Abstract
Inflammation provides a substrate for mechanisms that underlie the association of maternal diet during pregnancy with Attention Deficit-Hyperactivity Disorder (ADHD) symptoms in childhood. However, no previous study has quantified the proinflammatory potential of maternal diet as a risk factor for ADHD. Thus, we evaluated the association of maternal dietary inflammatory index (DII®) scores during pregnancy with ADHD symptoms in 4-year-old children born in two Mediterranean regions. We analyzed data from two population-based birth cohort studies—INMA (Environment and Childhood) four subcohorts in Spain (N = 2097), and RHEA study in Crete (Greece) (N = 444). The DII score of maternal diet was calculated based on validated food frequency questionnaires completed during pregnancy (12th and/or 32nd week of gestation). ADHD symptoms were assessed by ADHD-DSM-IV in INMA cohort and by ADHDT test in RHEA cohort, with questionnaires filled-out by teachers and parents, respectively. The associations between maternal DII and ADHD symptoms were analysed using multivariable-adjusted zero-inflated negative binomial regression models in each cohort study separately. Meta-analysis was conducted to combine data across the cohorts for fitting within one model. The DII was significantly higher in RHEA (RHEA = 2.09 [1.94, 2.24]) in comparison to INMA subcohorts (Asturias = − 1.52 [− 1.67, − 1.38]; Gipuzkoa = − 1.48 [− 1.64, − 1.33]; Sabadell = − 0.95 [− 1.07, − 0.83]; Valencia = − 0.76 [− 0.90, − 0.62]). Statistically significant reduced risk of inattention symptomatology (OR = 0.86; CI 95% = 0.77–0.96), hyperactivity symptomatology (OR = 0.82; CI 95% = 0.72–0.92) and total ADHD symptomatology (OR = 0.82; CI 95% = − 0.72 to 0.93) were observed with increased maternal DII in boys. No statistically significant associations were observed in girls between maternal DII and inattention, hyperactivity and total ADHD symptomatology. We found reduced risk of ADHD symptomatology with increased DII only in boys. This relationship requires further exploration in other settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last 20 years childhood neuropsychiatric disorders have increased significantly [7], with important consequences for the affected child and their families, and for the public health system. Attention deficit/hyperactivity disorder (ADHD) is the most frequent childhood-onset neuropsychiatric condition, with an estimated worldwide prevalence of approximately 5% in school-aged children [29], and males manifest more symptoms than females [22].
The etiology of ADHD probably is multifactorial, reflecting a complex interplay between genetic, lifestyle, nutritional, psychosocial and environmental factors [16, 35]. Growing evidence suggests that exposure to adverse environmental and psychological conditions, especially when exposure occurs in intrauterine life may increase the risk of developing ADHD in childhood [10]. Many pre and perinatal environmental or reproductive factors have been identified as risk factors, such as maternal smoking, alcohol and substance misuse, maternal stress or low birth weight and prematurity [38]. Exposure to environmental toxins such as lead may also increase the risk of ADHD [28], and of other adverse psychosocial outcomes [33, 37].
Maternal overweight and obesity before pregnancy are known risk factors for ADHD in the child [4, 30, 32]. More recent evidence further supports that nutrition during pregnancy is crucial for child neurodevelopment [17]. Previous studies have indicated that maternal dietary patterns and the consumption of specific food groups or nutrients during pregnancy are associated with cognitive and behavioral outcomes [5]. Many dietary components associated with child neurodevelopment have a known pro-inflammatory potential (i.e., fat products) and anti-inflammatory potential (i.e., fermented products) [9]; however, the joint contribution of diet-related inflammation with child neurodevelopmental outcomes has not been evaluated previously. This is an important evidence gap, as increased inflammation is thought to underlie, at least in part, the associations between maternal diet and overweight/obesity and ADHD [8, 14, 19, 31, 32]. Obese pregnant women have higher levels of circulating pro-inflammatory cytokines and both placental and intrauterine inflammation are related to altered fetal cytokine expression, fetal neuronal damage, and changes in neonatal brain gene expression [20].
