determined efficiencies were E (ttha0122) = 1.59, E (ttha1714) = 1.16, and E (ttha1934) = 1.98. The relative copy number of the gene (ttha0122 or ttha1714) in the mutant cell compared to the wild type is given by
where ∆C t(gene) is [C t(gene) for wild type − C t(gene) for mutant].
Estimation of spontaneous mutation frequency
The mutation frequency of T. thermophilus HB8 was estimated based on how frequently streptomycin-resistant mutants occurred, as calculated from the means of the modified Luria-Delbrück fluctuation test (Luria and Delbruck 1943). Pre-cultured wild type, ∆ttha0122, ∆ttha1300, and ∆ttha1714 strains of T. thermophilus HB8 were diluted 1:60 with 3 mL of TR medium (0.8 % polypeptone, 0.4 % yeast extract, 0.2 % NaCl; pH 7.2) and shaken at 70 °C for 2 h. After addition of 30 µL of 0 or 250 mM H2O2, cells were cultured at 70 °C for 5 h. One milliliter of each culture was spread on a plate containing 50 µg/mL streptomycin. Subsequently, the same cultures were diluted 1:105 with TR medium, and 100 µL of each diluted culture was spread on a drug-free plate. The plates were incubated at 70 °C for 20 h. The frequency of streptomycin-resistant mutants per 108 cells was calculated from the numbers of colonies formed on the streptomycin-containing and drug-free plates. The Mann–Whitney test was performed to statistically evaluate the results.
Growth of the mutants and measurement of sensitivity to oxidative stress
The wild type, ∆ttha0122, ∆ttha1300, and ∆ttha1714 strains of T. thermophilus HB8 were pre-cultured aerobically overnight at 70 °C in 4 mL of TT medium [0.8 % polypeptone, 0.4 % yeast extract, 0.2 % NaCl, 0.4 mM CaCl2, 0.4 mM MgCl2; pH 7.2 (Hashimoto et al. 2001)]. Then, 255 mL of fresh TT medium was inoculated with 0.5–1 mL of the pre-culture, and the cells were cultivated aerobically at 70 °C. The growth of the cells was monitored by measuring the absorbance at 600 nm (OD600) at various times during the culture.
To examine the sensitivity to H2O2, H2O2 was added to the culture medium at a final concentration of 10 mM at the mid-log phase, and OD600 was measured again to monitor the growth of T. thermophilus HB8.
Estimation of scavenging capability of the mutants
At the mid-log phase, H2O2 was added to a culture medium at a final concentration of 10 mM. A small volume of the culture medium was taken at 0, 1, 3, 5, 7, and 15 min after the addition, and the cells were immediately removed from the culture medium by filtration with a 0.22-µm cutoff filter and an aspirator. The concentration of residual H2O2 in the filtrate was measured by a horseradish peroxidase (HRP) enzyme assay with 2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) as a substrate. Approximately 100 µL of the diluted cell filtrate was added to 10 µL of 10 mM ABTS, 50 µL of 200 mM potassium phosphate (pH 7.0), and 20 µL of water. Subsequently, 20 µL of 2.5 μg/mL HRP was added to the solution. The absorbance at 414 nm was measured using a SpectraMax 190 Microplate Spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). The absorbance was converted to H2O2 concentration using a standard curve of H2O2 concentration (0–75 μM).
