Abstract
Animal studies suggest that prenatal vitamin D status may affect fetal brain growth. However, human studies are scarce with conflicting results. We aimed to investigate the association of maternal 25-hydroxyvitamin D [25(OH) D] levels with multiple neurodevelopmental outcomes at 4 years of age. We included 487 mother–child pairs from the prospective pregnancy cohort, “Rhea” in Crete, Greece. Maternal serum 25(OH) D concentrations were measured at the first prenatal visit (13 ± 2.4 weeks). Cognitive functions at 4 years were assessed by means of the McCarthy Scales of Children’s Abilities. Behavioral difficulties were assessed by means of Strengths and Difficulties Questionnaire and Attention Deficit Hyperactivity Disorder Test. Children of women in the high 25(OH) D tertile (>50.7 nmol/l) had 37% decreased number of hyperactivity–impulsivity symptoms (IRR 0.63, 95% CI 0.39, 0.99, p trend = 0.05) and 40% decreased number of total ADHD-like symptoms (IRR 0.60, 95% CI 0.37, 0.95, p trend = 0.03) at 4 years of age, compared to children of women in the low 25(OH) D tertile (<38.4 nmol/l), after adjustment for several confounders. Similar associations were found with the hyperactivity/inattention score of the SDQ questionnaire. Children of mothers with high 25(OH) D levels had also fewer total behavioral difficulties (beta-coeff: −1.25, 95% CI −2.32, −0.19) and externalizing symptoms (beta-coeff: −0.87, 95% CI −1.58, −0.15) at preschool age. The observed associations were stronger in girls than in boys (p for interaction < 0.1). No association was observed between maternal 25(OH) D concentrations and cognitive function in preschoolers. Our results suggest that high maternal vitamin D levels in early pregnancy may protect against behavioral difficulties, especially ADHD-like symptoms at preschool age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early pregnancy is a critical developmental time window for offspring growth and neurodevelopment [1]. Vitamin D has traditionally been viewed as a hormone essential for skeletal growth and calcium metabolism [2], but it has also multiple extraskeletal actions. As vitamin D amounts of the developing fetus are dependent on maternal stores, maternal vitamin D deficiency is of great concern for its consequences in the offspring. Recent data have shown a high prevalence of pregnant populations with vitamin D deficiency [3], even in countries with abundant sunshine [4]. In Europe, the prevalence of maternal vitamin D deficiency during pregnancy is similar or even higher in southern European countries, compared to central or northern countries, a phenomenon known as the vitamin D paradox in the Mediterranean region [5].
Low vitamin D concentrations during pregnancy have been related with fetal growth restriction [6], rickets [7], hypocalcaemia [8], respiratory tract infections [9], and allergic diseases [10]. Animal studies suggest that maternal vitamin D deficiency may impair fetus brain development [11]. Few epidemiological studies have examined so far the association between vitamin D status during pregnancy with offspring cognition [12,13,14,15,16,17,18,19] or behavioral difficulties [13,14,15,16, 20,21,22] with inconclusive results. Studies in the first half of pregnancy support an association of high maternal 25-hydroxyvitamin D [25(OH) D] levels, with improved mental and psychomotor development in infants [12], better receptive language development at 2 years of age [17], less language difficulties at 5 and 10 years of age [13], and a lower risk of developing ADHD-like symptoms in preschoolers [21]. Birth cohorts examining the impact of maternal 25(OH) D status in late pregnancy or cord blood levels on offspring neurodevelopment found very little [15, 18], or no association with offspring IQ [14] and no association with behavioral difficulties [14, 15] or ADHD diagnosis in mid childhood and adolescence [16, 22]. Differences in the sample size, timing of blood collection, and variation in outcome measures may partly explain the heterogeneity between studies. Additionally, most of them examined small sample sizes and had weak statistical power to detect a small to medium effect size.
We aimed to add to the above research more detailed data and investigate the associations of maternal 25(OH) D levels in early pregnancy with multiple neurodevelopmental outcomes, including neurocognitive function and behavioral difficulties at 4 years of age, in a prospective pregnancy cohort in Crete, Greece, after controlling for a wide range of confounders and effect modifiers.
Methods
Study design and population
The present study is part of the “Rhea” study, a prospective pregnancy cohort, at the prefecture of Heraklion, Crete, Greece. Detailed characteristics of the study population have been described elsewhere [23]. In brief, female residents who had become pregnant during the 12-month period starting in February 2007 participated in the study. Maternal inclusion criteria were the following: residents in the study area; pregnant women aged >16 years; no communication handicap. The study was approved by the Ethical Committee of the University Hospital of Heraklion (Crete, Greece) and all participants provided written informed consent after complete description of the study.