The dietary inflammatory index (DII®) was developed to capture the inflammatory potential of diets based on known associations of specific food groups and nutrients with serum inflammatory markers [34]. The DII therefore offers a unique opportunity to assess the overall association of a maternal proinflammatory diet with child health outcomes. Some outcomes have been studied, such as cortical bone outcomes [15], anthropometric indices [6], or muscle mass and strength [2], however, child neurodevelopment outcomes have not been previously examined. Given the pro-inflammatory potential of suspected nutrients, and evidence supporting a role of inflammation in the pathogenesis of ADHD, we hypothesised that a more pro-inflammatory diet during the mother’s pregnancy will increase ADHD symptomatology in her offspring. Thus, we evaluated the association between the DII scores of mothers’ diets during pregnancy and ADHD symptoms in their children at the age of 4 years in two Mediterranean regions (Spain and Greece).
Methods
Study participants
INMA
The population-based birth cohort study INMA (INfancia y Medio Ambiente—Environment and Childhood) recruited pregnant women from four Spanish regions (Asturias, Gipuzkoa, Sabadell and Valencia) between November 2003 and February 2008 [25]. Pregnant women were enrolled during the 1st trimester of pregnancy at primary health care centers or public hospitals, depending on the region, if they fulfilled these inclusion criteria: (1) age ≥ 16 years, (2) singleton pregnancy, (3) no assisted conception, (4) intention to deliver at the reference hospital, and (5) no communication problems. Written informed consent was signed by the mothers and the study was approved by the Hospital Ethics Committees in the participating regions and hospitals. Out of a total of 2644 mothers who were recruited in the first trimester of pregnancy, 2097 children were included in the present analysis after exclusion of neuropsychological tests of uncertain quality, due to less than optimal cooperation or poor quality test and basic pathologies.
RHEA
The RHEA Study in Crete (Greece) (Chatzi et al. 2017) enrolled pregnant women during the 1st trimester of pregnancy at hospitals or private clinics if they were: (1) resident in the study areas, (2) ≥ 16 years of age, and (3) without communication handicap. The study was approved by the ethical committee of the University Hospital in Heraklion, Crete (Greece) and all participants provided written, informed consent. A total of 1606 mothers were originally enrolled and a subgroup of 612 children performed the neuropsychological development test at age 4 years. After excluding data from women who did not have exposure information, had a specific pathology, or a low-quality test, a total of 444 children were included in the analysis.
Dietary inflammatory index
DII scores were calculated based on dietary data [34] collected in the first and third trimesters of pregnancy (12th and 32nd week of gestation) in the INMA cohorts and in the 2nd trimester of pregnancy (14th–18th week of gestation) in the RHEA cohort using a validated food frequency questionnaire, [11, 21, 40]. Thirty-two nutrients were accounted for the DII calculation in the RHEA and INMA cohort including both pro-inflammatory factors (such as alcohol, carbohydrate and saturated fats) and anti-inflammatory factors (such as certain vitamins, fiber and green tea). We excluded oregano intake for the DII calculation in the INMA cohort, and caffeine intake for the DII calculation in the RHEA cohort because this information had not been collected in the respective FFQs (Food Frequency Questionaires).
The method of calculation is described in detail elsewhere [34]. Briefly, the scoring algorithm was based on extensive review of the literature published from 1950 to 2010 focused on the effect of diet on six inflammatory biomarkers (IL-1β, IL-4, IL-6, IL-10, TNF-α, and CRP). A total of 45 food parameters, including macronutrients and micronutrients, were scored according to whether they increased (+ 1), decreased (− 1), or had no effect (0) on these inflammatory biomarkers. These inflammatory effect scores were weighted based on study design. To avoid the arbitrariness resulting from simply using raw consumption amounts, intakes of foods and nutrition were standardized to a representative range of dietary intake based on actual human consumption in 11 populations living in different countries across the world that provided an estimate of a mean and standard deviation for each parameter. These values were converted to a proportion (with values from 0 to 1). Each proportion was doubled, and then 1 was subtracted to achieve a symmetrical distribution around a mean of ≈ 0. For each individual food parameter, this score was then multiplied by the respective food parameter effect score, derived from the literature review, to obtain a food parameter-specific DII score. All of the food parameter-specific DII scores were then summed to create the overall DII score for every participant in the study, DII = b1 × n1 + b2 × n2……….. b32 × n32, where b refers to the literature-derived inflammatory effects score for each of the evaluable food parameters and n refers to the food parameter-specific centered percentiles, which were derived from the dietary data from the mother. For comparative purposes between the two cohort studies, we used the average mean of the first and third pregnancy trimester DIIs in INMA as a better proxy of maternal diet throughout pregnancy. Energy adjusted-DII (E-DII) was calculated by converting all nutrients from the studies and the global database to per 1000 kcal and then calculating the DII.
