Abstract
Polyhydroxyalkanoates (PHAs) are macromolecules produced by bacteria as means for storing carbon and energy in intracellular granules. PHAs have physical properties similar to those of plastics and have become of interest to industry as materials for environmentally friendly bioplastic production. There is an ongoing search for new PHA-producing bacterial strains and PHA-synthesizing enzymes tolerating extreme conditions to find ways of producing PHAs at cold temperatures and high solute concentrations. Moreover, the study of PHA producers in the sea-ice biome can aid in understanding the microbial ecology of carbon cycling in ice-associated ecosystems. In this study, PHA producers and PHA synthase genes were examined under the extreme environmental conditions of sea ice and cold seawater to find evidence of PHA production in an environment requiring adaptation to high salinity and cold temperatures. Sea ice and cold estuarine water samples were collected from the northern Baltic Sea and evidence of PHA production was gathered, using microscopy with Nile Blue A staining of PHA-granules and PCR assays detecting PHA-synthesis genes. The PHA granules and PHA synthases were found at all sampling locations, in both sea ice and water, and throughout the sampling period spanning over 10 years. Our study shows, for the first time, that PHA synthesis occurs in Baltic Sea cold-adapted bacteria in their natural environment, which makes the Baltic Sea and its cold environments an interesting choice in the quest for PHA-synthesizing bacteria and synthesis genes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyhydroxyalkanoates (PHAs) are structurally simple biopolyesters that are deposited as water-insoluble granules within the cells of bacteria and archaea (Anderson and Dawes 1990; Madison and Huisman 1999). Sustainable and biodegradable PHAs have become an attractive alternative to petrochemistry-derived plastic materials. However, the production costs of PHAs are still high compared with that of traditional plastics (Koller et al. 2011). In theory, biopolymer production can be made more economically feasible and sustainable, using cold-adapted bacterial strains and enzymes functioning efficiently at low temperatures (Feller and Gerday 2003; Margesin and Feller 2010). Bio-prospecting of PHA-producing bacterial strains and PHA synthases for more profitable production of PHAs at cold temperatures and high solute concentrations is ongoing.
PHA synthases, encoded by phaC genes and sometimes an additional gene phaE or phaR, are the key enzymes that produce PHA (Rehm 2003). PhaCs catalyze the conversion of (R)-3-hydroxyacyl-CoA substrates to PHAs. The synthases have been assigned to four classes (Rehm 2003) and produce over 150 chemically different monomers (Rehm 2010). This results in an enormous variation in the physical properties of PHA polymers, which is beneficial, considering the use of PHAs as bioplastics. The major function of PHAs in bacteria is carbon and energy storage, and apart from this, they facilitate the transport of calcium phosphate and DNA across membranes by forming membrane-spanning calcium polyphosphate channels contributing to stress and osmotic resistances of the cells (Tal and Okon 1985; Reusch and Sadoff 1988; Ayub et al. 2009).
PHA increases fitness during environmental stress caused by cold temperatures, osmotic pressure and carbon limitation in PHA-producing bacteria, both in planktonic and biofilm cultures (Tal and Okon 1985; Lee 1996; Kadouri et al. 2005; Ayub et al. 2009; Tribelli and Lopez 2011). Sea ice and cold seawater are extreme environments, with all three stressors affecting the growth of both planktonic and biofilm-associated cells (Pomeroy and Wiebe 2001; Feller and Gerday 2003; Krell et al. 2007; Deming 2010). Many sea-ice bacteria inhabit small brine channels and have adapted to a biofilm like lifestyle with high organism densities and extracellular polymeric substance (EPS) production (Krembs and Deming 2008; Underwood et al. 2010; Krembs et al. 2011). Carbon availability and cold temperatures act as interactive limiting factors of growth, but bacteria can still grow in cold water, and even in ice, unless both substrate availability and temperature are extremely low at the same time (Pomeroy and Wiebe 2001). Sea ice has lower temperatures than the underlying seawater, but the concentrations of macronutrients and bioavailable carbon substrates can be higher (Cota et al. 1987; Pomeroy and Wiebe 2001) enabling sea-ice bacteria to grow and presumably store carbon in the form of PHA.
The ability to produce PHAs is known to be a common occurrence among soil and sediment bacteria. Correspondingly in marine environments, PHA or PHA-producing organisms have mainly been isolated from estuarine sediments and estuarine microbial mats (Berlanga et al. 2006; Villanueva et al. 2007; Koller et al. 2011). Only some bacterial isolates from marine pelagic waters are known to produce PHA in culture (Godoy et al. 2003; Cho and Giovannoni 2004; Arahal et al. 2007), but no systematic research on the occurrence of PHAs in marine pelagic bacterial communities has so far been conducted. The same applies for the PHA producers found in cold environments. Cold-adapted Polaromonas sp. (Mattes et al. 2008), Photobacterium profundum (Vezzi et al. 2005), Sphingopyxis alaskensis (Ting et al. 2010; Godoy et al. 2003), Pseudomonas extremaustralis, (Lopez et al. 2009; Tribelli et al. 2012) and Rhodoferax ferrireducens (Finneran et al. 2003; Risso et al. 2009) either produce PHAs in culture or possess the metabolic pathway for PHA production, but the production of PHAs by cold-adapted organisms has not been systematically studied.
