Abstract
This study aims to examine regional gray matter (GM) changes over a period of 2 years in patients diagnosed with early-onset first-episode psychosis (EO-FEP), and to identify baseline predictors of abnormalities at the follow-up. Fifty-nine patients with EO-FEP aged 11–17 years were assessed. Magnetic resonance imaging was carried out at admission and 2 years later. Changes over time were assessed with voxel-based morphometry. Fifty-nine patients (34 schizophrenia—SCZ, 15 bipolar disorder—BP, and 10 other psychotic disorders) and 70 healthy controls were assessed. At baseline no differences were found between the EO-FEP groups and control subjects. Over time, SCZ patients presented a larger GM decrease in the orbitofrontal cortex, anterior midline frontal cortex, cingulate, left caudate, and thalamus. BP patients also had a larger GM decrease in the right putamen, right orbitofrontal cortex, and anterior and midline region of the right superior frontal gyrus and left caudate, but with fewer areas showing significant differences than in the comparison between SCZ and controls. In the cross-sectional analysis, only SCZ patients showed differences with respect to controls in some GM areas. Significant baseline predictors of a 2-year reduction in GM were IQ and working memory. EO-FEP patients did not show differences in GM compared to controls at baseline. Both SCZ and BP patients showed a greater decrease in specific areas during the first 2 years. At follow-up, only SCZ patients differed significantly from controls in specific brain areas. The GM reduction was predicted by baseline cognitive variables.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several studies have examined structural brain abnormalities in patients with long-duration schizophrenia (SCZ) and have shown reduced global gray matter (GM) and white matter (WM) volumes in both adults [1, 2] and children [3, 4]. Studies of patients with first-episode psychosis (FEP) offer the opportunity to detect brain abnormalities at a very early stage without the influence of potential confounding variables (i.e., medication).
Cross-sectional studies using voxel-based morphometry (VBM) neuroimaging in early-onset first-episode psychosis (EO-FEP) are scarce and have found very few abnormalities in specific areas [5,6,7]. Moreover, very few longitudinal studies have been conducted with EO-FEP patients, and none have used VBM methodology. Using a region of interest (ROI) methodology, Reig et al. [8] studied 21 FEP patients both at baseline and after 2-year follow-up and found a progression in frontal lobe changes, especially in male patients. Arango et al. [9], with the same methodology, found that compared with controls FEP-SCZ patients showed greater GM volume loss in the frontal lobe, total GM, and increases in the left frontal CSF and total volume during the 2-year follow-up. No differences were found between FEP-BP patients and controls during the 2-year follow-up. Brain abnormalities were related to markers of poorer prognosis such as greater GM volume loss, with more weeks of hospitalization or less improvement in PANSS negative and general subscale scores. In their meta-analysis of longitudinal MRI studies in EO-FEP patients with ROI methodology, Fraguas et al. [10] concluded that the frontal GM was the only region showing significant differences in volume change over time between patients and controls.
Some studies with adult FEP patients have found that cognitive baseline variables are good predictors of GM reduction at follow-up. In a longitudinal study, Ayesa-Arriola et al. [11] found no morphometric differences in GM at baseline between cognitively impaired and cognitively preserved FEP patients, but patients who were cognitively impaired at baseline presented greater progressive brain volume loss after 3 years in the parietal area and in total GM volume. Gutierrez-Galve et al. [12, 13], using surface-based morphometry (SBM), showed that progressive cortical thinning in the superior and inferior frontal and superior temporal cortex was predicted by lower baseline IQ and working memory.
To the best of our knowledge, no previous longitudinal studies with EO-FEP patients have used VBM methodology. Only a few follow-up studies in children and adolescent FEP samples have been carried out to date. The studies published are mainly cross-sectional and have been conducted in young adult samples with a mean age between 18 and 24 years or with a wide age range. Analyses of predictors of GM volume are even fewer in number and those published present some methodological issues.