Purification of MnCAT from T. thermophilus HB8
T. thermophilus HB8 cells were grown in a culture medium containing polypeptone, yeast extract, and NaCl. Approximately 100 g of frozen T. thermophilus cells was suspended and disrupted by sonication in 500 mL of Buffer A (20 mM Tris–HCl, 100 mM NaCl, 10 mM EDTA, and 10 mM beta-mercaptoethanol; pH 8.0). The cell lysate was ultracentrifuged (200,000×g) for 30 min at 4 °C. Ammonium sulfate was added to the clear supernatant in a stepwise manner to produce 30, 45, 60, and 75 % saturation. After stirring for an hour, the precipitate was recovered by centrifugation at each stage. The supernatant containing 75 % ammonium sulfate was left overnight and the resulting precipitate was collected by centrifugation. Each precipitate was dissolved in Buffer B (20 mM Tris–HCl, 1 mM EDTA, and 10 mM beta-mercaptoethanol; pH 8.0). The five protein fractions were separately dialyzed overnight against Buffer B. To find the MnCAT-containing fraction, H2O2-scavenging activity was measured using a ferric-xylenol orange method (Gay et al. 1999). A 1:1000 diluted solution of each fraction was incubated at room temperature for 5 min in 50 mM potassium phosphate (pH 7.0) containing 41 μM H2O2. The reaction was stopped by adding H2SO4. The resulting solution was mixed at a 1:1 ratio with a solution containing 0.25 mM xylenol orange, 0.1 mM ferrous ammonium sulfate, and 25 mM H2SO4. After 30 min, the absorbance at 560 nm was measured to determine the H2O2-scavenging activity. Three fractions with activity (45–60, 60–75, and 75 % overnight) were separately loaded onto a TOYOPEARL SuperQ-650 M column (Tosoh Bioscience, Tokyo, Japan) equilibrated with Buffer B and eluted with a linear gradient of 0–1 M NaCl. H2O2-scavenging activity was measured again to find the MnCAT-containing fractions. All the fractions with activity were pooled, desalted into Buffer B, and loaded to a Resource Q column (GE Healthcare, Buckinghamshire, England) equilibrated with Buffer B. Proteins were eluted with a linear gradient of 0–0.25 M NaCl. The MnCAT-containing fractions were pooled, desalted into 10 mM sodium phosphate buffer, 150 mM NaCl, 1 mM EDTA, and 10 mM beta-mercaptoethanol (pH 7.0), and loaded onto a Bio-Scale CHT10-I column (Bio-Rad) equilibrated with the same buffer. The proteins were eluted with a linear gradient of 10–250 mM sodium phosphate. The MnCAT-containing fractions were pooled, desalted against 20 mM 2-morpholinoethanesulfonic acid (MES), 1 mM EDTA, and 10 mM beta-mercaptoethanol (pH 6.0), and loaded onto a RESOURCE S column (GE Healthcare) equilibrated with the same buffer. Proteins were eluted with a linear gradient of 0–0.25 M NaCl. The MnCAT-containing fractions were pooled and subjected to gel filtration on a HiLoad 16/60 Superdex 200 pg column (GE Healthcare) equilibrated with 20 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, and 10 mM beta-mercaptoethanol (pH 8.0). The MnCAT-containing preparation was polished on a Bio-Scale CHT5-I column (Bio-Rad Laboratories, Hercules, CA) with a linear gradient of 10–250 mM sodium phosphate, followed by desalting against 20 mM MOPS, 1 mM EDTA, and 10 mM beta-mercaptoethanol (pH 7.0) using a HiPrep 26/10 Desalting column (GE Healthcare). The protein concentration of MnCAT was determined with a molecular extinction coefficient at 280 nm (30,337 M−1 cm−1) that is calculated according to the formula provided by Kuramitsu et al. (1990). The final concentration was 33 mg/mL. The proteins were separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and identified by peptide mass fingerprinting using MALDI-TOF MS (Ultraflex, Bruker Daltonics, Bremen, Germany).
Characterization of MnCAT
The metal content of MnCAT was determined by CIROS-160EOP inductively coupled plasma spectrometry (Rigaku, Tokyo, Japan). The H2O2-scavenging activity was measured using a SpectraMax 190 Microplate Spectrophotometer (Molecular Devices). MnCAT (50 nM) was incubated with various concentrations of H2O2 in 50 mM potassium phosphate (pH 7.0) at 25 °C. The decomposition of H2O2 was monitored at 240 nm (ε 240 = 43.6 M−1 cm−1) (Beers and Sizer 1952), and the steady-state kinetics of MnCAT were analyzed. The K m and k cat values were determined using the Lineweaver–Burk plot.
Preparation and characterization of possible HPX homologue
The recombinant form of the possible HPX homologue (TTHA1714) was expressed in E. coli BL21(DE3) and was produced as the apo-form (Ebihara et al. 2005). The holo-form of the possible HPX homologue was prepared in vitro by reconstitution of the intact preparation with hemin (Ebihara et al. 2005). Total protein concentration was determined using the bicinchoninic acid method (Smith et al. 1985). Heme concentration was determined by pyridine hemochrome analysis (Berry and Trumpower 1987). Peroxidase activity was measured at 25 °C using a U-3010 spectrophotometer (Hitachi, Tokyo, Japan) and a 1-cm quartz cuvette. Reaction mixtures contained 1 μM reconstituted possible HPX homologue, 2 mM reductant substrate (ABTS or guaiacol), 2 mM H2O2, and 50 mM potassium phosphate (pH 7.0). Oxidations of ABTS and guaiacol were determined from the increase in absorbance at 730 nm (ε 730 = 1.4 × 104 M−1 cm−1) and at 470 nm (ε 470 = 3.8 × 103 M−1 cm−1), respectively (Matsui et al. 1999). The reaction was initiated by the addition of H2O2. Only the linear rate was used to calculate the activity. Intact possible HPX homologue (apo-form), hemin, myoglobin, and HRP were used as controls of peroxidase activity.