In a recent study, Kaartokallio et al. (2013) found evidence for PHA production in subarctic sea ice in Greenland fjord, based on microscopic detection of Nile Blue A-stained intracellular granules in environmental samples. However, their study did not cover the identification of PHA synthase genes from sea ice. Before the field data presented by Kaartokallio et al. (2013), the overall occurrence of PHA production in sea ice had only been indicated by genomic evidence from the psychrophilic bacterium Colwellia psychrerythrea 34H (Methe et al. 2005).
The aim of this study was to obtain evidence for PHA production in the extreme and cold environments of the Northern Baltic Sea by searching for PHA granules and PHA synthase genes in environmental samples. To that end, we collected samples from Baltic Sea ice and complemented them with stored sea ice and estuarine samples spanning over 10 years and three geographical locations. From this versatile data set we found evidence for putative PHA production in the sea ice and cold estuarine waters of the northern Baltic Sea.
Materials and methods
Sampling
Samples were collected from seasonal sea ice and cold waters in three coastal locations in the northern Baltic Sea in March between years 2000 and 2013. New samples dedicated for this study were collected in year 2013. Samples for the other years originated from earlier sampling campaigns and were adequately stored. This strategy was chosen to be able to elucidate the temporal and spatial occurrence of PHA production. Sea-ice sampling is logistically difficult and costly and by utilizing stored samples, the available data set could be significantly augmented. Sampling and selection of stored samples were targeted to lower ice layer samples (0–15 cm above the ice-water interface) collected during the months of March and early April to match the presumed maximum biomass and carbon availability in sea ice (Granskog et al. 2006). All sea-ice sampling sites were pristine and represented natural sea-ice conditions in the area. Total ice thickness at the sampling sites varied from 45 to 55 cm. Water sampling was conducted in May 2011 shortly after sea-ice breakup in an estuary receiving water from agriculture-dominated catchment with moderate to high anthropogenic influence (Asmala et al. 2013). Information on the sampling sites and sample types obtained is given in Table 1 and Fig. 1. Sea-ice samples from years 2000 and 2003, identified as ice SWF-M, originated from the sample sets published previously (Kaartokallio 2004; Kaartokallio et al. 2007).
The ice samples were collected by a Cold Regions Research and Engineering Laboratory (CRREL)-type ice auger and the ice cores were sectioned with hand saw and placed in acid-washed polyethylene jars. In the laboratory, the ice was allowed to melt in the dark at +4 °C. Water samples were collected from a small boat with a standard water sampler from a 1-m depth in 1-l polycarbonate bottles. The water Western Finland (WF) 2 and 3 samples were prefiltered through a 0.8-μm filter by a peristaltic pump and a 3 + 0.8-μm nominal pore size Sartoclean GF capsule filter (Sartorius, Göttingen, Germany).
To obtain community DNA, melted ice and water were filtered onto 0.2-μm pore size individually packed sterile mixed cellulose ester filters (d 47 mm, Whatman, Little Chalfont, UK). The filters were packed into sterile cryovials and stored at −80 °C until DNA extraction. Community DNA samples from year 2004 were extracted soon after sampling and stored as extracts at −80 °C until analysis. Subsamples for microscopy were transferred to 20-ml glass vials, fixed with 1 % (final concentration) electron microscopy grade glutaraldehyde and stored at +4 °C in the dark until analysis.
Microscopy
PHA was detected, using a modification of the widely used Nile Blue A staining method, by Ostle and Holt (1982) as follows: 5 ml of each glutaraldehyde-fixed sample was filtered onto a black 0.2-μm polycarbonate filter (Osmonics, Penang, Malaysia) and stained for 15 min with 1 % (w/v) aqueous solution of Nile Blue A sulfate (Sigma Aldrich, St. Louis, MO, USA). After staining, the filters were rinsed three times with deionized water to remove excess stain. The filters were examined, using a Leitz Aristoplan epifluorescence microscope under green excitation (M3 filter) and PL Fluotar 100 × 12.5/20 oil-immersion objective (Leica Microsystems Gmbh, Wetzlar, Germany). Cells with brightly fluorescing bodies within them were imaged, using a Photometrics CH250/A charge-coupled device camera (Photometrics, Tuscon, AZ, USA) connected to a Leitz Aristoplan epifluorescence microscope and PMIS image-acquisition software. Total bacterial abundance was measured with Acridine Orange direct count (Hobbie et al. 1977). Prior to counting, 5–10 ml of each sample was filtered onto a black 0.2-μm polycarbonate filter (Osmonics, Penang, Malaysia) and stained for 5 min with 0.015 % acridine orange solution. The filters were examined with the same microscope setup as for PHA but using blue excitation and I3 filter. Total bacterial abundance and abundance of PHA granule-containing cells were calculated from at least 20 counting fields on a New Porton E11 counting grid. Only bacteria with clearly defined and organized spherical fluorescent granules within the cell were counted as granule-containing cells.