The aims of the present study were (1) to expand our previous global structural findings regarding progressive brain abnormalities during the first years of a psychotic episode [9] using a VBM methodology that allows for comprehensive exploration of both whole brain and specific areas and (2) to analyze whether baseline variables can predict GM reduction in specific areas at follow-up. Our hypotheses were: (1) that EO-FEP-SCZ patients would show a greater decrease in global GM volumes and in fronto-subcortical regions (i.e., orbitofrontal, anterior, and superior frontal cortex, putamen, caudate, and thalamus) than controls and that BP patients would be less differentiated from controls; and (2) that the decrease in GM in the affected areas at follow-up would be predicted by the subject’s performance on IQ and working memory at baseline.
Experimental procedures
Participants
As part of the Child and Adolescent First-Episode Psychosis Study (CAFEPS) [14], MRI data were obtained from 92 patients and 94 controls at baseline. Two years later, a second neuroimaging assessment was carried out in 59 patients and 70 controls. The MRI attrition and further details about the sample and inclusion and exclusion criteria are provided in supplemental material section 1. All parents or legal guardians gave written informed consent before the study began and all patients agreed to participate.
Based on the results of the Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime versions [15] at 2-year follow-up, patients were divided into three diagnostic categories: the schizophrenia spectrum group (SCZ) (N = 34), which included 9 patients diagnosed with schizoaffective disorder, and 25 patients diagnosed with schizophrenia; the bipolar group (BP) (N = 15); and the other psychosis group (N = 10), including 3 cases of depression with psychotic features and 7 cases of psychoses not otherwise specified.
Clinical and socioeconomic status assessment
Clinical assessment included the following scales: Kiddie Schedule for Affective Disorders and Schizophrenia, Present and Lifetime versions (K-SADS-PL) [15], the Spanish version of the Positive and Negative Symptom Scale (PANSS) [16], and the Premorbid Adjustment Scale (PAS) [17]. We also determined the duration of untreated psychosis and estimated socioeconomic status (SES) using the Hollingshead–Redlich scale [18].
A complete description of each clinical scale is provided in supplemental material section 2.
Cognitive assessment
The cognitive assessment was performed at baseline. A complete description of the methodology has been provided elsewhere [19]. The cognitive domains assessed were general intelligence quotient (IQ), working memory, verbal memory, and executive functions. Further details of each cognitive domain can be found in supplemental material section 3.
VBM analysis
Details of image acquisition and further information about VBM analysis are provided in supplemental material sections 4 and 5. All 3D images were manually re-orientated to stereotactic space (i.e., the anterior–posterior axis was parallel to the anterior commissure–posterior commissure line, and the inter-hemispheric fissure was aligned on the other two axes) using SPM8 (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, University College London, UK, http://www.fil.ion.ucl.ac.uk/spm).
Statistical analyses of images Statistics were computed using SPM8. Cross-sectional analysis was performed via two-sample t tests to assess GM volume differences between the control group and diagnostic groups. To assess longitudinal GM volume differences, a flexible factorial model was used between the control and diagnostic groups.
An explicit mask was calculated to select which voxels should undergo statistical analysis. The aim was to exclude any voxels that were unlikely to belong to each tissue class. A mask was created for each analysis group and GM probability map using a 0.2 threshold. The threshold was set by visual inspection of pre-processed images and assessed by visual inspection of the mask image resulting from the SPM statistical analysis. A mean image of GM was then calculated and used as an external mask after binarization. Age, sex, TIV, and scan site were used as nuisance covariates to control the potential effect of this variable on the two-sample t test. The statistical threshold criterion was p < 0.001 uncorrected, using a cutoff of p < 0.05, FWE (family-wise error) correction for multiple comparisons at the cluster level.
Anatomical localization was done using projection onto the corresponding whole-brain customized template. Labels and the corresponding percentage of voxels belonging to the labeled regions were obtained for each significant GM cluster using the AAL Toolbox [20].
MRI volume calculation
Total intracranial cerebral volume (ICV), gray matter (GM), white matter (WM), and cerebral spinal fluid (CSF) were calculated using GM, WM, and CSF probability maps from the new segment tool of SPM8, using customized tissue probability maps.
Statistical analysis of numerical data
Categorical socio-demographic variables were analyzed using the χ 2 test. Continuous clinical variables were compared between groups via MANCOVA, with the variables that differed between groups (age) as covariates. Post hoc analysis was performed with the Bonferroni correction for multiple comparisons.