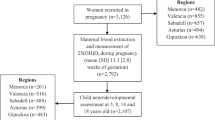
In total, 879 singletons participated at the 4-year follow-up, from October 2011 to January 2013, during which neurodevelopmental assessment was performed in 875 children (99.5%). We excluded 26 children with diagnosed neurodevelopmental disorders (i.e., pervasive developmental disorder), other severe medical disorders (i.e. plagiocephalus, microcephaly, hydrocephalus, brain tumor) and/or incomplete examination. Thus, 849 mother–child pairs were available for our analysis. From those, sufficient maternal serum from early pregnancy for 25(OH) D measurement was available for 497 mothers. We further excluded ten mother–child pairs with missing data for possible confounders. Thus, a cohort of 487 mother–child pairs (98% of the children with maternal 25(OH) D data and neurodevelopmental assessment) was available for the present analysis (Fig. 1). We observed no difference between the children included in the analysis and those that were excluded, except breastfeeding duration which was shorter in participants (Table S1).
Measures
Maternal 25(OH) D concentrations in early pregnancy
Maternal non-fasting serum samples during early pregnancy (13 ± 2.4 weeks) were collected in serum gel separator (BD 367958) tubes, centrifuged and stored at −80 °C until assayed. We used chemiluminescent immunoassay (CLIA) test (DiaSorin, Cat. No. 310600) to measure the total amount of 25(OH) D (both serum 25(OH) D2 and 25(OH) D3) [24]. The analytical range for the 25(OH) D assay was 10–375 nmol/L. Inter- and intra-assay precision were <10 and <5%, respectively. We found a weak, but significant correlation between maternal vitamin D serum concentration and dietary intake as measured from a Food Frequency Questionnaire, which included 250 food items and was completed at the end of the first trimester of pregnancy (Spearman’s ρ = 0.12; p = 0.006). FFQ did not take into account vitamin D taken from supplements. We asked about intake of vitamin D supplements in a separate questionnaire; however, none of the study participants were using such supplements. Maternal vitamin D concentration was treated as categorical divided into tertiles: tertile 1: <38.4 nmol/l; tertile 2: 38.4–50.7 nmol/l; tertile 3: >50.7 nmol/l.
Behavioral difficulties at 4 years of age
Information on children’s behavior at 4 years of age was obtained via maternal report on two standardized child behavior scales. The parent version of the Strengths and Difficulties Questionnaire (SDQ) [25] is a 25-item behavioral screening instrument designed for children aged 3–16 years. It consists of five subscales generating scores for emotional symptoms, conduct problems, hyperactivity/inattention, peer relations problems, and prosocial behavior; all but the last one are summed to generate a total difficulties score, with a high score being less favorable (range 0–40). Two additional scores were calculated: the internalizing problems score by adding up the emotional and peer relationships subscales (range 0–20) and the externalizing problems score by adding up the conduct and hyperactivity subscales (range 0–20). The prosocial behavior scale provides information on protective factors of the child; a low score is less favorable. The SDQ has been translated and adapted for the Greek population [26]. Internal consistency (Cronbach’s alpha) varied between 0.38 and 0.70.
The Attention Deficit Hyperactivity Disorder Test (ADHDT) [27] is designed to identify and evaluate ADHD symptoms in ages 3–23 years. It is composed of 36 items in three subscales; (1) hyperactivity, (2) inattention, and (3) impulsivity. All items were rated on a three-point scale (0 = never or rarely, 1 = mild, or 2 = severe).The ADHDT has been translated and adapted for the Greek population [28]. We used the ADHD Criteria of Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) to categorize ADHD-like symptoms. Three quantitative traits were generated for use in our analyses: (1) a count of the number of hyperactive–impulsive symptoms, (2) a count of the number of inattentive symptoms, and (3) a count of the total number of ADHD-like symptoms. In all three cases, a binary measure indicating the presence (severe) or absence (never/rarely or mild) of each symptom was measured and the totals were generated by summing over all symptoms, making the maximum number of symptoms 9, 9, and 18, respectively.