DNA purification and storage
DNA from the ice SWF and water WF samples was isolated from filters, using a PowerSoil® DNA Isolation Kit (MO BIO Laboratories, Inc., Carlsbad, CA, USA), according to the manufacturer’s instructions. DNA from ice the northeastern Sweden (NES) samples was isolated from filters using a DNeasy tissue kit (Qiagen, Hilden, Germany). The DNA concentrations were measured, using NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The isolated DNA was stored at −80 °C.
Primer design
Nucleotide sequences for all bacterial genes annotated as phaC were retrieved from the National Center for Biotechnology Information (NCBI) GenBank nucleotide database. The sequences were aligned with Multiple Sequence Comparison by log Expectation (MUSCLE; Edgar 2004) and divided into groups, based on sequence similarity. The groups were realigned and closely related alignments were joined together into groups containing the majority of the different phaC sequences of the respective class of the PHA synthase (I–IV). The groups which had the most species were selected for each class. The alignments were checked and corrected manually, using BioEdit (Tom Hall, Ibis Biosciences, Carlsbad, CA, USA).
Class I phaC of β-proteobacteria, class II of γ-proteobacteria (Rehm 2003), class III of Gram-positive bacteria and class IV of Bacillus megaterium were selected for primer design. Primers for the specific amplification of target phaC class I, II, III and IV sequences were designed with Primer Basic Local Alignment Search Tool (Primer-BLAST) (Ye et al. 2012), using Primer3 (Koressaar and Remm 2007; Untergasser et al. 2012), based on consensus sequences of the alignments of the different phaC classes. The designed primers were compared to the sequence alignment of the phaC group and the primers that were located in conserved regions of the genes were selected. The selected primers were: phaCI730F (CGCCCTGCATCAACAAGTTC) and phaCI1218R (GTAGTTCCAGACCAGGTCGTT), phaCII36F (GAGCGAAAAACAGTACGCCA) and phaCII1056R (CATCGGTGGGTAGTTCTGGT), phaCIII110F (CAGAGCCGCAAGTCGGATTA) and phaCIII1068R (AGCCAATCTCCAATCGTCGG), and phaCIV9F (TCCTTACGTGCAAGAGTGGG) and phaCIV921R (ATCACGGCTAGCAGCAATGT). The phaC classes I–IV are designated in the primer name.
Primer efficiency and specificity were checked with colony PCR, using cultured bacteria which possess the phaC gene of the phaC class of interest. Cupriavidus metallidurans CH34 was used for testing class I phaC primers, Pseudomonas fluorescens and Pseudomonas aeruginosa for class II, Bacillus cereus for class III and Bacillus megaterium for class IV. The expected PCR product sizes using the bacteria mentioned above as templates in colony PCR were: 488 bp for phaCI730F and 1218R, 1021 bp for phaCII36F and 1056R (from P. fluorescens), 958 bp for phaCIII110F and 1068R, and 913 bp for phaCIV9F and 921R pairs.
PCR and cloning
The PCR reactions were performed in a 20-µl volume containing 4–50 ng of template DNA, 0.4 U Phusion High-Fidelity DNA Polymerase (Thermo Scientific), 1× HF buffer for Phusion, 200 µM of each dNTP, and of each forward and reverse primer filled to end volume with Milli-Q water (Merck Millipore, Billerica, MA, USA). Positive controls were performed with colony PCR, using cultured bacteria that were used also in primer testing.
The cycling conditions were as follows: 30 s at 98 °C followed by 40 cycles of 10 s at 98 °C, 10 s at 65 °C and 15 s at 72 °C, and a final elongation step at 72 °C for 10 min. The reactions were repeated twice and the amplified PCR products checked by gel electrophoresis to confirm the results.
The purified PCR products were pooled and ligated into a SmaI-digested pUC19 vector. The resulting constructs were transformed into Escherichia coli and the plasmid DNA was purified using a QIAprep Spin Miniprep Kit (Qiagen).
Sequencing and sequence analysis
Five phaC class I and II clones were sequenced at the Institute of Biotechnology (University of Helsinki, Finland). The sequences were processed using the Staden package (Staden 1996). The four different sequences were deposited in GenBank with accession numbers KM065446-KM065449. A maximum likelihood phylogenetic tree was constructed from the partial phaC nucleotide sequences of this study and select class I, II, III and IV PHA synthase producer phaC sequences retrieved from GenBank. The Synechocystis sp. phaC gene was used as an out-group. The phaC sequences were aligned using Multiple Alignment with Fast Fourier Transform (MAFFT) version 7.058b (Katoh et al. 2002; Katoh and Standley 2013). Ambiguous and highly variable regions were removed, using Block Mapping and Gathering with Entropy (BGME) software (Criscuolo and Gribaldo 2010). A maximum likelihood phylogenetic tree with 100 bootstrap resamplings was generated with Randomized Axelerated Maximum Likelihood (RAxML) version 7.7.6 (Stamatakis et al. 2005), using the generalized time reversible (GTR) nucleotide substitution model with a gamma model of rate heterogeneity.