Results
Disruptions of the ttha0122, ttha1300, and ttha1714 genes of T. thermophilus HB8
In order to test their H2O2-scavenging abilities, we tried to disrupt the ttha0122, ttha1300, and ttha1714 genes of T. thermophilus HB8. Gene disruption was carried out by substituting the target gene with the thermostable kanamycin-resistant gene, HTK (Fig. 1a). Three cycles of gene disruption were carried out to obtain the disruption mutants. Ohtani et al. (2010) showed that T. thermophilus HB8 is a polyploid organism. Therefore, we examined the extent to which the gene was disrupted in the mutants obtained. Using standard PCR methods, a DNA fragment of HTK was amplified from the isolated genomic DNA of all mutants (Fig. 1b, lanes 3, 7, and 11), suggesting that the relevant gene was disrupted by homologous recombination, as expected. On the other hand, DNA fragments of ttha0122 and ttha1714 were amplified from the genomic DNA of the ∆ttha0122 and ∆ttha1714 strains, respectively (Fig. 1b, lanes 9 and 13). A DNA fragment of ttha1300 was not amplified from the genomic DNA of ∆ttha1300 strain (Fig. 1b, lane 5).
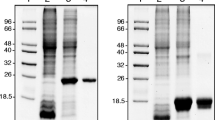
Disruptions of ttha1300, ttha0122, and ttha1714 examined by PCR. a A schematic representation of the amplified regions in wild type and mutants. Arrows represent primers used for PCR. Primer sets 1 and 2, 3 and 4, and 5 and 6 were used for examination of the ttha1300, ttha0122, and ttha1714 disruptions, respectively. b Amplified DNA fragments were analyzed by agarose gel electrophoresis. PCR reactions contained genomic DNA isolated from the wild type (W), ∆ttha1300 (∆1300), ∆ttha0122 (∆0122), or ∆ttha1714 (∆1714) strains, as well as primer set 1 (lanes 2 and 3), 2 (lanes 4 and 5), 3 (lanes 6 and 7), 4 (lanes 8 and 9), 5 (lanes 10 and 11), and 6 (lanes 12 and 13). Lane 1 DNA size marker. The lengths of the amplified fragments showed good concordance with the theoretical lengths of the targeted regions
Characterization by PCR revealed that the genomes of the kanamycin-resistant mutants obtained by ttha0122- and ttha1714-gene disruption retained a certain number of wild type copies of ttha0122 and ttha1714, respectively. In order to estimate the number of ttha0122 and ttha1714 genes remaining in the mutants, quantitative real-time PCR was performed. The ttha1934 gene was used as a reference gene. Compared with the wild type, 31 % of ttha0122 and 11 % of ttha1714 were present in ∆ttha0122 and ∆ttha1714, respectively (Table 1). Our results indicate that some, but not all, copies of mncat and hpx were disrupted in ∆ttha0122 and ∆ttha1714, while all copies of ttha1300 were disrupted in ∆ttha1300.
Phenotypes of the mutants
To investigate whether each protein contributes to protection from oxidative stress, we measured the spontaneous mutation frequencies of ∆ttha1300, ∆ttha0122, and ∆ttha1714 by measuring the spontaneous generation of streptomycin-resistant mutants. Streptomycin resistance can be acquired by single-base substitutions including AT-CG transversion and AT-GC transition mutations (Bonny et al. 1991). These mutations can be generated by oxidative DNA damage, such as the formation of 8-oxoguanine and 5-formyluracil (Wallace 2002). The wild type strain showed a similar mutation frequency under normal and oxidative stress conditions (Fig. 2). This result indicates that the H2O2-induced oxidative stress protection mechanism works wells in the wild type. Compared to the wild type, ∆ttha1300 showed no significant increase in mutation frequency under either condition. On the other hand, ∆ttha0122 and ∆ttha1714 exhibited statistically significant increases in the mutation frequencies (approximately 8- and 6-fold higher, respectively) under normal conditions when compared to the wild type (Fig. 2). Moreover, these increases in mutation frequencies were more significant under oxidative stress conditions (Fig. 2). These results indicate that under normal conditions, MnCAT (TTHA1012) and a possible HPX homologue (TTHA1714) contribute to protection from oxidative DNA damage. Furthermore, the fact that ∆ttha1300 did not show a significant increase in spontaneous mutation frequency (Fig. 2) indicates that this possible peroxiredoxin homologue (TTHA1300) does not significantly contribute to oxidative stress protection under our experimental conditions.