Volume differences between each patient group and controls at baseline and at 2-year follow-up were assessed via univariate analysis of variance, with age, sex, total intracranial volume at first scan, and site where MRI was performed as no-interest covariates. The inter-scan interval was also included as a covariate in the statistical analysis at follow-up. Longitudinal changes in clinical variables and volume measures within the patient and control groups were examined using paired t tests.
To assess differences in longitudinal changes in GM among the three diagnostic groups and controls, a volume change variable was calculated. This variable was the result of subtracting the follow-up volume from the baseline volume. Differences in change between each patient group and controls were tested via univariate analysis, with age, sex, total intracranial volume at first scan, site, and inter-scan interval as covariates. This methodology has been used previously [9] and is preferred to the repeated measures ANCOVA because the confounding effect of the scanner site is minimized by using the within-subjects change values. In order to test the influence of the antipsychotic medication on our results, a partial correlation analysis was conducted between the cumulative doses of chlorpromazine and all significant results. Age, sex, TIC at first scan and site where the MRI was performed were included as covariates in the analysis. To test the predictive value of baseline variables with respect to GM volume at follow-up, linear regression analyses were performed with clinical and cognitive variables as potential predictors in the patient and control groups. All statistical analyses were performed using the SPSS 20.0 package.
Results
Socio-demographic and general clinical characteristics in subgroups of EO-FEP patients and controls
The different subgroups of EO-FEP patients and controls were similar in sex, parental SES, handedness, ethnicity, and educational level (see Table 1). Differences in mean age were statistically significant, with BP patients being significantly older than controls, but not older than the SCZ or other psychosis groups. Regarding estimated IQ, all three patient groups scored lower than controls, whereas on the PAS the SCZ and BP patients showed worse premorbid adjustment than the other psychosis group, and all of them had worse scores than controls.
Comparison of EO-FEP subgroups in relation to clinical variables at baseline and at follow-up and in change on clinical scales during follow-up
Table 2 shows the comparison of EO-FEP patients with a diagnosis of SCZ, BP or other psychotic disorders at first assessment and at the 2-year follow-up with respect to clinical variables. The duration of illness before entering the study, number of weeks hospitalized, and cumulative antipsychotic dose in chlorpromazine equivalents during the 2-year follow-up were not significantly different between the groups. The table also shows psychopharmacological treatment at both baseline and follow-up; the large majority of patients were treated with second-generation antipsychotics. At baseline there were no statistically significant differences between diagnostic groups on any PANSS subscale. At follow-up, SCZ patients scored higher on negative symptoms and on the Total PANSS score.
Comparison of EO-FEP subgroups and controls in whole-brain volumes at baseline and follow-up and in change during the follow-up period
Table 3 shows the global cerebral volumes in all groups. At baseline, the SCZ and BP subgroups had significantly more CSF volume than control subjects. There were no differences in GM or WM. The other psychosis group did not show any differences with respect to controls. During follow-up, control subjects showed a decrease in GM and an increase in WM volume. SCZ patients had a significantly larger decrease in GM and a significantly smaller increase in WM in comparison with controls. BP patients had a significantly smaller increase in WM and significantly larger increase in CSF than controls. There were no differences in changes between controls and the other psychosis group. In the cross-sectional analysis at follow-up, SCZ patients showed significant differences compared with controls, specifically in terms of smaller total intracranial volume, GM and WM volumes, and more CSF. BP patients only had less global WM and more CSF than controls, while the other psychosis group showed no significant differences in cerebral volumes compared with controls. Correlations between GM, WM, CSF, and cumulative doses of chlorpromazine levels were conducted to assess the influence of the antipsychotic level and whole-brain volumes. No significant results were observed in any of the analyses.
Comparison of voxel-based regional analysis of gray matter between the EO-FEP subgroups and controls at baseline
In the regional VBM study at first assessment, there were no significant differences in GM between SCZ, BP, and other psychosis patients and control subjects.