Results
Detection of PHA granules
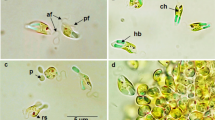
Cells containing putative PHA granules, i.e. brightly fluorescing intracellular inclusions observed following the Nile Blue A stain, were found at all three sampling locations, both sea ice and water and throughout the sampling years (Table 2; Fig. 2). The PHA granules occurred in defined bacterial morphotypes, some of which were consistent across all samples. Three main morphotypes of PHA-containing cells were found: (1) long thin filaments with numerous PHA granules inside the cells (Fig. 2a, c, d, g), (2) shorter filaments or elongated rods with 3–8 granules inside the cell (Fig. 2b, e–h), (3) and cells with singlet and doublet granules (Fig. 2d, e, g, h). All main morphotypes occurred in the ice and water samples but single- and double-granule-containing cells were more abundant in water samples, whereas the filamentous morphotypes were common in ice samples. Granule-containing bacteria were often associated with diatoms or EPS matrices in ice samples. On average, 18 % (n = 12) of all bacterial cells in ice and 5 % (n = 3) in water samples contained PHA granules. In ice samples, the share of granule-bearing cells varied from 8 to 27 % between years and sampling locations. Nile Blue A can also selectively stain intracellular neutral lipid or wax ester inclusions accumulated by limited number of bacterial phyla that, however, do not generally occur in sea ice (Ishige et al. 2003; Wältermann and Steinbüchel 2005; Deming 2010). In a study by Alvarez et al. (1997), 73 % of studied psychrophilic hydrocarbon-degrading marine bacterial strains were able to produce PHA, whereas only 1 % accumulated other lipids or wax esters.
Photomicrographs of Nile Blue A-stained bacterial cells containing putative PHA granules. Sample location is indicated in the panels (WF western Finland, unfiltered, NES north eastern Sweden, SWF southwestern Finland. SWF-M = additional samples from years 2000 and 2003 with only microscopy data available). Stars denote samples with community DNA obtained and the presence of phaC genes confirmed by PCR. Various bacterial morphotypes containing PHA granules are shown; insets in panels f and g display additional morphotypes in the respective samples. Bacteria attached to EPS matrices are shown in panels b and h
Presence of phaC genes
The phaC primers tested with strains possessing phaC genes of classes I–IV were all specific and yielded products of expected sizes in colony PCR done with the phaC-possessing bacteria Pseudomonas fluorescens, Cupriavidus metallidurans, Bacillus megaterium and Bacillus cereus.
The PCR screening of class III and class IV phaC genes from the ice and water samples with phaCIII110-1068 and phaCIV9-921 did not yield products. However, the screening of class I and class II genes done with phaCI730-1218 and phaCII36-1056 primer pairs yielded PCR products from several environmental samples. PCR products were obtained from all sampling locations, time points and ice and water samples (Table 2), which support the microscopy results suggesting the existence of PHA granules. The phaC class I type genes were found at all three sampling stations in the Baltic Sea, and from both sea ice and estuarine water, and from all sampling years where DNA samples were available. The phaC class II type genes were found from fewer samples than class I type. Class II type was found only in the SWF ice and WF water samples but not from the NES ice samples. All the ice SWF samples, and the WF water sample that had not been prefiltered, had the phaC class II genes.
Sequence analysis
To get further information about the PHA-synthase genes, the phaC clones were sequenced. The four phaC class I clones contained three different phaC gene sequences (PHAI1, PHAI2, PHAI3) and clustered together with the phaC class I gene sequences of β-proteobacteria (Fig. 3). The sequences were most similar to Rhodoferax ferrireducens, Polaromonas naphthalenivorans and Polaromonas sp. phaC class I sequences (Table 3). The PHAII clone sequence clustered with the phaC class II genes of γ-proteobacteria (Fig. 3) and was most similar to Pseudomonas extremaustralis strain 14-3 phaC class II (Table 3). The maximum likelihood phylogenetic tree of the phaC genes and PHA clone sequences is shown in Fig. 3. All the phaC genes that were found differed from the nucleotide sequences retrieved from Genbank. The highest similarity of 85.2 % was found between the phaC class II gene of Pseudomonas extremaustralis and clone PHAII (Table 3), which suggests that none of the phaC genes were from previously sequenced PHA producers or of a previously sequenced phaC type.