Neurodevelopmental assessment at 4 years of age
Children’s cognitive and motor development was assessed by means of the McCarthy Scales of Children’s Abilities (MSCA) at 4 years of age [29]. The MSCA is developed for children of ages 2½–8½ years, and includes five conventional subscales (verbal, perceptual performance, quantitative, memory, and motor). A general cognitive score was calculated by combining the verbal, perceptual performance, and quantitative scores [29]. Raw scores were standardized for child’s age at test administration using a method for the estimation of age-specific reference intervals based on fractional polynomials. Standardized residuals were then typified having a mean of 100 points with a 15 SD to homogenize the scales. Higher scores represent better general cognition, language, or psychomotor development. The inter-rater reliability was very high for all scales (intra-class correlation coefficient ≥0.973). MSCA translation and cross-cultural adaptation were conducted according to the internationally recommended methodology. Internal consistency (Cronbach’s alpha) varied between 0.76 and 0.89. Confirmatory factor analysis supported good fit of the model (χ2/df = 2, CFI = 0.83, GFI = 0.97, RMSEA = 0.034) [30].
Procedure
Women were invited to provide blood and urine samples and to participate in a face-to-face interview at the first prenatal visit (mean (SD): 12.4 (1.6) weeks). Maternal intelligence quotient (IQ) was measured using the Raven’s Standard Progressive Matrices test at the 4-year follow-up [31]. Children’s cognitive and motor function at 4 years of age was evaluated by two trained psychologists through the McCarthy Scales of Children Abilities. The administration time ranged from 40 to 60 min. The examiners, also, noted critical comments about the difficulties or special conditions of the neurodevelopmental assessment, so as to evaluate the “quality of assessment” such as: no difficulties, difficulties due to physical problems (e.g., physical illness, tiredness, asleep), difficulties due to behavior problems (e.g., nervousness, shyness). Inter-observer variability was tested in a subsample of 12 children and was <1%. Additional information on children’s behavior and ADHD-like symptoms was obtained via maternal report on the SDQ and ADHDT questionnaires. All testing was done at the University Hospital of Heraklion, and Medical Health Centres in the prefecture of Heraklion, Crete, Greece.
Potential covariates
Potential covariates included characteristics that have an established or potential association with the exposure and/or outcomes of interest including: maternal age at delivery (years), maternal education (low: ≤6 years of school; medium: ≤12 years of school; high: university or technical college degree), maternal origin (Greek; non-Greek), smoking during pregnancy (yes; no), parity (multiparous; primiparous), maternal pre-pregnancy BMI (kgr/m2), maternal IQ measured using the Raven’s Standard Progressive Matrices (Raven and Court 1996), first-trimester serum TSH levels, child’s sex (male; female), birth weight (kgr), prematurity (preterm; non-preterm), and any breastfeeding duration (months, information on breastfeeding duration was collected during the 9th and the 18th months follow-up).
Statistical analysis
The primary outcomes of interest were SDQ scores, ADHD-like symptoms, and MSCA scores, at 4 years of age. SDQ and MSCA scores were treated as continuous variables, whereas ADHD-like symptoms were treated as quantitative traits. The primary exposure of interest was maternal 25(OH) D in early pregnancy. The distribution of mean 25(OH) D concentration was plotted by calendar month and showed a seasonal variation (Fig S1). As 25(OH) D concentrations followed a sinusoidal pattern, we fitted a cosinor model to the data to predict “deseasonalized” annual mean 25(OH) D concentration for each participant adjusted for month at blood collection.
Descriptive statistics were used to summarize the baseline characteristics of our study population. Bivariate comparisons of normally distributed variables were tested with ANOVA and non-normally distributed variables were tested with non-parametric Kruskal–Wallis test, whereas categorical variables were tested with Pearson’s Chi-square test. Generalized additive models (GAMs) were applied to explore the shape of the relationships between 25(OH) D concentration in maternal serum and outcomes under study. These models did not indicate clear linear relationships (p gain defined as the difference in normalized deviance between the GAM model and the linear model for the same predictor < 0.10); thus, maternal 25(OH) D concentration was treated as categorical divided into tertiles. To test the dose–response relationship of 25(OH) D concentrations and outcomes of interest, p for trend was assessed (p < 0.10).
We used multivariate linear regression models to assess the association (beta-coefficient, 95% CI) of 25(OH) D levels in early pregnancy on SDQ and standardized MCSA scores at 4 years of age. We also examined the risk [incidence rate ratio (IRR), 95%] of the number of ADHD-like symptoms in association with 25(OH) D levels in early pregnancy using multivariate negative binomial regression models. Covariates were selected if they showed at least marginally significant association (p < 0.1) with exposures and outcomes of interest or if they modified the coefficient of maternal 25(OH) D concentration by at least 10% when included in the crude model. Information about child sex and age, the examiner who conducted the developmental testing, and quality of neurodevelopmental assessment were included as a priori confounders. Based on the previous criteria, we created two models: (1) the crude model, minimally adjusted for child sex and age for SDQ scores and ADHD-like symptoms as outcomes, and child sex, examiner, and quality of assessment for MCSA scores; (2) the adjusted model additionally adjusted for maternal age, education, parity, smoking during pregnancy, and pre-pregnancy BMI.