Comparison of volume changes in the longitudinal voxel-based regional analysis of gray matter between EO-FEP subgroups and control subjects
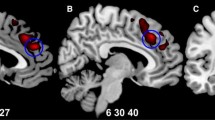
As shown in Table 4, there was a statistically significant greater reduction in five clusters of GM in SCZ patients in comparison with controls. The largest cluster, with the highest number of voxels, involved the left orbitofrontal and olfactory cortex, the midline and anterior region of the left superior frontal gyrus, and the right anterior cingulate; the second cluster involved the midline region of the right superior frontal cortex, the inferior orbitofrontal cortex, the rectus gyrus, the right inferior frontal gyrus, and the right postcentral areas; the third cluster involved the left caudate and thalamus; the fourth cluster involved the right hippocampus and left thalamus; and the fifth involved the left inferior gyrus, the left precentral and postcentral area, and the left insula. Figure 1a shows differences in changes between SCZ patients and Controls. The BP group showed a larger decrease in GM than controls in three clusters, but with a smaller number of voxels than was observed in the two largest clusters for SCZ patients. These three clusters were as follows: one involving the right putamen, rectus gyrus, and the midline anterior part of the right superior frontal gyrus; another involving the left caudate; and a third in the left occipital area. Figure 1b shows the differences in changes between BP patients and controls. In the direct comparison between SCZ and BP patients, only one cluster showed differences between the two groups at peak level (MNI coordinate: −55, −5, 22), namely that in the left postcentral and precentral area (p FWE-corr = 0.016; T = 5.62).
Longitudinal study: Regions with a significantly greater decrease in volume in EO-FEP schizophrenia (a) and bipolar disorder (b) patients compared with control subjects during follow-up, overlaid onto the SPM8 canonical single-subject T1 image. The color bar represents the t score: yellow indicates higher statistical significance than orange or red. The statistical parametric maps follow the standard neuroradiological representation, with the left side of the images corresponding to the left hemisphere. Maps are generated with an uncorrected p < 0.001
Regarding the other psychosis subgroup, there were no significant differences with respect to controls in changes in GM between baseline and follow-up assessment. Correlations between all significant clusters and chlorpromazine levels were performed and no significant results were observed.
Comparison of volumes in the voxel-based regional analysis of gray matter between EO-FEP subgroups and control subjects at the 2-year follow-up
At the follow-up assessment (Table 5), SCZ patients showed smaller volumes than controls in four clusters: one involved the right and left thalamus; a further two involved the right and left superior frontal cortex, right and left orbitofrontal cortex, and the anterior and medial part of the cingulum; and a fourth involved the right and left rectus and the orbitofrontal cortex. Figure 2 shows the differences between SCZ patients and controls at the 2-year follow-up. BP patients and the other psychosis group showed no significant differences in GM with respect to controls at the follow-up assessment. In the direct comparison between SCZ and BP patients, there were no significant differences in any specific GM area. No significant correlations were observed between significant GM clusters and chlorpromazine levels.
Regions of decreased gray matter volume at the 2-year follow-up in EO-FEP schizophrenia patients compared with control subjects are overlaid onto the SPM8 canonical single-subject T1 image. The color bar represents the t score: Yellow indicates higher statistical significance than orange or red. The statistical parametric maps follow the standard neuroradiological representation, with the left side of the images corresponding to the left hemisphere. Maps are generated with an uncorrected p < 0.001
Baseline predictors of volume reductions at follow-up in the longitudinal voxel-based regional analysis of gray matter in SCZ patients
In SCZ patients, low IQ at baseline predicted GM reductions at follow-up in the right and left rectus and orbitofrontal cortex (cluster 1 38 −20; R 2 = 0.205; F 1,30 = 7.745; p = 0.009) and in the right and left superior frontal and orbitofrontal cortex (cluster −1 51 13 R 2 = 0.246; F 1,30 = 9.775; p = 0.004). Low performance in the working memory domain was a significant predictor of a GM volume decrease in the right and left superior and orbitofrontal cortex at the 2-year follow-up (cluster −1 51 13; R 2 = 0.218; F 1,30 = 8.631; p = 0.006). These associations were not present in the healthy control group, except for lower working memory performance and GM volume decrease in the right and left superior and orbitofrontal cortex (cluster −1 51 13; R 2 = 0.061; F 1,67 = 4.360; p = 0.041), although in the control group working memory explained only 6.1% of the variance, whereas in the SCZ group it explained 24.6% (Fig. 3). Other baseline variables such as negative symptoms (PANSS), premorbid adjustment, verbal memory, or executive function were not significant predictors of GM reduction at follow-up. Correlations between GM reduction at follow-up in the different voxels and cumulative antipsychotic dose in chlorpromazine equivalents were not significant.