ADHD symptoms
ADHD symptoms were assessed by ADHD-DSM-IV (18 items) in INMA cohort and by Attention-Deficit/Hyperactivity Disorder Test (ADHDT) (36 items) in RHEA cohort at around 4.5 years of age, with questionnaires filled-out by teachers and mothers, respectively. The ADHD-DSM-IV symptom data were gathered using the Diagnostic Criteria for ADHD of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (ADHD-DSM-IV) [3]. We used the DSM-IV symptom checklist for ADHD as a rating scale without further modifications, found to be psychometrically useful in a prior validation study comparing reports of parents and teachers [1]. It is an 18-item checklist designed to identify inattention (1–9), and hyperactivity–impulsivity (10–18) symptoms in children. Each ADHD symptom is rated on a four-point Likert scale (0 = never/rarely, 1 = sometimes, 2 = often, or 3 = very often). ADHDT [23] is a 36-item questionnaire designed to identify and evaluate ADHD symptoms in ages 3–23 years according to the DSM-IV criteria for ADHD. It contains three subscales: hyperactivity (13 items), impulsivity (10 items) and inattention (13 items). All 36 items are summed to generate an index for total ADHD difficulties. The ADHDT has been adapted in the Greek population [27]. In this case each item is rated on a three-point Likert scale (0 = not a problem, 1 = mild problem, or 2 = severe problem).
For all tests, higher scores indicate more ADHD symptoms. The continuous measure of ADHD symptoms was calculated as the sum of the score of each item. This measure ranges from 0 to 54 in the case of ADHD-DSM-IV (INMA cohort) and from 0 to 72 in ADHDT (RHEA cohort) test. The categorical measure of ADHD symptoms was calculated recoding the list of symptoms as 0 (symptom absent when the response is 0 or 1) or 1 (symptom present when the response is 2 or 3) [24]. Children rated with six or more symptoms of each dimension were classified as inattentive, hyperactive or inattentive and hyperactive taking into account the criteria of Diagnostic Criteria for ADHD of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (ADHD-DSM-IV) [3].
Covariates
Information related to sociodemographic and anthropometric characteristics, diet, and lifestyle was collected through questionnaires administered during pregnancy and later, when children were around 14 months and 4-years-old in INMA and 9–12 months and 4-years-old in RHEA. All questionnaires and measurements were conducted face-to-face by trained interviewers. Child’s sex (male or female), weeks at birth (preterm or not), cesarean (yes/no), gestational age and pre-pregnancy body mass index (BMI) calculated in kg/m2 and classified as < 25 (normal), 25–29.9 (overweight) and ≥ 30 (obesity). Child BMI z-scores specific for age and sex were calculated using the WHO growth standards, and a BMI z-scores above the 85th percentile, were afterwards classified as overweight (including obesity) [18]. Information about passive smoking at home at 4 years (yes/no), duration of breastfeeding and working status at 4 years (yes/no), were collected in a subsequent interview when infants were around 14 months and 4-years-old in INMA and 9–12 months and 4.5-years-old in RHEA. Information on maternal age at delivery and education (low/medium/high), maternal working status (yes/no) during pregnancy and at child age 4 years, maternal origin (from country cohort/other) and residence (rural/urban), maternal smoking during pregnancy (yes/no), parity, BMI, pre-pregnancy diabetes (yes/no), gestational diabetes and hypertension (yes/no) was obtained through questionnaires administered during pregnancy or 4 years and from medical records.
Statistical analysis
As preliminary analyses, we compared socio-demographic characteristics of the mother and child across the cohorts of INMA (Asturias, Gipuzkoa, Sabadell and Valencia) and RHEA. The difference in the mean of inattention, hyperactivity, impulsivity and ADHD symptoms according the mother and child characteristics were analyzed using the Student t test and one-way ANOVA.