Because relations of 25(OH) D concentration with offspring neurodevelopmental outcomes could be confounded by maternal IQ, we repeated the analysis after adjusting for maternal IQ in a subsample (n = 218) for which information was available. To check for residual confounding, we also adjusted our final models for physical activity, maternal alcohol intake, and total energy intake during pregnancy as well as children's BMI at 4 years of age. We further examined potential heterogeneity in associations related to maternal pre-pregnancy BMI, maternal TSH levels in early pregnancy, child’s sex, birth weight z-score, and breastfeeding duration, by including a multiplicative interaction term in the models (statistically significant effect modification if p value < 0.10) and by stratifying the sample accordingly.
All hypotheses testing were conducted assuming a 0.05 significance level and a two-sided alternative hypothesis. Due to multiple hypotheses testing, Benjamini–Hochberg correction was performed post hoc to control for false discovery rate (FDR = 0.25). We used Stata S.E. version 13 for the statistical analyses (Stata Corp, Texas, USA).
Missing data
In the initial 842 SDQ and ADHDT questionnaires completed by parents at 4 years of age, there were missing values in 1–17 items of the ADHDT questionnaire for 114 subjects and in 1–9 items of the SDQ questionnaire for 57 subjects, respectively. Missing items were imputed to minimize the impact of lack of data and the identification of the number of ADHD-like symptoms based on imputed data. We applied ordinal logistic chained equations to multiply imputed missing values (mi impute procedure in STATA 13.0), and 20 imputed data sets were generated. We have repeated the analysis using cases with complete data and interpretation of results remained unchanged, even though some associations lost statistical significance due to sample size reduction (data not shown).
Results
Sample characteristics
The socio-demographic characteristics of our study population are described in Table 1. Most mothers had a medium education level (52.4%), 46% were primiparous, and 35% were smokers during pregnancy. The mean (SD) concentration of maternal circulating 25(OH) D was 46.3 (15.4) nmol/l. The second tertile higher threshold (50.7 nmol/l) corresponded well with the recently used definition of vitamin D deficiency as a 25(OH) D concentration <50 nmol/l [31], indicating that two-thirds of pregnant women suffered from vitamin D deficiency. Women in the low 25(OH) D tertile (<38.4 nmol/l) had a higher mean BMI pre-pregnancy and were more likely to breastfeed their children for a shorter interval. We included 257 (52.7%) boys and 230 (47.3%) girls in the present analysis; the mean (SD) age was 4.2 (0.2) years and the mean (SD) birth weight was 3.2(0.5) kg.
Behavioral difficulties
Compared with children of mothers in the low 25(OH) D tertile (<38.4 nmol/l) in early pregnancy children of mothers in the high 25(OH) D tertile (>50.7 nmol/l) had 37% fewer hyperactivity–impulsivity symptoms (IRR 0.63, 95% CI 0.39, 0.99, p trend = 0.05) and 40% fewer total ADHD-like symptoms (IRR 0.60, 95% CI 0.37, 0.95, p trend = 0.03) at preschool age, after adjustment for several confounders (Fig. 2).
Association [incidence rate ratio (IRR) with 95% confidence interval] of maternal 25(OH) D concentrations with ADHD-like symptoms in a all children, b males, and c females at 4 years of age. All models were adjusted for child age at assessment, maternal age, maternal education, parity, smoking during pregnancy, and maternal body mass index pre-pregnancy. The models including all children were additionally adjusted for child sex
Similar associations were observed between 25(OH) D tertiles in early pregnancy and hyperactivity/inattention subscale score of SDQ questionnaire at preschool age (Table 2). Additionally, children of mothers in the high 25(OH) D tertile in early pregnancy had a significant score reduction in total behavioral difficulties (beta-coeff: −1.25, 95% CI −2.32, −0.19) and more specifically in the scale of externalizing symptoms (beta-coeff: −0.87, 95% CI −1.58, −0.15) at 4 years of age (Table 2). Effect estimates of the crude models for the associations between maternal vitamin D concentrations and behavioral outcomes under study did not differ substantially from the final models adjusted for maternal and child characteristics (Table 2, Table S2).
Neuropsychological outcomes
We did not find a significant association between maternal 25(OH) D tertiles in early pregnancy and offspring cognitive and motor function at preschool age in crude models, although we observed a trend of higher scores in almost all MSCA subscales among children of women in the high 25(OH) D tertile (>50.7 nmoll/l) (Table S2). Effect estimates remained to a large extent the same after adjustment for maternal and child characteristics (Table S3).