Spontaneous mutation frequencies of the wild type, ∆ttha1300, ∆ttha0122, and ∆ttha1714 strains. The spontaneous mutation frequencies were evaluated by measuring the frequency of streptomycin-resistant (StrR) mutants, as described in the “Materials and methods”. Normal conditions (without H2O2), white bar; oxidative stress conditions (with H2O2), gray bar. Error bars indicate the standard deviation of the results (n = 3 wild type, ∆ttha1300, ∆ttha0122 strains analyzed; n = 4 ∆ttha1714 strain analyzed). *P = 0.05 by Mann–Whitney test
Next, we compared the growth curves of the wild type, ∆ttha1300, ∆ttha0122, and ∆ttha1714 strains (Fig. 3). We observed that ∆ttha1300 and ∆ttha0122 grew at a rate similar to that of the wild type (Fig. 3b, c), while ∆ttha1714 grew slower than the wild type, throughout the culture (Fig. 3d, filled circles). Notably, after a long culture period, the culture medium of ∆ttha1714 turned red, compared to the wild type (Fig. 4).
Effects of gene disruption on cell growth and sensitivity to H2O2-induced oxidative stress. Growth curves of the wild type (a), ∆ttha1300 (b), ∆ttha0122 (c), and ∆ttha1714 (d) strains were obtained by OD600 measurement. The wild type was cultured in parallel with each gene mutant. The wild type, grown under normal conditions (open squares); the wild type, grown with H2O2 treatment (open triangles), the gene mutant, grown under normal conditions (filled circles); the gene mutant, grown with H2O2 treatment (filled triangles). To impose oxidative stress, H2O2 was added at a final concentration of 10 mM at the mid-log phase (the time indicated by the arrow, 300 min for the wild type and ∆ttha0122; 360 min for ∆ttha1300 and ∆ttha1714)
Cultures of the wild type and ∆ttha1714. The wild type and ∆ttha1714 were cultured aerobically overnight (12.5 h) at 70 °C in 4 mL TT medium. Cultures 1 and 2 are the wild type (duplicate). Cultures 3–5, ∆ttha1714 (triplicate). A large amount of the culture medium (255 mL) of ∆ttha1714 also became red
To examine the sensitivity to H2O2-induced oxidative stress, we added H2O2 to the culture medium at the mid-log phase and monitored the cell growth. H2O2 rapidly diffuses across the cell membrane (Imlay 2008; Seaver and Imlay 2001b). Thus, when H2O2 is added to the culture medium, it can enter the cells and cause oxidative stress (Imlay 2008). When the wild type was treated with 5 mM H2O2, it grew at a rate similar to that of the untreated control (Supplemental Fig. S1). When the wild type was treated with 10 mM H2O2, it showed slight growth retardation and was not able to recover by the end of the culture period (Fig. 3a). These results indicate that the oxidative stress imposed by 10 mM H2O2 is large enough to cause a significant change in cell growth. After exposure to H2O2, ∆ttha1300 and ∆ttha1714 showed a growth defect similar to that shown by the wild type (Fig. 3b, d). On the other hand, ∆ttha0122 grew very poorly under oxidative stress and exhibited the most significant growth defect of all the mutants (Fig. 3c). These results indicate that mutants with reduced levels of MnCAT are highly sensitive to H2O2-induced oxidative stress.
Since the cell membrane is permeable to H2O2 (Imlay 2008; Seaver and Imlay 2001b), H2O2 must be detoxified by enzymes within the cells. To extrapolate the H2O2-scavenging capability of the wild type and of each mutant, we measured the H2O2 concentration in the culture medium over time after addition of H2O2. Both ∆ttha1300 and ∆ttha1714 scavenged H2O2 as well as the wild type (Fig. 5). On the other hand, ∆ttha0122 exhibited virtually no scavenging activity (Fig. 5, filled circles). H2O2 was not degraded in the culture medium that was not inoculated with T. thermophilus cells (Fig. 5, open squares), or in the filtrate where T. thermophilus cells were removed from the culture medium harvested at the mid-log phase (data not shown). These results indicate that MnCAT (TTHA0122) is a strong scavenger of H2O2 in T. thermophilus HB8. The presence of MnCAT in ∆ttha1300 and ∆ttha1714 is likely responsible for their scavenging capability.