Discussion
Our study shows the presence of PHA synthesis genes and PHA granules in the extreme northern Baltic Sea ice and cold pelagic seawater environments. Evidence of PHA production was gathered, using microscopy with Nile Blue A staining of PHA granules and PCR assays done with primers designed for this study. Our findings support previous results from Greenland subarctic sea ice by (Kaartokallio et al. 2013) on the occurrence of PHA production in pristine sea-ice environments.
PHA granules were found at all the sampling locations and time points in the sea-ice and water samples, and granule-containing cells formed a significant portion of all bacterial cells, especially in sea ice. The granule-containing bacterial cells found in ice and estuarine water samples corresponded to morphotypes found previously in sea ice (Kaartokallio et al. 2013). Many granule-containing bacteria had a specific filamentous-like morphology, resembling that of a Rhodoferax antarcticus strain isolated from a permanently frozen Antarctic lake (Madigan et al. 2000), which is closely related to known PHA-producing species in the same genus Rhodoferax (Madigan et al. 2000; Finneran et al. 2003; Risso et al. 2009).
Osmotic pressure, carbon availability, and cold temperatures act as limiting factors of growth, but bacteria can still grow in cold water, and even in ice, unless both substrate availability and temperature are extremely low at the same time (Pomeroy and Wiebe 2001). We observed putative PHA granules in all of the sea-ice and water samples. Occurrence of the PHA granules in freezing temperatures of the sea ice may partly be explained by sea ice having a higher concentration of bioavailable carbon compounds than the underlying sea water (Cota et al. 1987; Pomeroy and Wiebe 2001). In the sea-ice samples, the PHA granule-containing bacteria were often associated with diatoms or EPS matrices, pointing to the presence of an ample available carbon substrate supply needed to sustain PHA production. Available organic carbon in sea ice is a mixture of carbohydrates, proteinaceous material and humic substances (Calace et al. 2001; Stedmon et al. 2011). The carbohydrate pool consists mainly of nitrogen poor polysaccharides, and it is thought that the nitrogen-rich proteinaceous pool is degraded much faster (Thomas et al. 1995; Stedmon et al. 2011). Thus, the proteinaceous pool consisting of proteins, peptides and amino acids originating from ice organisms is a potential substrate pool used for PHA synthesis in sea ice. Free amino acids have also been shown to occur in high concentrations in sea ice (Amon et al. 2001). The finding of putative PHA-granules in environmental samples suggests that active PHA production occurs in sea ice and cold estuarine water environments, despite the extreme low temperatures and variable osmotic pressure (in sea ice).
The microscopic evidence for PHA production based on the finding of putative PHA granules was corroborated by the detection of phaC genes. The phaC class I genes were found in ice and both prefiltered and unfiltered water samples. The class I genes were found at all four sampling stations throughout the sampling period. The class I genes often occurred concurrently with long thin filament like cells resembling Rhodoferax antarcticus (Madigan et al. 2000). The results suggest that bacteria inhabiting the Baltic Sea ice and cold estuarine water possess PHA class I synthases, affiliated to certain β-proteobacteria (Rehm 2003), i.e. Rhodoferax.
The class II PHA synthase genes were found only at one of the ice-sampling locations (SWF, 2013) and from unfiltered estuarine water from WF collected in 2010. Thus, class II genes were not as widely distributed among the sampling locations and years as the class I genes. Bacteria carrying class II genes could have been excluded from the 0.8-µm prefiltered WF samples and possibly therefore the genes were not found in our PCR assay. The prefiltration could have excluded the phaC class II producers if the cells were large or attached to EPS or particles. In microscopy, PHA granule-containing bacteria were often associated with EPS and diatom-resembling particles in the unfiltered ice samples. In addition to the possible effects of the prefiltration on the detection of the phaC class II producers, we cannot rule out the possibility that our PCR assay may not have revealed the full diversity of the class II genes.
The inability to detect class III and class IV phaC genes with our primers may be due to the lack on class III and IV phaC sequence data from Gram-negative bacteria available from these environments to be used for primer design. However, the class III and IV phaCs might not be as common in sea ice and cold sea water bacteria, and the genes not detected because of the limited sensitivity of PCR.
The phaC class I and II clone sequences obtained resembled the phaC genes of cold-adapted bacteria, but due to the low nucleotide sequence similarities they seem to represent previously unsequenced types of phaCs. The phaC class I genes with the most similarities to our sequences were from three cold-adapted β-proteobacteria: Polaromonas naphthalenivorans, Polaromonas sp., and Rhodoferax ferrireducens. The phaC class II sequence also was similar to a corresponding class II phaC sequence from a cold-adapted Pseudomonas extremaustralis belonging phylogenetically to the γ-proteobacteria. Previous phylogenetic studies of phaC sequences have shown that phaC trees are for the most part congruent with 16 s rRNA gene-based trees (Kadouri et al. 2005). Therefore, it is reasonable to assume that the bacteria encoding the phaC clone sequences found in this study could be related to the phaC-possessing bacteria with the highest sequence similarities to the clone sequences. In addition to the phylogenetic evidence, genera Polaromonas and Rhodoferax are known to inhabit sea ice (Deming 2010) and are likely adapted to the high osmotic pressures typical of the sea-ice primary habitat, the brine channel system. However, the possibility of horizontal gene transfer of PHA synthases cannot be excluded (Koller et al. 2011), so no definite conclusions can be made about the identities of the bacteria encoding the phaC genes.