Due to the excess of zeros values in symptoms, the association between maternal DII and the symptoms was analysed using multivariable-adjusted zero-inflated negative binomial regression models [39]. The zero-inflated negative binomial regression generates two separate models. First, a logit model is generated for the “zero” cases, predicting whether an individual would be in this group. Then, a negative binomial model is generated to predict the counts for those individuals who are not certain zeros. Separate models were created for each cohort (INMA subcohorts and RHEA) due to the measurement differences in the test administered for the evaluation of ADHD symptomatology. We adjusted the models for those variables with p value ≤ 0.20 in the bivariate analysis. These variables were maintained in the final fully adjusted models if they changed the magnitude of the DII-ADHD effect estimate by more than 10%. The final fully adjusted models in both INMA and RHEA cohorts were adjusted by child’s age at the moment of the test, and mother’s variables: pre-pregnancy BMI, age at delivery, working status, educational level, parity and smoking during pregnancy. The interaction between sex and DII was significant for some models so results are presented separately for boys and girls. The estimated effects were meta-analyzed to obtain an overall estimation for the association between de symptoms and DII across all the study areas. Results of meta-analyses are reported for the zero models in Figs. 1 and 2 for boys and girls, respectively. We used Cochran’s Q-test to test heterogeneity among cohorts. If the Q-test was not significant (p > 0.05), fixed-effects analysis was used. Statistical analyses were conducted using the Statistical Package R version 3.5.1 and the meta-analysis was performed in Stata with the package ‘metan’.
Results
The sample consisted of data from 2541 children and their mothers, encompassing 2,097 mother child pairs from the INMA cohort (Asturias = 531; Gipuzkoa = 377; Sabadell = 631; Valencia = 558) and 444 from the RHEA cohort. Descriptive DII data in each cohort and the comparison between them are presented in Supplementary Fig. 1. The DII was significantly higher in RHEA (RHEA = 2.09 [1.94, 2.24]) in comparison to INMA subcohorts (Asturias = − 1.52 [− 1.67, − 1.38]; Gipuzkoa = − 1.48 [− 1.64, − 1.33]; Sabadell = − 0.95 [− 1.07, − 0.83]; Valencia = − 0.76 [− 0.90, − 0.62]).
Descriptive statistics and comparative analysis of child and maternal characteristics in each cohort are presented in Table 1. In the bivariate analysis, scores for several behaviors were significantly related to child and mother characteristics; boys presented with higher symptomatology of inattention, hyperactivity and impulsivity in both the INMA and RHEA cohorts. In both cohorts children with normal BMI (based on WHO criteria) presented with fewer symptoms in total ADHD compared to overweight and obese children, and children exposed to passive smoking at home had more total ADHD symptoms. Children with more than 3.43 months breastfeeding duration presented with fewer symptoms of hyperactivity, impulsivity and total symptomatology in the RHEA cohort. High maternal education and working status during pregnancy (yes) were associated with fewer symptoms in both cohorts.
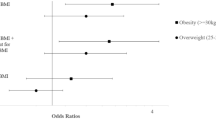
Figure 1 shows the results of the meta-analysis of multivariable-adjusted zero-inflated negative binomial regression models of DII and Inattention, Hyperactivity and total ADHD in boys. After adjusting models by children´s age at the time of the test, region, pre-pregnancy BMI, maternal age at delivery, working status during pregnancy, educational level, parity and smoking during pregnancy, statistically significant inverse associations were observed between maternal DII and inattention symptomatology (OR = 0.86; 95% CI = 0.77–0.96), maternal DII and hyperactivity symptomatology (OR = 0.82; 95% CI = 0.72–0.92) and maternal DII and total ADHD symptomatology (OR = 0.82; 95% CI = 0.72–0.93) in boys.
Figure 2 shows the results of the meta-analysis of multivariable-adjusted zero-inflated negative binomial regression models of DII and inattention, hyperactivity and total ADHD in girls. No statistically significant associations were observed between maternal DII and inattention symptomatology (OR CI 95% 1.04 (0.88–1.19)), maternal DII and hyperactivity symptomatology (OR CI 95% 0.89 (0.76–1.02)) and maternal DII and total ADHD symptomatology (OR CI 95% 0.89 (0.76–1.02)).
Discussion
The present study assessed the association between maternal DII and ADHD symptomatology in 2541 children and their mothers from the Spanish INMA cohort and the Greek RHEA cohort. Significant differences were seen between the Spanish and Greek cohorts suggesting a more pro-inflammatory diet of mothers in Greece comparing to mothers in Spain. A higher proportion of mothers with high adherence to the Mediterranean diet was observed in RHEA cohort in a previous study [12], indicating differences in the diet of Greek and Spanish pregnant women.