H2O2-scavenging capability of the wild type and the mutants. A small volume of culture medium was taken at various time points after addition of H2O2 (final concentration, 10 mM), and the concentration of residual H2O2 in the culture filtrate was measured by an enzyme assay described in the “Materials and methods”. Controls without inoculation (open squares); the wild type (filled squares); ∆ttha1300 (filled triangles), ∆ttha0122 (filled circles), and ∆ttha1714 (filled diamonds)
Characterization of MnCAT purified from T. thermophilus HB8 cells
To confirm its H2O2-scavenging capability in vitro, we purified MnCAT from the T. thermophilus HB8 cells using the successive steps of ammonium sulfate fractionation, anion-exchange column chromatography, hydroxyapatite column chromatography, cation-exchange column chromatography, and gel filtration. A protein with H2O2-scavenging activity was purified to near homogeneity on SDS-PAGE (Fig. 6). The apparent molecular weight was 37 × 103. The major band was identified as MnCAT by peptide mass fingerprinting using MALDI-TOF MS. A gel filtration analysis gave an apparent molecular weight of 140 × 103 for the purified MnCAT. Inductively coupled plasma spectrometry of MnCAT preparation showed a Mn ion/MnCAT ratio of 0.52. The steady-state kinetics of this preparation was performed using H2O2 as a substrate (Table 2). The K m value of this preparation was similar to that of MnCAT in a previous study although the k cat value was much lower (Shank et al. 1994). The k cat/K m of this preparation was 6.3 × 104 M−1 s−1 (Table 2). These results indicate that T. thermophilus possesses an intracellular protein, MnCAT, which scavenges H2O2 in vitro.
SDS-PAGE analysis of MnCAT preparations from T. thermophilus HB8. The pooled fractions after each purification step were subjected to 12 % SDS-PAGE followed by Coomassie Brilliant Blue staining. Lane 1 molecular weight marker, lane 2 pellet of cell lysate, lane 3 supernatant of cell lysate, lane 4 30 % ammonium sulfate fraction, lane 5 30–45 % ammonium sulfate fraction, lane 6 45–60 % ammonium sulfate fraction, lane 7 60–75 % ammonium sulfate fraction, lane 8 60–75 % ammonium sulfate fraction (left overnight), lane 9 supernatant of the 75 % ammonium sulfate fraction, lane 10 pooled fractions after TOYOPEARL SuperQ-650 M column chromatography, lane 11 after Resource Q column chromatography, lane 12 after Bio-Scale CHT10-I column chromatography, lane 13 after Resource S column chromatography, lane 14 after HiLoad 16/60 Superdex 200 pg column chromatography, lane 15 after Bio-Scale CHT5-I column chromatography, and lane 16 final preparation. Molecular weights of the marker proteins are shown on the left. Arrowhead shown on the right represents the size of MnCAT
Characterization of the possible HPX homologue
To examine if the possible HPX homologue functions as a peroxidase, we expressed the recombinant form of this protein using an E. coli expression system. Since this protein was produced in an apo-form, the intact preparation (apo-form) was reconstituted with hemin. The peroxidase activity of the reconstituted preparation was measured using ABTS or guaiacol as a reductant substrate and H2O2 as an oxidant substrate (Supplemental Fig. S2). For both reductant substrates, the reconstituted preparation showed a larger increase in absorbance than the intact preparation and hemin. The absorbance increase detected with myoglobin is consistent with a previous report in which myoglobin showed peroxidase activity with ABTS and guaiacol (Matsui et al. 1999).
Table 3 shows a comparison of peroxidase activities measured using reconstituted and intact preparations, hemin, myoglobin, and HRP. The heme content of the reconstituted possible HPX homologue was estimated to be 0.59 mol mol−1 protein (data not shown). Peroxidase activities of reconstituted preparation were higher than those of hemin and myoglobin, although much smaller than those of HRP, a typical peroxidase (Table 3). These results indicate that the possible HPX homologue (TTHA1714) functions as a peroxidase with H2O2 as an oxidant substrate.