Effect modification–sensitivity analyses
Further analyses showed that the observed associations were more pronounced in girls than in boys (p for interaction <0.10, Table S4). We saw no evidence for a multiplicative interaction of maternal vitamin D tertiles in early pregnancy with maternal pre-pregnancy BMI, TSH levels, child’s birth weight z score and breastfeeding duration.
Further adjustment for maternal intelligence in the subsample (n = 218) for which maternal IQ data were available did not change the direction of associations, though confidence intervals were wider, probably due to small sample size (Table S5). The observed associations remained substantially the same, after adjustment for physical activity, alcohol intake during pregnancy, total energy intake during pregnancy, and children’s BMI at 4 years of age, although some of our main findings lost statistical significance, probably due to sample size reduction (Table S6). Because the effect of season is close to significant (Table 1) for our analysis, we repeated the analysis by including season in regression models and the associations remained the same (Table S7). To elucidate whether gestational diabetes, gestational hypertension, or prematurity modified the observed results, we performed a sensitivity analysis in which we excluded (a) women diagnosed with gestational diabetes (n = 41), (b) women diagnosed with gestational hypertension (n = 21), and (c) children born preterm (<37 gestational weeks, n = 58). We found no substantial differences in observed estimates (data not shown).
Discussion
In this prospective pregnancy cohort, we examined different domains of child neuropsychological and behavioral development at preschool age affected by vitamin D levels in early pregnancy. To our knowledge, this is the first study to examine the impact of maternal vitamin D status in early pregnancy on both cognitive function and behavioral difficulties in preschoolers. We showed that exposure to high 25(OH) D levels (>50.7 nmol/l) was associated with reduced number of hyperactivity–impulsivity and total ADHD-like symptoms, as well as total behavioral difficulties at preschool age. Our findings suggest that a vitamin D cutoff value of 50 nmol/l may be essential not only for bone health [32], but also for prevention of behavioral difficulties in the offspring. The observed associations persisted after adjustment for several maternal confounders and pre-pregnancy BMI. We also showed for the first time that child sex may modify the impact of vitamin D status in early pregnancy on offspring behavior at 4 years of age. We did not find a strong association between maternal vitamin D levels in early pregnancy and child cognitive and motor function at 4 years of age.
Although maternal vitamin D status has an important role in early brain development, data on the association between child neurodevelopment and vitamin D status in early pregnancy are limited. Previous studies support that high maternal 25(OH) D levels in the first half of pregnancy were associated with improved mental and psychomotor development in infants [12], better receptive language development at 2 years of age [17], and less language difficulties at 5 and 10 years of age [13]. We examined a wide broad of cognitive abilities at 4 years of age and also found a trend of higher scores among children of women in the high 25(OH) D tertile. However, our results did not reach statistical significance probably due to the small sample size. We also found an inverse relationship between maternal vitamin D status in early pregnancy and behavioral difficulties, including ADHD-like symptoms at 4 years of age. Our results are consistent with the findings of Morales et al. [21], who found a significant association between high 25(OH) D levels in early pregnancy and reduced number of ADHD-like symptoms in preschool children. However, we investigated more aspects of child behavior in our study and additionally found that high vitamin D levels in early pregnancy were associated with a reduced number of total behavioral problems, including externalizing symptoms at 4 years of age. Further adjustment for maternal IQ did not change the direction of the associations, suggesting a limited role of maternal genetic confounding.
It is well known that early pregnancy is a time window of particular vulnerability, as cortical structures critical to cognitive function and behavioral regulation are first formed. Maternal vitamin D performs a number of biological functions that are fundamental to early brain development [11], including proliferation and differentiation of brain cells [33], regulation of axonal growth [34], calcium signaling within the brain, and neurotrophic and neuroprotective actions [34]. In animal models, prenatal vitamin D deficiency has been associated with morphological changes [33] that may persist despite a postnatal return to normal vitamin D levels, resulting in abnormal behaviors in adulthood [35]. However, studies in humans are limited and plausible biological mechanisms are not clear yet.
In our study, we showed for the first time that child sex may modify the impact of maternal vitamin D status on offspring neurodevelopment. Higher levels of 25(OH) D in early pregnancy had a stronger protective effect on behavioral difficulties in females compared to males, especially on hyperactivity/inattention, externalizing symptoms and total ADHD-like symptoms. Limited data in adults have shown that immunomodulatory effects of vitamin D are significantly stronger in females than in males in multiple sclerosis patients supporting estrogen-promoted differences on vitamin D metabolism and action [36]. Whether there is also a functional synergy between estradiol and vitamin D action on prenatal brain development remains to be investigated.