The bacteria found in cold environments encoding phaC genes with highest similarities to our phaC sequences have wide carbon substrate ranges, including cellobiose, phenols, alkenes and chlorinated hydrocarbons (Alfreider et al. 2002; Connon et al. 2005; Lopez et al. 2009; Risso et al. 2009; Tribelli et al. 2012). It is known that extremophiles inhabiting cold environments can have wide substrate ranges, making them interesting choices for industrial applications using low-cost carbon sources (Margesin and Feller 2010). The cold tolerance and wide substrate ranges of some PHA producers, such as those found in cold environments, expand our views on PHA production using various carbon sources, including hydrocarbon-contaminated substrates, and in cold temperatures, potentially lowering the production costs of biodegradable plastics.
Our study presents evidence of PHA production in the Baltic Sea by cold-adapted bacteria. The observed geographical and temporal coverage of phaC genes and PHA-granules in the Northern Baltic Sea, together with earlier evidence of putative PHA-production found in Greenland sea ice (Kaartokallio et al. 2013), suggest that PHA production occurs in different sea-ice environments of the Northern hemisphere. Moreover, taken the vast annual areal coverage of sea ice, studying PHA-production may be important in understanding microbial ecology and biogeochemistry of ice-covered seas, e.g. carbon export from melting sea ice and survival of sea-ice bacteria in the water column. In respect to biotechnological applications of the PHA-synthesizing bacteria, sea ice and cold sea water could be interesting environments for bio-prospecting of PHA-producing bacteria with diverse metabolic capabilities, e.g. for use in bioplastic production on previously unusable or hydrocarbon-polluted waste materials under extreme conditions.
References
Alfreider A, Vogt C, Babel W (2002) Microbial diversity in an in situ reactor system treating monochlorobenzene contaminated groundwater as revealed by 16S ribosomal DNA analysis. Syst Appl Microbiol 25:232–240
Alvarez HM, Pucci OH, Steinbüchel A (1997) Lipid storage compounds in marine bacteria. Appl Microbiol Biotechnol 47:132–139
Amon RMW, Fitznar HP, Benner R (2001) Linkages among the bioreactivity, chemical composition, and diagenetic state of marine dissolved organic matter. Limnol Oceanogr 46:287–297
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Arahal DR, Lekunberri I, Gonzalez JM, Pascual J, Pujalte MJ, Pedros-Alio C, Pinhassi J (2007) Neptuniibacter caesariensis gen. nov., sp nov., a novel marine genome-sequenced gammaproteobacterium. Int J Syst Evol Microbiol 57:1000–1006
Asmala E, Autio R, Kaartokallio H, Pitkanen L, Stedmon CA, Thomas DN (2013) Bioavailability of riverine dissolved organic matter in three Baltic Sea estuaries and the effect of catchment land use. Biogeosciences 10:6969–6986
Ayub ND, Tribelli PM, Lopez NI (2009) Polyhydroxyalkanoates are essential for maintenance of redox state in the Antarctic bacterium Pseudomonas sp 14-3 during low temperature adaptation. Extremophiles 13:59–66
Berlanga M, Montero MT, Fernandez-Borrell J, Guerrero R (2006) Rapid spectrofluorometric screening of poly-hydroxyalkanoate-producing bacteria from microbial mats. Int Microbiol 9:95–102
Calace N, Castrovinci D, Maresca V, Petronio BM, Pietroletti M, Scardala S (2001) Aquatic humic substances in pack ice-seawater-sediment system. Int J Environ Anal Chem 79:315–329
Cho JC, Giovannoni SJ (2004) Robiginitalea biformatagen. nov., sp nov., a novel marine bacterium in the family Flavobacteriaceae with a higher G+C content. Int J Syst Evol Microbiol 54:1101–1106
Connon S, Tovanabootr A, Dolan M, Vergin K, Giovannoni S, Semprini L (2005) Bacterial community composition determined by culture-independent and -dependent methods during propane-stimulated bioremediation in trichloroethene-contaminated groundwater. Environ Microbiol 7:165–178
Cota GF, Prinsenberg SJ, Bennett EB, Loder JW, Lewis MR, Anning JL, Watson NHF, Harris LR (1987) Nutrient fluxes during extended blooms of Arctic ice algae. J Geophys Res Oceans 92:1951–1962
Criscuolo A, Gribaldo S (2010) BMGE (block mapping and gathering with entropy): a new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol Biol 10:210