We found statistically significant inverse associations between maternal DII and inattention, hyperactivity and total ADHD symptomatology only in boys. These results suggest that a higher DII during pregnancy could be associated with less ADHD symptomatology, which is inconsistent with our primary hypothesis and requires further exploration in other settings.
Taking into consideration evidence that supports the pro-inflammatory potential of diet and its effect on the pathogenesis of ADHD, we expected that higher levels of maternal DII would have a positive relation with ADHD symptomatology in children. Recent studies in some other children outcomes, have observed positive associations between DII and anthropometric indices such as children BMI, waist circumference, hip circumference and parental BMI [6], inverse association between DII and skeletal muscle mass in boys [2], or increased risk for wheeze in adults and children with allergic (atopic) wheeze [26]. A potential limitation of the current state of literature is that most previous findings have been reported from cross-sectional studies of adults and it is yet unclear to what extent maternal DII over the pregnancy period may be associated with health outcomes in the child at later ages. In our study, we examined for the first time, associations with child ADHD symptomatology and we observed associations suggesting that a more pro-inflammatory diet may be linked to lower risk for ADHD symptoms in boys. It is yet unclear whether this association may be indeed causal. However, one possible explanation for our findings may be that within an overall Mediterranean diet pattern, that characterizes our two study regions and it is generally considered as a healthy diet, the pro-inflammatory potential captured by dietary inflammatory indexes may not necessarily be associated with detrimental health effects. It may be also possible that those mothers’ children with higher DII score also had a lower diet quality (e.g., low Mediterranean Dietary Score, MDS) which could be related to ADHD symptoms in boys. It may be also possible that boys followed a higher quality diet from birth to the age of 4–5 years. Unfortunately, we were not able to explore the potential confounding effects of the adherence to Mediterranean diet of mothers and children, which should be considered as a study limitation.
Due to the genetic component of ADHD, information about parents´ ADHD symptomatology would be interesting to include in the models. Unfortunately, this information is not available it is considered as a limitation of the study.
Given the smaller sample size of the RHEA cohort, it is not clear whether the absence of significant associations were due to limited power, the generally higher DII range observed in the RHEA cohort or other methodological limitations (e.g., differences in ADHD assessment tests or FFQs).
The main strengths of this study include the use of the previously validated DII that permits to evaluating the association of the overall anti- or pro-inflammatory diet potential on health outcomes. Also, data were derived from the large sample size and analysis of two large Mediterranean-based cohorts. Another strength is the use of standardized and validated questionnaires for assessment of ADHD symptoms in children in both INMA and RHEA cohorts. However, in spite of the fact that both cohorts used standardized, validated and instruments based on DSM IV [3] (American Psychiatric Association 1994), there are differences in the distribution of the items in each dimension of the test and this has been a limitation in our study. In INMA cohort ADHD symptoms were assessed by ADHD-DSM-IV (18 items) and in RHEA cohort by Attention-Deficit/Hyperactivity Disorder Test (ADHDT) (36 items). In ADHD-DSM-IV there are nine items for inattention, and another nine items for hyperactivity–impulsivity, and for Attention-Deficit/Hyperactivity Disorder Test (ADHDT), there are 13 items for Hyperactivity, 10 for Impulsivity and 13 items for inattention. This is the reason why we could not perform pooled-analysed analysis of the INMA and RHEA cohorts, thus precluding that may have permitted to provide even more precise effect estimates, especially in regard to interactions.
In conclusion, we found sex-specific inverse association between maternal DII and ADHD symptomatology, with associations seen only in boys, and going to the opposite direction to what was expected. More specifically, we found that higher maternal DII during pregnancy, suggesting an overall more pro-inflammatory diet, may have a protective effect on the behavioral symptoms in boys. However, at the moment we are not able to provide a plausible mechanism through which maternal DII could potentially protect against ADHD, and taking into account that the odds values in different cohorts are close to 1, we consider that it can be a chance finding. Further research is needed to replicate the findings from this study and clarify the potential impact of anti- and pro-inflammatory dietary patterns on child health.