Discussion
T. thermophilus HB8 possesses at least three enzymes that can scavenge H2O2: MnCAT (TTHA0122), a possible peroxiredoxin homologue (TTHA1300), and a possible HPX homologue (TTHA1714). To investigate the roles of these proteins, we attempted to delete the ttha0122, ttha1300, and ttha1714 genes from the T. thermophilus HB8 chromosome by homologous recombination. This method has been widely used to disrupt genes of interest in T. thermophilus HB8 (Agari et al. 2008, 2011; Fukui et al. 2011; Nakane et al. 2011; Shimada et al. 2010). In this study, we completely disrupted the ttha1300 gene, but were unable to delete every copy of ttha0122 or ttha1714 (Fig. 1b; Table 1). Our inability to completely delete ttha0122 is consistent with a previous study, which reported unsuccessful attempts to knock out the mncat gene in T. thermophilus HB27 (Moreno et al. 2004). Partial disruption of a gene has been previously reported with the deletion of recJ in Deinococcus radiodurans (Cao et al. 2010), an extremely radioresistant and polyploid bacterium (Hansen 1978) closely related to the genus Thermus (Omelchenko et al. 2005). Our inability to produce ttha0122 and ttha1714 null mutants suggests that their gene products exert essential functions in T. thermophilus HB8.
The ∆ttha0122 and ∆ttha1714 strains showed conspicuous phenotypes despite containing residual wild type genes. The partial disruption of each gene caused a significant increase in the spontaneous mutation frequency: eightfold higher for ∆ttha0122 and sixfold higher for ∆ttha1714 under normal conditions (Fig. 2). Nakane et al. (2011) measured the spontaneous mutation rate for the ∆mutM strain of T. thermophilus HB8. MutM removes an oxidatively damaged base, 8-oxoguanine, from DNA (Morita et al. 2010). While ∆mutM shows no significant increase in its spontaneous mutation frequency under the normal conditions, it exhibits a roughly threefold increase in the rate of spontaneous mutations under oxidative conditions (Nakane et al. 2011). Notably, this increase is less than that of ∆ttha0122 and ∆ttha1714 under normal conditions (Fig. 2). These findings indicate that the oxidative stress occurring in ∆ttha0122 and ∆ttha1714 under normal conditions is high enough to cause mutagenic effects and suggest an important role for MnCAT and the possible HPX homologue in scavenging ROS.
Moreno et al. (2004) used an antisense RNA to inhibit the function of MnCAT in T. thermophilus HB27 and showed that this causes the cells to be more sensitive to H2O2. Consistent with this previous report, ∆ttha0122 was highly sensitive to H2O2-induced oxidative stress in our study (Fig. 3c). MnCAT (TTHA0122) is annotated as an intracellular enzyme that degrades H2O2. To confirm its presence, we purified it from T. thermophilus HB8 cells (Fig. 6). Enzymatic analysis showed that it scavenges H2O2 with substantial catalytic efficiency (Table 2). The virtual absence of scavenging activity in ∆ttha0122 (Fig. 5) is probably due to the decreased amount of MnCAT in the mutant strain. Collectively, our results indicate that MnCAT is the primary scavenger for intracellular H2O2 in T. thermophilus.
Figure 5 shows that the HPX mutant, ∆ttha1714, scavenges H2O2 as well as the wild type. This finding indicates that this possible HPX homologue is not the primary H2O2 scavenger. However, it does help T. thermophilus cells cope with oxidative stress, since ∆ttha1714 showed a significant increase in spontaneous mutation frequency (Fig. 2). Based on the similarities in amino acid sequence and three-dimensional structure, TTHA1714 is considered as a possible HPX homologue, and is classified as a dye-decolorizing peroxidase that uses H2O2 to degrade various compounds such as an anthraquinone-type dye (Sugano 2009). Enzymatic analysis suggests that TTHA1714 acts as a peroxidase with H2O2 as an oxidant substrate (Table 3). The culture medium of ∆ttha1714 turned red after a long culture period (Fig. 4). Decreased levels of the possible HPX homologue in ∆ttha1714 may cause the accumulation of a red-colored dye and H2O2, leading to hydroxyl radical production and oxidative damage.
Agari et al. (2010) used DNA microarray analysis to identify a host of genes under the control of the oxidative stress-responsible transcriptional activator, SdrP, from T. thermophilus HB8. Since the mncat and hpx genes are not included in the list, another transcriptional regulator probably controls the expression of these two genes.
In aerobically growing cells, various types of ROS scavengers contribute to diminish oxidative damage and maintain aerobic metabolism. In accordance with a previous study by Moreno et al. (2004), our results provide further evidence that MnCAT is the primary scavenger for H2O2 and serves essential functions in T. thermophilus. It is noteworthy in this study that both MnCAT and the possible HPX homologue exhibited a similar and significant increase in spontaneous mutation frequency even under normal conditions (Fig. 2). This finding indicates that the protective role of the possible HPX homologue against oxidative stress is comparable to that of MnCAT. Although present in the HPX mutant (∆ttha1714), MnCAT may not be able to complement the deficiency of the possible HPX homologue in the mutant. Based on these findings, we propose that not only MnCAT but also the possible HPX homologue, a member of dye-decolorizing peroxidase, acts as ROS scavengers to protect T. thermophilus cells from oxidative stress. This is the first study on the involvement of the possible HPX homologue TTHA1714 in protecting cells from oxidative stress in this thermophile. Further biochemical and gene expression analyses are warranted to understand the discrete roles of these scavenging enzymes.