With the vitamin D deficiency epidemic among pregnant women, the present results have important public health implications, which may be more profound in countries with higher prevalence of vitamin D deficiency. Despite a hypothetical excess of sunshine hours in the Mediterranean region, maternal hypovitaminosis D remains common in pregnant populations of these countries [5]. In our study, we found that almost two-thirds of pregnant women (n = 313) had vitamin D deficiency [25(OH) D levels < 50 nmol/l] in early pregnancy [31]. Possible reasons for this paradox could be maternal darker skin pigmentation, poor dietary vitamin D intake, veiled clothing reducing sunshine exposure, and increased prevalence of obesity [5]. In addition, preventive strategies for maternal vitamin D deficiency in the Mediterranean region are lacking so far, as hypovitaminosis D is largely unrecognized and underrated in several South European countries [5].
The strengths of our study include its prospective population-based study design, well-established neurodevelopmental outcome measures, and control for several maternal and child characteristics. The inclusion of maternal intelligence, although available for a subsample of the total population, should be considered as an additional strength. We estimated maternal vitamin D status in early pregnancy by measuring circulating 25(OH) D concentration, a reliable indicator of vitamin D synthesis and intake. We minimized the potential effect of season in our results by using the deseasonalized variable of 25(OH) D in our analysis. Neurodevelopment assessment at preschool age was performed with the use of MCSA [29], a valid, standardized psychometric test which provides both a general level of child’s intellectual functioning and an assessment of separate neurodevelopmental domains. In the present analysis, we used standardized neurodevelopmental scales (mean of 100 points with a 15 SD). There is extensive literature on the public health impact of a 1-point loss of a neuropsychological scale, but most are based on the effects of lead exposure on IQ [37]. Although a seemingly small change of a 1-point decrease in IQ score might not be relevant at the individual level, at the population level it is possible to shift the distribution of IQ to the left and increase the number of persons below the normal range [38].
A limitation of our study is the assessment of children’s ADHD symptoms and behavioral difficulties by parent-reported measures, which could be different from assessments made by a health-care professional. However, both ADHDT and SDQ questionnaire are well established and widely used screening tools with high specificity and sensitivity. Although we incorporated extensive information on potential social and environmental factors that are associated with child neurodevelopment, we acknowledge that residual confounding because of other unmeasured confounders such as social class and child’s vitamin D status may still occur.
In conclusion, our findings support a possible inverse relationship between vitamin D levels in early pregnancy and behavioral problems, especially hyperactivity/inattention, externalizing symptoms, and total ADHD-like symptoms in early childhood. These associations are more pronounced in females and may have important implications from a public health perspective. Whether these findings translate into long-term increased risk of abnormal behavior for the offspring of vitamin D-deficient women is unknown. However, unlike other causes of neurodevelopmental disorders, maternal vitamin D deficiency may be prevented. We speculate that appropriate supplementation during pregnancy may reduce the incidence of behavioral difficulties and ADHD-like symptoms later in life. Further investigation is needed to assess the long-term effects of vitamin D supplements in pregnancy on neuropsychological and behavioral development in offspring.