References
Amador-Campos JA, Forns-Santacana M, Guàrdia-Olmos J, Peró-Cebollero M (2006) DSM-IV attention deficit hyperactivity disorder symptoms: agreement between informants in prevalence and factor structure at different ages. J Psychopathol Behav Assess 28:23–32
Amakye WK, Zhang Z, Wei Y, Shivappa N, Hebert JR, Wang J, Su Y, Mao L (2018) The relationship between dietary inflammatory index (DII) and muscle mass and strength in Chinese children aged 6–9 years. Asia Pac J Clin Nutr 27:1315–1324. https://doi.org/10.6133/apjcn.201811_27(6).0019
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Association, Washington, DC
Andersen CH, Thomsen PH, Nohr EA, Lemcke S (2018) Maternal body mass index before pregnancy as a risk factor for ADHD and autism in children. Eur Child Adolesc Psychiatry 27:139–148. https://doi.org/10.1007/s00787-017-1027-6
Anjos T, Altmäe S, Emmett P, Tiemeier H, Closa-Monasterolo R, Luque V, Wiseman S, Pérez-García M, Lattka E, Demmelmair H, Egan B, Straub N, Szajewska H, Evans J, Horton C, Paus T, Isaacs E, van Klinken JW, Koletzko B, Campoy C (2013) Nutrition and neurodevelopment in children: focus on NUTRIMENTHE project. Eur J Nutr 52:1825–1842. https://doi.org/10.1007/s00394-013-0560-4
Aslani Z, Qorbani M, Hébert JR, Shivappa N, Motlagh ME, Asayesh H, Mahdavi-Gorabi A, Kelishadi R (2019) Association of dietary inflammatory index with anthropometric indices in children and adolescents: the weight disorder survey of the childhood and adolescence surveillance and prevention of adult non- communicable disease (CASPIAN)-IV study. Br J Nutr 121:340–350. https://doi.org/10.1017/S0007114518003240
Atladottir HO, Gyllenberg D, Langridge A, Sandin S, Hansen SN, Leonard H, Gissler M, Reichenberg A, Schendel DE, Bourke J, Hultman CM, Grice DE, Buxbaum JD, Parner ET (2015) The increasing prevalence of reported diagnoses of childhood psychiatric disorders: a descriptive multinational comparison. Eur Child Adolesc Psychiatry 24:173–183. https://doi.org/10.1007/s00787-014-0553-8
Bilbo SD, Tsang V (2010) Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J 24:2104–2115
Bordoni A, Danesi F, Dardevet D, Dupont D, Fernandez AS, Gille D, Nunes dos Santos C, Pinto P, Re R, Rémond D, Shahar DR, Vergères G (2017) Dairy products and inflammation: a review of the clinical evidence. Crit Rev Food Sci Nutr 57(12):2497–2525. https://doi.org/10.1080/10408398.2014.967385
Buss C, Entringer S, Poggi Davis E, Hobel CJ, Swanson JM, Wadhwa PD, Sandman CA (2012) Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms. PLoS ONE 7:e37758. https://doi.org/10.1371/journal.pone.0037758
Chatzi L, Melaki V, Sarri K et al (2011) Dietary patterns during pregnancy and the risk of postpartum depression: the mother–child ‘Rhea’ cohort in Crete, Greece. Public Health Nutr 14:1663–1670. https://doi.org/10.1017/S1368980010003629
Chatzi L, Garcia R, Roumeliotaki T, Basterrechea M, Begiristain H, Iñiguez C, Vioque J, Kogevinas M, Sunyer J (2013) Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: INMA (Spain) and RHEA (Greece) mother–child cohort studies. Br J Nutr 110:2058–2068. https://doi.org/10.1017/S0007114513001426
Chatzi L, Leventakou V, Vafeiadi M, Koutra K, Roumeliotaki T, Chalkiadaki G, Karachaliou M, Daraki V, Kyriklaki A, Kampouri M, Fthenou E, Sarri K, Vassilaki M, Fasoulaki M, Bitsios P, Koutis A, Stephanou EG, Kogevinas M (2017) Cohort Profile: The Mother-Child Cohort in Crete, Greece (Rhea Study). Int J Epidemiol 46:1392–1393. https://doi.org/10.1093/ije/dyx084
Chen Q, Sjolander A, Langstrom N, Rodriguez A, Serlachius E, D’Onofrio BM et al (2014) Maternal pre-pregnancy body mass index and offspring attention deficit hyperactivity disorder: a population-based cohort study usinga sibling-comparison design. Int J Epidemiol 43:83–90. https://doi.org/10.1093/ije/dyt152
Coheley LM, Shivappa N, Hebert JR, Lewis RD (2019) Dietary inflammatory index® and cortical bone outcomes in healthy adolescent children. Osteoporos Int. https://doi.org/10.1007/s00198-019-04946-3
Cortese S, Vincenzi B (2011) Obesity and ADHD: Clinical and Neurobiological Implications. In: Stanford C, Tannock R (Eds) Behavioral Neuroscience of Attention Deficit Hyperactivity Disorder and Its Treatment. Current Topics in Behavioral Neurosciences, vol 9. Springer, Berlin, Heidelberg.