Abbreviations
- ROS:
-
Reactive oxygen species
- MnCAT:
-
Manganese-containing catalase
- HPX:
-
Heme peroxidase
- HTK :
-
Thermostable kanamycin-resistance gene
- HRP:
-
Horseradish peroxidase
- ABTS:
-
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- MES:
-
2-morpholinoethanesulfonic acid
- MOPS:
-
3-(N-morpholino)propanesulfonic acid
- SDS-PAGE:
-
SDS-polyacrylamide gel electrophoresis
- MALDI-TOF MS:
-
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
References
Agari Y, Kashihara A, Yokoyama S, Kuramitsu S, Shinkai A (2008) Global gene expression mediated by Thermus thermophilus SdrP, a CRP/FNR family transcriptional regulator. Mol Microbiol 70:60–75. doi:10.1111/j.1365-2958.2008.06388.x
Agari Y, Kuramitsu S, Shinkai A (2010) Identification of novel genes regulated by the oxidative stress-responsive transcriptional activator SdrP in Thermus thermophilus HB8. FEMS Microbiol Lett 313:127–134. doi:10.1111/j.1574-6968.2010.02133.x
Agari Y, Agari K, Sakamoto K, Kuramitsu S, Shinkai A (2011) TetR-family transcriptional repressor Thermus thermophilus FadR controls fatty acid degradation. Microbiology 157:1589–1601. doi:10.1099/mic.0.048017-0
Beers RF Jr, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Berry EA, Trumpower BL (1987) Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal Biochem 161:1–15
Bonny C, Montandon PE, Marc-Martin S, Stutz E (1991) Analysis of streptomycin-resistance of Escherichia coli mutants. Biochim Biophys Acta 1089:213–219
Cao Z, Mueller CW, Julin DA (2010) Analysis of the recJ gene and protein from Deinococcus radiodurans. DNA Repair (Amst) 9:66–75. doi:10.1016/j.dnarep.2009.10.009
Chelikani P, Fita I, Loewen PC (2004) Diversity of structures and properties among catalases. Cell Mol Life Sci 61:192–208. doi:10.1007/s00018-003-3206-5
Ebihara A et al (2005) Structure-based functional identification of a novel heme-binding protein from Thermus thermophilus HB8. J Struct Funct Genom 6:21–32. doi:10.1007/s10969-005-1103-x
Fukui K, Wakamatsu T, Agari Y, Masui R, Kuramitsu S (2011) Inactivation of the DNA repair genes mutS, mutL or the anti-recombination gene mutS2 leads to activation of vitamin B1 biosynthesis genes. PLoS ONE 6:e19053. doi:10.1371/journal.pone.0019053
Gay C, Collins J, Gebicki JM (1999) Hydroperoxide assay with the ferric-xylenol orange complex. Anal Biochem 273:149–155. doi:10.1006/abio.1999.4208
Giorgio M, Trinei M, Migliaccio E, Pelicci PG (2007) Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol 8:722–728
Hansen MT (1978) Multiplicity of genome equivalents in the radiation-resistant bacterium Micrococcus radiodurans. J Bacteriol 134:71–75
Hashimoto Y, Yano T, Kuramitsu S, Kagamiyama H (2001) Disruption of Thermus thermophilus genes by homologous recombination using a thermostable kanamycin-resistant marker. FEBS Lett 506:231–234
Hoseki J, Yano T, Koyama Y, Kuramitsu S, Kagamiyama H (1999) Directed evolution of thermostable kanamycin-resistance gene: a convenient selection marker for Thermus thermophilus. J Biochem 126:951–956
Imlay JA (2003) Pathways of oxidative damage. Annu Rev Microbiol 57:395–418
Imlay JA (2008) Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 77:755–776. doi:10.1146/annurev.biochem.77.061606.161055
Kuramitsu S, Hiromi K, Hayashi H, Morino Y, Kagamiyama H (1990) Pre-steady-state kinetics of Escherichia coli aspartate aminotransferase catalyzed reactions and thermodynamic aspects of its substrate specificity. Biochemistry 29:5469–5476
Luria SE, Delbruck M (1943) Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28:491–511
Matsui T, Ozaki S, Liong E, Phillips GN Jr, Watanabe Y (1999) Effects of the location of distal histidine in the reaction of myoglobin with hydrogen peroxide. J Biol Chem 274:2838–2844