Discussion
The main findings in this longitudinal study with children and adolescents are that SCZ EO-FEP patients present larger decreases in GM than control subjects, and especially in the orbitofrontal cortex, anterior midline frontal cortex, cingulate and left caudate and thalamus, and that BP EO-FEP patients also present larger decreases of GM than controls in the right putamen, right orbitofrontal cortex and anterior and midline region of the right superior frontal gyrus, left caudate and left occipital area, but with less extensive differences than in the comparison between SCZ and controls. After the 2-year follow-up, only SCZ patients showed regional differences with respect to controls in the orbitofrontal cortex, the anterior and midline areas of the superior frontal cortex and thalami, and these abnormalities were predicted by lower IQ and working memory performance at baseline.
In our study, the assessment of whole-brain volumes was included to test the progress of GM, WM, CSF in FEP and healthy controls and to include these results as covariates in some of the following statistical analyses. Thus, at first assessment, SCZ and BP subgroups had significantly more CSF volume than control subjects, but there were no differences in global GM or WM. This is in agreement with some other studies that did not find global GM or WM differences between FEP patients and controls at baseline (cross-sectional analysis) at these young ages and with a very short duration of the disorder [5, 7, 21].
In the regional VBM study, at the first assessment there were no significant differences in GM between SCZ, BP or other psychosis patients and control subjects. This result corroborates the findings of Pagsberg et al. [5] who, also using VMB methodology, found no abnormalities in specific areas in adolescent FEP patients. Nevertheless, other authors have found small significant differences with respect to controls. Janssen et al. [6] showed lower GM volumes in the left medial frontal gyrus in FEP-SCZ and BP and also in the left middle frontal gyrus in SCZ patients in comparison with controls, while in a study of EO-FEP-SCZ patients with a duration of illness of 9 months Tang et al. [7] found that patients only had lower GM volume in a small voxel at the left and middle and superior temporal gyrus. In contrast, in adult cross-sectional studies with VMB, a reduction in GM has been observed in different frontal and medial regions, anterior cingulate, and fronto-subcortical regions such as medial frontal, caudate, thalamus, cingulated gyrus, parahippocampal gyrus, hippocampus, insula and putamen [22,23,24]. These differences in the results at baseline between adolescent and adult FEP patients in cross-sectional studies may be because the disorder duration may be longer in adult samples even if long-term symptoms are not clinically obvious and therefore patients are not referred to treatment. Recruiting adolescent samples probably ensures that the duration of sub-threshold psychosis before baseline assessment is indeed short. Moreover, the idea that the duration of disorder is crucial to find structural abnormalities is underscored by the finding of the reduction during the 2-year follow-up in the present study, which was significantly higher in EO-FEP-SCZ and BP patients than in control subjects.
In our study, whole-brain GM change at the 2-year follow-up decreased more and WM increased less in SCZ patients than in healthy controls. GM results obtained in this study are in line with those obtained by the study with the ROI methodology [21]. Nevertheless, although Arango et al. found a significant increase in WM volume in all samples (FEP and healthy controls), their study failed to find significant differences between FEP groups and controls. Differences in WM results between our study and Arango’s study may be due to methodological issues, since the authors used software developed in-house [25, 26] and stated that with this methodology the multicenter reproducibility of measurements was much higher for GM than WM. As has been well established by previous literature [27, 28], in the general population as well as in our control sample, GM undergoes a pre-pubertal increase and a post-pubertal decrease due to synaptic pruning processes, while WM increases over time as a result of myelination processes [29]. Thus, some authors have hypothesized that in SCZ samples, the GM loss pattern is the same, but is abnormally accelerated [28]. An overall difference with respect to controls in GM changes during the first 2 years of the disorder, especially in the frontal and parietal lobes, has already been reported in Arango et al. [21] and support the idea that SCZ or BP causes deviations from normal brain development, suggesting a neurodevelopmental basis for these disorders.