Abbreviations
- ADHD:
-
Attention deficit hyperactivity disorder
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- DSM-IV:
-
Diagnostic and Statistical Manual of Mental Disorders
- GAMs:
-
Generalized additive models
- IQ:
-
Intelligence quotient
- IRR:
-
Incidence rate ratio
- MSCA:
-
McCarthy Scales of Children’s Abilities
- SDQ:
-
Strengths and Difficulties Questionnaire
- SD:
-
Standard deviation
- TSH:
-
Thyroid stimulating hormone
References
Kubota T, Miyake K, Hariya N, Mochizuki K (2015) Understanding the epigenetics of neurodevelopmental disorders and DOHaD. J Dev Orig Health Dis 6(2):96–104. doi:10.1017/S2040174415000057
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357(3):266–281. doi:10.1056/NEJMra070553
Urrutia RP, Thorp JM (2012) Vitamin D in pregnancy: current concepts. Curr Opin Obstet Gynecol 24(2):57–64. doi:10.1097/GCO.0b013e3283505ab3
Hamilton SA, McNeil R, Hollis BW, Davis DJ, Winkler J, Cook C, Warner G, Bivens B, McShane P, Wagner CL (2010) Profound vitamin D deficiency in a diverse group of women during pregnancy living in a sun-rich environment at latitude 32°N. Int J Endocrinol 2010:917428. doi:10.1155/2010/917428
Karras SN, Anagnostis P, Annweiler C, Naughton DP, Petroczi A, Bili E, Harizopoulou V, Tarlatzis BC, Persinaki A, Papadopoulou F, Goulis DG (2014) Maternal vitamin D status during pregnancy: the Mediterranean reality. Eur J Clin Nutr 68(8):864–869. doi:10.1038/ejcn.2014.80
Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM (2013) Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ 346:f1169. doi:10.1136/bmj.f1169
Paterson CR, Ayoub D (2015) Congenital rickets due to vitamin D deficiency in the mothers. Clin Nutr 34(5):793–798. doi:10.1016/j.clnu.2014.12.006
Camadoo L, Tibbott R, Isaza F (2007) Maternal vitamin D deficiency associated with neonatal hypocalcaemic convulsions. Nutr J 6:23. doi:10.1186/1475-2891-6-23
Morales E, Romieu I, Guerra S, Ballester F, Rebagliato M, Vioque J, Tardon A, Rodriguez Delhi C, Arranz L, Torrent M, Espada M, Basterrechea M, Sunyer J (2012) Maternal vitamin D status in pregnancy and risk of lower respiratory tract infections, wheezing, and asthma in offspring. Epidemiology 23(1):64–71. doi:10.1097/EDE.0b013e31823a44d3
Sharief S, Jariwala S, Kumar J, Muntner P, Melamed ML (2011) Vitamin D levels and food and environmental allergies in the United States: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol 127(5):1195–1202. doi:10.1016/j.jaci.2011.01.017
Groves NJ, McGrath JJ, Burne TH (2014) Vitamin D as a neurosteroid affecting the developing and adult brain. Annu Rev Nutr 34:117–141. doi:10.1146/annurev-nutr-071813-105557
Morales E, Guxens M, Llop S, Rodriguez-Bernal CL, Tardon A, Riano I, Ibarluzea J, Lertxundi N, Espada M, Rodriguez A, Sunyer J (2012) Circulating 25-hydroxyvitamin D3 in pregnancy and infant neuropsychological development. Pediatrics 130(4):e913–e920. doi:10.1542/peds.2011-3289
Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Kusel MM, Hart PH (2012) Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics 129(3):485–493. doi:10.1542/peds.2011-2644
Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C (2008) Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr 62(1):68–77. doi:10.1038/sj.ejcn.1602680
Keim SA, Bodnar LM, Klebanoff MA (2014) Maternal and cord blood 25(OH)-vitamin D concentrations in relation to child development and behaviour. Paediatr Perinat Epidemiol 28(5):434–444. doi:10.1111/ppe.12135
Strom M, Halldorsson TI, Hansen S, Granstrom C, Maslova E, Petersen SB, Cohen AS, Olsen SF (2014) Vitamin D measured in maternal serum and offspring neurodevelopmental outcomes: a prospective study with long-term follow-up. Ann Nutr Metab 64(3–4):254–261. doi:10.1159/000365030
Tylavsky FA, Kocak M, Murphy LE, Graff JC, Palmer FB, Volgyi E, Diaz-Thomas AM, Ferry RJ (2015) Gestational vitamin 25(OH)D status as a risk factor for receptive language development: a 24-month, longitudinal, observational study. Nutrients 7(12):9918–9930. doi:10.3390/nu7125499
Zhu P, Tong SL, Hao JH, Tao RX, Huang K, Hu WB, Zhou QF, Jiang XM, Tao FB (2015) Cord blood vitamin D and neurocognitive development are nonlinearly related in toddlers. J Nutr 145(6):1232–1238. doi:10.3945/jn.114.208801
Gould JF, Anderson AJ, Yelland LN, Smithers LG, Skeaff CM, Zhou SJ, Gibson RA, Makrides M (2016) Association of cord blood vitamin D with early childhood growth and neurodevelopment. J Paediatr Child Health. doi:10.1111/jpc.13308
Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Hart PH, Kusel MM (2013) Maternal vitamin D levels and the autism phenotype among offspring. J Autism Dev Disord 43(7):1495–1504. doi:10.1007/s10803-012-1676-8