Deming JW (2010) Sea ice bacteria and viruses. In: Thomas DN, Dieckmann GS (eds) Sea ice, 2nd edn. Wiley-Blackwell, Oxford, pp 247–282
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Feller G, Gerday C (2003) Psychrophilic enzymes: Hot topics in cold adaptation. Nat Rev Microbiol 1:200–208
Finneran KT, Johnsen CV, Lovley DR (2003) Rhodoferax ferrireducens sp nov., a psychrotolerant, facultatively anaerobic bacterium that oxidizes acetate with the reduction of Fe(III). Int J Syst Evol Microbiol 53:669–673
Godoy F, Vancanneyt M, Martinez M, Steinbuchel A, Swings J, Rehm BHA (2003) Sphingopyxis chilensis sp nov., a chlorophenol-degrading bacterium that accumulates polyhydroxyalkanoate, and transfer of Sphingomonas alaskensis to Sphingopyxis alaskensis comb. nov. Int J Syst Evol Microbiol 53:473–477
Granskog M, Kaartokallio H, Kuosa H, Thomas DN, Vainio J (2006) Sea ice in the Baltic Sea—a review. Estuar Coast Shelf Sci 70:145–160
Hobbie JE, Daley RJ, Jasper S (1977) Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol 33:1225–1228
Ishige T, Tani A, Sakai Y, Kato N (2003) Wax ester production by bacteria. Curr Op Microbiol 6:244–250
Kaartokallio H (2004) Food web components, and physical and chemical properties of Baltic Sea ice. Mar Ecol Prog Ser 273:49–63
Kaartokallio H, Kuosa H, Thomas DN, Granskog MA, Kivi K (2007) Biomass, composition and activity of organism assemblages along a salinity gradient in sea ice subjected to river discharge in the Baltic Sea. Polar Biol 30:183–197
Kaartokallio H, Sogaard DH, Norman L, Rysgaard S, Tison J-, Delille B, Thomas DN (2013) Short-term variability in bacterial abundance, cell properties, and incorporation of leucine and thymidine in subarctic sea ice. Aquat Microb Ecol 71:57–73
Kadouri D, Jurkevitch E, Okon Y, Castro-Sowinski S (2005) Ecological and agricultural significance of bacterial polyhydroxyalkanoates. Crit Rev Microbiol 31:55–67
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066
Koller M, Gasser I, Schmid F, Berg G (2011) Linking ecology with economy: insights into polyhydroxyalkanoate-producing microorganisms. Eng Life Sci 11:222–237
Koressaar T, Remm M (2007) Enhancements and modifications of primer design program Primer3. Bioinformatics 23:1289–1291
Krell A, Funck D, Plettner I, John U, Dieckmann G (2007) Regulation of proline metabolism under salt stress in the psychrophilic diatom Fragilariopsis cylindrus (Bacillariophyceae). J Phycol 43:753–762
Krembs C, Deming JW (2008) The role of exopolymers in microbial adaptation to sea ice. In: Margesin R, Schinner F, Marx J et al (eds) Psychrophiles: from biodiversity to biotechnology. Springer, Heidelberg, pp 247–264
Krembs C, Eicken H, Deming JW (2011) Exopolymer alteration of physical properties of sea ice and implications for ice habitability and biogeochemistry in a warmer Arctic. Proc Natl Acad Sci USA 108:3653–3658
Lee SY (1996) Bacterial polyhydroxyalkanoates. Biotechnol Bioeng 49:1–14
Lopez NI, Julia Pettinari M, Stackebrandt E, Tribelli PM, Potter M, Steinbuechel A, Mendez BS (2009) Pseudomonas extremaustralis sp. nov., a poly(3-hydroxybutyrate) producer isolated from an Antarctic environment. Curr Microbiol 59:514–519
Madigan MT, Jung DO, Woese CR, Achenbach LA (2000) Rhodoferax antarcticus sp. nov., a moderately psychrophilic purple nonsulfur bacterium isolated from an Antarctic microbial mat. Arch Microbiol 173:269–277
Madison LL, Huisman GW (1999) Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol R 63:21–53
Margesin R, Feller G (2010) Biotechnological applications of psychrophiles. Environ Technol 31:835–844
Mattes TE, Alexander AK, Richardson PM, Munk AC, Han CS, Stothard P, Coleman NV (2008) The genome of Polaromonas sp strain JS666: Insights into the evolution of a hydrocarbon- and xenobiotic-degrading bacterium, and features of relevance to biotechnology. Appl Environ Microbiol 74:6405–6416
Methe BA, Nelson KE, Deming JW, Momen B, Melamud E, Zhang XJ, Moult J, Madupu R, Nelson WC, Dodson RJ, Brinkac LM, Daugherty SC, Durkin AS, DeBoy RT, Kolonay JF, Sullivan SA, Zhou LW, Davidsen TM, Wu M, Huston AL, Lewis M, Weaver B, Weidman JF, Khouri H, Utterback TR, Feldblyum TV, Fraser CM (2005) The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc Natl Acad Sci USA 102:10913–10918
Ostle AG, Holt JG (1982) Nile blue A as a fluorescent stain for poly-beta-hydroxybutyrate. Appl Environ Microbiol 44:238–241