DeCapo M, Thompson JR, Dunn G, Sullivan EL (2019) Perinatal nutrition and programmed risk for neuropsychiatric disorders: a focus on animal models. Biol Psychiat 85:122–134. https://doi.org/10.1016/j.biopsych.2018.08.006
De Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J (2007) Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85:660–667
Edlow AG (2017) Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat Diagn 37:95–110. https://doi.org/10.1002/pd.4932
Elovitz MA, Brown AG, Breen K et al (2011) Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci 29:663–671
Fernández-Barrés S, Romaguera D, Valvi D, Martínez D, Vioque J, Navarrete- Muñoz EM, Amiano P, Gonzalez-Palacios S, Guxens M, Pereda E, Riaño I, Tardón A, Iñiguez C, Arija V, Sunyer J, Vrijheid M (2016) Mediterraneandietary pattern in pregnant women and offspring risk of overweight and abdominal obesity in early childhood: the INMA birth cohort study. Pediatr Obesity 11(6):491–499. https://doi.org/10.1111/ijpo.12092
Gershon J, Gershon J (2002) A meta-analytic review of gender differences in ADHD. J Atten Disord 5(3):143–154. https://doi.org/10.1177/108705470200500302
Gilliam JE (1995) Attention-deficit hyperactivity disorder test. Pro-Ed, Austin
Gomez R (2007) Testing gender differential item functioning for ordinal and binary scored parent rated ADHD symptoms. Personal Individ Differ 42:733–742. https://doi.org/10.1016/j.paid.2006.08.011
Guxens M, Ballester F, Espada M et al (2012) Cohort profile: the INMA–INfancia y Medio Ambiente-(Environment and Childhood) project. Int J Epidemiol 41:930–940. https://doi.org/10.1093/ije/dyr054
Han YY, Forno E, Shivappa N, Wirth MD, Hébert JR, Celedón JC (2018) The dietary inflammatory index and current wheeze among children and adults in the United States. J Allergy Clin Immunol Pract 6:834–841. https://doi.org/10.1016/j.jaip.2017.12.029
Maniadaki K, Kakouros E (2002) Translation and adaptation of the Attention Deficit HyperactivityDisorderTest (ADHDT; Giliam, 1995). In: Stalikas A, Triliva S, Roussi P (eds) The psychometric Instruments in Greece. Ellinika Grammata, Athens, pp 102–103
Park JH, Seo JH, Hong YS, Kim YM, Kang JW, Yoo JH, Chueh HW, Lee JH, Kwak MJ, Kim J, Woo HD, Kim DW, Bang YR, Choe BM (2016) Blood lead concentrations and attention deficit hyperactivity disorder in Korean children: a hospital-based case control study. BMC Pediatr. https://doi.org/10.1186/s12887-016-0696-5
Polanczyk G, Rohde LA (2007) Epidemiology of attention-deficit/hyperactivity disorder across the lifespan. Curr Opin Psychiatry 20:386–392. https://doi.org/10.1097/YCO.0b013e3281568d7a
Pugh SJ, Hutcheon JA, Richardson GA, Brooks MM, Himes KP, Day NL, Bodnar LM (2016) Gestational weight gain, prepregnancy body mass index and offspring attention-deficit hyperactivity disorder symptoms and behaviour at age 10. BJOG 123:2094–2103. https://doi.org/10.1111/1471-0528.13909
Rodriguez A, Miettunen J, Henriksen TB, Olsen J, Obel C, Taanila A, Ebeling H, Linnet KM, Moilanen I, Jarvelin MR (2008) Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: evidence from three prospective pregnancy cohorts. Int J Obes 32:550–557. https://doi.org/10.1038/sj.ijo.0803741
Rodriguez A (2010) Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J Child Psychol Psychiatry 51:134–143. https://doi.org/10.1111/j.1469-7610.2009.02133.x
Rutter M, Beckett C, Castle J, Colvert E, Kreppner J, Mehta M, Stevens S, Sonuga-Barke E (2007) Effects of profound early institutional deprivation: an overview of findings from a UK longitudinal study of Romanian adoptees. Eur J Dev Psychol 4:332–350