Morales E, Julvez J, Torrent M, Ballester F, Rodriguez-Bernal CL, Andiarena A, Vegas O, Castilla AM, Rodriguez-Dehli C, Tardon A, Sunyer J (2015) Vitamin D in pregnancy and attention deficit hyperactivity disorder-like symptoms in childhood. Epidemiology 26(4):458–465. doi:10.1097/EDE.0000000000000292
Gustafsson P, Rylander L, Lindh CH, Jonsson BA, Ode A, Olofsson P, Ivarsson SA, Rignell-Hydbom A, Haglund N, Kallen K (2015) Vitamin D status at birth and future risk of attention deficit/hyperactivity disorder (ADHD). PLoS ONE 10(10):e0140164. doi:10.1371/journal.pone.0140164
Chatzi L, Plana E, Daraki V, Karakosta P, Alegkakis D, Tsatsanis C, Kafatos A, Koutis A, Kogevinas M (2009) Metabolic syndrome in early pregnancy and risk of preterm birth. Am J Epidemiol 170(7):829–836. doi:10.1093/aje/kwp211
Katrinaki M, Kampa M, Margioris A, Castanas E, Malliaraki N (2016) Vitamin D levels in a large Mediterranean cohort: reconsidering normal cut-off values. Hormones (Athens) 15(2):205–223. doi:10.14310/horm.2002.1674
Goodman R (1997) The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry 38(5):581–586
Mpipou-Nakou I, Stogiannidou A, Kisseoglou G (2001) Strengths and difficulties of school-age children in the family and school context. Psychologia 8(4):506–525
Gilliam JE (1995) Examiners manual for the attention-deficit/hyperactivity disorder test: a method for identifying individuals with ADHD. pro-Ed, Austin
Maniadaki K, Kakouros E (2002) Translation and adaptation of the attention deficit hyperactivity disorder test (ADHDT; Giliam, 1995). In: Stalikas A, Triliva S, Roussi P (eds) The psychometric Instruments in Greece. Ellinika Grammata, Athens, pp 102–103
McCarthy D (1972) Manual for the McCarthy scales of children’s abilities. Psychological Corp, New York
Kampouri M, Kyriklaki A, Roumeliotaki T, Koutra K, Anousaki D, Sarri K, Vassilaki M, Kogevinas M, Chatzi L (2017) Patterns of early-life social and environmental exposures and child cognitive development, Rhea birth cohort, Crete, Greece. Child Dev. doi:10.1111/cdev.12782
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96(7):1911–1930. doi:10.1210/jc.2011-0385
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96(1):53–58. doi:10.1210/jc.2010-2704
Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F (2003) Vitamin D3 and brain development. Neuroscience 118(3):641–653
Eyles DW, Burne TH, McGrath JJ (2013) Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol 34(1):47–64. doi:10.1016/j.yfrne.2012.07.001
O’Loan J, Eyles DW, Kesby J, Ko P, McGrath JJ, Burne TH (2007) Vitamin D deficiency during various stages of pregnancy in the rat; its impact on development and behaviour in adult offspring. Psychoneuroendocrinology 32(3):227–234. doi:10.1016/j.psyneuen.2006.12.006
Correale J, Ysrraelit MC, Gaitan MI (2010) Gender differences in 1,25 dihydroxyvitamin D3 immunomodulatory effects in multiple sclerosis patients and healthy subjects. J Immunol 185(8):4948–4958. doi:10.4049/jimmunol.1000588
Grandjean P, Landrigan PJ (2006) Developmental neurotoxicity of industrial chemicals. The Lancet 368(9553):2167–2178
Bellinger DC (2012) A strategy for comparing the contributions of environmental chemicals and other risk factors to neurodevelopment of children. Environ Health Perspect 120(4):501
Acknowledgements
The authors would particularly like to thank all the cohort participants for their generous collaboration. The Rhea project was financially supported by European projects (EU FP6-2003-Food-3-NewGeneris, EU FP6. STREP Hiwate, EU FP7 ENV.2007.1.2.2.2. Project No 211250 Escape, EU FP7-2008-ENV-1.2.1.4 Envirogenomarkers, EU FP7-HEALTH-2009- single stage CHICOS, EU FP7 ENV.2008.1.2.1.6. Proposal No 226285 ENRIECO, EUFP7- HEALTH-2012 Proposal No 308333 HELIX), MeDALL (FP7 European Union project, No. 264357), and the Greek Ministry of Health (Program of Prevention of obesity and neurodevelopmental disorders in preschool children, in Heraklion district, Crete, Greece: 2011–2014; “Rhea Plus”: Primary Prevention Program of Environmental Risk Factors for Reproductive Health, and Child Health: 2012–2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Daraki, V., Roumeliotaki, T., Koutra, K. et al. High maternal vitamin D levels in early pregnancy may protect against behavioral difficulties at preschool age: the Rhea mother–child cohort, Crete, Greece. Eur Child Adolesc Psychiatry 27, 79–88 (2018). https://doi.org/10.1007/s00787-017-1023-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-017-1023-x