Pomeroy LR, Wiebe WJ (2001) Temperature and substrates as interactive limiting factors for marine heterotrophic bacteria. Aquat Microb Ecol 23:187–204
Rehm BHA (2003) Polyester synthases: natural catalysts for plastics. Biochem J 376:15–33
Rehm BHA (2010) Bacterial polymers: biosynthesis, modifications and applications. Nat Rev Microbiol 8:578–592
Reusch RN, Sadoff HL (1988) Putative structure and functions of a poly-beta-hydroxybutyrate calcium polyphosphate channel in bacterial plasma membranes. Proc Natl Acad Sci USA 85:4176–4180
Risso C, Sun J, Zhuang K, Mahadevan R, DeBoy R, Ismail W, Shrivastava S, Huot H, Kothari S, Daugherty S, Bui O, Schilling CH, Lovley DR, Methe BA (2009) Genome-scale comparison and constraint-based metabolic reconstruction of the facultative anaerobic Fe(III)-reducer Rhodoferax ferrireducens. BMC Genom 10:447
Staden R (1996) The Staden sequence analysis package. Mol Biotechnol 5:233–241
Stamatakis A, Ott M, Ludwig T (2005) RAxML-OMP: An efficient program for phylogenetic inference on SMPs. In: 8th International Conference on Parallel Computing Technologies 3606, pp 288–302
Stedmon CA, Thomas DN, Papadimitriou S, Granskog MA, Dieckmann GS (2011) Using fluorescence to characterize dissolved organic matter in Antarctic sea ice brines. J Geophys Res Biogeo 116:G03027
Tal S, Okon Y (1985) Production of the reserve material poly-beta-hydroxybutyrate and its function in Azospirillum brasilense Cd. Can J Microbiol 31:608–613
Thomas DN, Lara RJ, Eicken H, Kattner G, Skoog A (1995) Dissolved organic matter in Arctic multiyear sea ice during winter—major components and relationship to ice characteristics. Polar Biol 15:477–483
Ting L, Williams TJ, Cowley MJ, Lauro FM, Guilhaus M, Raftery MJ, Cavicchioli R (2010) Cold adaptation in the marine bacterium, Sphingopyxis alaskensis, assessed using quantitative proteomics. Environ Microbiol 12:2658–2676
Tribelli PM, Lopez NI (2011) Poly(3-hydroxybutyrate) influences biofilm formation and motility in the novel Antarctic species Pseudomonas extremaustralis under cold conditions. Extremophiles 15:541–547
Tribelli PM, Raiger Iustman LJ, Catone MV, Di Martino C, Revale S, Mendez BS, Lopez NI (2012) Genome sequence of the polyhydroxybutyrate producer Pseudomonas extremaustralis, a highly stress-resistant Antarctic bacterium. J Bacteriol 194:2381–2382
Underwood GJC, Fietz S, Papadimitriou S, Thomas DN, Dieckmann GS (2010) Distribution and composition of dissolved extracellular polymeric substances (EPS) in Antarctic sea ice. Mar Ecol Prog Ser 404:1–19
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3—new capabilities and interfaces. Nucleic Acids Res 40:e115
Vezzi A, Campanaro S, D’Angelo M, Simonato F, Vitulo N, Lauro FM, Cestaro A, Malacrida G, Simionati B, Cannata N, Romualdi C, Bartlett DH, Valle G (2005) Life at depth: Photobacterium profundum genome sequence and expression analysis. Science 307:1459–1461
Villanueva L, Navarrete A, Urmeneta J, Geyer R, White DC, Guerrero R (2007) Monitoring diel variations of physiological status and bacterial diversity in an estuarine microbial mat: an integrated biomarker analysis. Microb Ecol 54:523–531
Wältermann M, Steinbüchel A (2005) Neutral lipid bodies in prokaryotes: recent insights into structure, formation, and relationship to eukaryotic lipid deposits. J Bacteriol 187:3607–3619
Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL (2012) Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinform 13:134
Acknowledgments
Walter and Andrée de Nottbeck foundation and Academy of Finland FidiPro-programme provided funding for fieldwork and sample analysis for HK. AK, KP and MV were funded by Academy of Finland project grant. David Thomas and the WE research group are acknowledged for collaboration and logistic support in the sample collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Atomi.
The research was funded by the Academy of Finland, and University of Helsinki and Walter and Andrée de Nottbeck foundation.
Rights and permissions
About this article
Cite this article
Pärnänen, K., Karkman, A., Virta, M. et al. Discovery of bacterial polyhydroxyalkanoate synthase (PhaC)-encoding genes from seasonal Baltic Sea ice and cold estuarine waters. Extremophiles 19, 197–206 (2015). https://doi.org/10.1007/s00792-014-0699-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-014-0699-9