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR (2014) Designing and developing a literature-derived population-based dietary inflammatory index. Public Health Nutr 17(6):1689–1696
Spencer TJ, Biederman J, Mick E (2007) Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol 32:631–642. https://doi.org/10.1093/jpepsy/jsm005
Steck S, Shivappa N, Tabung F, Harmon EB, Wirth M, Hurley T, Hebert J (2014) The dietary inflammatory index: a new tool for assessing diet quality based on inflammatory potential. Digest 49:1–9
Thapar A, Cooper M, Eyre O, Langley K (2013) Practitioner review: What have we learnt about the causes of ADHD? J Child Psychol Psychiatry 54:3–16. https://doi.org/10.1111/j.1469-7610.2012.02611.x
Thapar A, Cooper M, Jefferies R, Stergiakouli E (2012) What causes attention deficit hyperactivity disorder? Arch Dis Child 97:260–265. https://doi.org/10.1136/archdischild-2011-300482
Vilor-Tejedor N, Alemany S, Forns J, Cáceres A, Murcia M, Maciá D, Pujol J, Sunyer J, González JR (2016) Assessment of susceptibility risk factors for ADHD in imaging genetic studies. J Atten Disord. https://doi.org/10.1177/1087054716664408
Vioque J, Navarrete-Muñoz EM, Gimenez-Monzó D, García-de-la-Hera M, Granado F, Young IS, Ramón R, Ballester F, Murcia M, RebagliatoIñiguez MC (2013) Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr J 12:26. https://doi.org/10.1186/1475-2891-12-26
Acknowledgements
The authors would particularly like to thank all the cohort participants for their generous collaboration. A full roster of the INMA Project Investigators can be found at http://www.proyectoinma.org/presentacioninma/listadoinvestigadores/en_listadonvestigadores.html[V1] and of the Rhea project Investigators at http://www.rhea.gr/en/about-rhea/the-rhea-team/.
Funding
This study was funded by grants from Spanish Ministry of Health-Instituto de Salud Carlos III (Red INMA G03/176, CB06/02/0041, FIS-PI041436, FIS- PI081151, FIS- PI042018, FISPI09/02311, FIS-PI06/0867, FIS-PS09/00090, FIS-FEDER PI11/1007 FIS-FEDER 03/1615, 04/1509, 04/1112, 04/1931, 05/1079, 05/1052, 06/1213, 07/0314, and 09/02647), Generalitat de Catalunya- CIRIT 1999SGR 00241, Conselleria de Sanitat Generalitat Valenciana, FISS-FEDER PI13/02429, FISS-FEDER PI18/00909, Universidad de Oviedo, Obra Social Cajastur-LIBERBANK, Department of Health of the Basque Government (2005111093 and 2009111069), the Provincial Government of Gipuzkoa (DFG06/004 and DFG08/001), Fundación Roger Torné, and Alicia Koplowitz Foundation 2017. Drs. Valvi and Chatzi are currently receiving funding from the National Institute of Environmental Health Sciences (R21 ES029328, R21 ES028903).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina to develop computer and smart phone applications for patient counselling and dietary intervention in clinical settings. Dr. Nitin Shivappa is an employee of CHI. All other authors declare that they have no conflict of interest.
Ethical approval
The INMA Project was approved by the Hospital Ethics Committees in the participating regions and hospitals. The RHEA study was approved by the ethical committee of the University Hospital in Heraklion, Crete (Greece).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lertxundi, N., Molinuevo, A., Valvi, D. et al. Dietary inflammatory index of mothers during pregnancy and Attention Deficit-Hyperactivity Disorder symptoms in the child at preschool age: a prospective investigation in the INMA and RHEA cohorts. Eur Child Adolesc Psychiatry 31, 615–624 (2022). https://doi.org/10.1007/s00787-020-01705-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-020-01705-2