Mishra S, Imlay J (2012) Why do bacteria use so many enzymes to scavenge hydrogen peroxide? Arch Biochem Biophys 525:145–160. doi:10.1016/j.abb.2012.04.014
Moreno R, Hidalgo A, Cava F, Fernandez-Lafuente R, Guisan JM, Berenguer J (2004) Use of an antisense RNA strategy to investigate the functional significance of Mn-catalase in the extreme thermophile Thermus thermophilus. J Bacteriol 186:7804–7806. doi:10.1128/JB.186.22.7804-7806.2004
Morita R et al (2010) Molecular mechanisms of the whole DNA repair system: a comparison of bacterial and eukaryotic systems. J Nucleic Acids 2010:179594. doi:10.4061/2010/179594
Nakane S, Wakamatsu T, Masui R, Kuramitsu S, Fukui K (2011) In vivo, in vitro, and X-ray crystallographic analyses suggest the involvement of an uncharacterized triose-phosphate isomerase (TIM) barrel protein in protection against oxidative stress. J Biol Chem 286:41636–41646. doi:10.1074/jbc.M111.293886
Ohtani N, Tomita M, Itaya M (2010) An extreme thermophile, Thermus thermophilus, is a polyploid bacterium. J Bacteriol 192:5499–5505. doi:10.1128/JB.00662-10
Omelchenko MV et al (2005) Comparative genomics of Thermus thermophilus and Deinococcus radiodurans: divergent routes of adaptation to thermophily and radiation resistance. BMC Evol Biol 5:57. doi:10.1186/1471-2148-5-57
Oshima T, Imahori K (1974) Description of Thermus thermophilus (Yoshida and Oshima) comb. nov., a nonsporulating thermophilic bacterium from a Japanese thermal spa. Int J Syst Bacteriol 24:102–112
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Seaver LC, Imlay JA (2001a) Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol 183:7173–7181. doi:10.1128/JB.183.24.7173-7181.2001
Seaver LC, Imlay JA (2001b) Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J Bacteriol 183:7182–7189. doi:10.1128/JB.183.24.7182-7189.2001
Shank M, Barynin V, Dismukes GC (1994) Protein coordination to manganese determines the high catalytic rate of dimanganese catalases. Comparison to functional catalase mimics. Biochemistry 33:15433–15436
Shimada A, Masui R, Nakagawa N, Takahata Y, Kim K, Kuramitsu S, Fukui K (2010) A novel single-stranded DNA-specific 3ʹ-5ʹ exonuclease, Thermus thermophilus exonuclease I, is involved in several DNA repair pathways. Nucleic Acids Res 38:5692–5705. doi:10.1093/nar/gkq350
Smith PK et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Sugano Y (2009) DyP-type peroxidases comprise a novel heme peroxidase family. Cell Mol Life Sci 66:1387–1403. doi:10.1007/s00018-008-8651-8
Wallace SS (2002) Biological consequences of free radical-damaged DNA bases. Free Radic Biol Med 33:1–14
Whittaker JW (2012) Non-heme manganese catalase–the ‘other’ catalase. Arch Biochem Biophys 525:111–120. doi:10.1016/j.abb.2011.12.008
Wood ZA, Schroder E, Robin Harris J, Poole LB (2003) Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci 28:32–40
Yokoyama S et al (2000) Structural genomics projects in Japan. Nat Struct Biol 7(Suppl):943–945
Zubieta C et al (2007a) Identification and structural characterization of heme binding in a novel dye-decolorizing peroxidase, TyrA. Proteins 69:234–243. doi:10.1002/prot.21673
Zubieta C et al (2007b) Crystal structures of two novel dye-decolorizing peroxidases reveal a beta-barrel fold with a conserved heme-binding motif. Proteins 69:223–233. doi:10.1002/prot.21550
Acknowledgments
We thank all the members of SR System Biology Research Group, RIKEN SPring-8, Harima Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Albers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ebihara, A., Manzoku, M., Fukui, K. et al. Roles of Mn-catalase and a possible heme peroxidase homologue in protection from oxidative stress in Thermus thermophilus . Extremophiles 19, 775–785 (2015). https://doi.org/10.1007/s00792-015-0753-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-015-0753-2