Regarding voxel-based region analysis of change at the 2-year follow-up, the present study is the first one to use this methodology in EO-FEP patients and indicates some of the regions that show greater decreases than in controls during these two first years in SCZ and BP adolescents. Regarding SCZ patients, the areas affected are both orbitofrontal cortices, the anterior and midline area of the left superior frontal gyrus and the precentral and postcentral gyrus, the right superior and inferior frontal gyrus, the right postcentral area, the left caudate and thalamus and the right hippocampus. These areas are quite similar to some of the already mentioned areas that have been found to be decreased in adult FEP-SCZ patients in cross-sectional studies with VMB [22,23,24]. In a longitudinal study with adults using VBM, Mane et al. [30] studied FEP-SCZ patients, and after 4 years found an excessive GM decrease in FEP-SCZ patients in the left superior temporal gyrus and right orbitofrontal gyrus. So in view of the previous literature, our results might suggest that the changes observed could be an early biomarker in schizophrenia [31].
As we hypothesized at the 2-year follow-up, BP patients showed less extensive differences with respect to controls with smaller voxel sizes showing a decrease of GM than SCZ patients. The regions that showed a greater decrease than controls during follow-up were in the frontal and limbic structures; the decreases were more marked in SCZ patients, though the difference was not great. In their cross-sectional study, Watson et al. [24] pointed out that FEP-BP did not differ from controls in any area, and our study with ROI methodology [21] found no differences between controls and FEP-BP patients. With regard to the group of other psychosis patients, there were no differences with respect to controls in their GM changes during the 2-year follow-up.
With regard to the cross-sectional analysis at the 2-year follow-up, only SCZ patients showed differences with respect to controls in specific GM regions, such as the superior frontal, orbitofrontal cortex and the anterior and medial part of the cingulum and thalamus. These regions partially correspond to the already mentioned areas found in cross-sectional studies of adult patients as mentioned elsewhere [22,23,24]. No significant differences were detected in the VBM analysis of BP samples. These data may be in line with those obtained by McDonald et al. [32] who hypothesized that bipolar psychosis may be predominantly a WM disorder, while schizophrenia involves both GM and WM disturbances. Moreover, methodological issues such as the size of our BP sample may have meant that our study lacked the statistical power to show significant differences in GM in the VBM analysis.
Regarding the prediction of GM change in specific regions according to baseline clinical and cognitive variables, only IQ and performance in working memory tasks were significant predictors of GM reductions in the superior frontal, orbitofrontal cortex and anterior and medial part of the cingulum and thalamus in SCZ patients. In control subjects only working memory was a significant predictor of GM volume in the orbitofrontal cortex and anterior and medial part of the cingulum areas, but the variance explained was much lower than in SCZ patients. The majority of studies assessing the relationship between cognitive variables and GM reduction are cross-sectional, but a few studies in adult FEP patients have shown that some baseline cognitive variables predict GM reduction at follow-up. In a longitudinal study, Ayesa-Arriola et al. [11] found that patients classified as cognitively impaired with a global measure, including major cognitive domains of verbal and working memory, executive functions, speed of procession and attention at baseline, presented greater progressive brain volume loss after 3 years. In another study, Gutierrez-Galve [12] showed that cortical changes in the superior and inferior frontal and in the lower extent superior temporal cortex were predicted by baseline IQ and working memory. Intelligence as a global measure is closely related to the frontal and parietal areas, and patients with schizophrenia have lower premorbid IQ than controls [33]. Our findings show that lower IQ, as a measure of lower cognitive reserve, can predict a more severe disorder in relation to GM abnormalities. Other clinical variables at baseline such as premorbid adjustment, duration of illness or negative PANSS score did not predict reduction of GM in any specific area. Some adult patient studies did not find any relationship between symptom severity or duration of illness and cortical abnormalities [13, 34], although other studies with longer duration of illness in their sample did find a correlation with gray matter abnormalities [35]. Moreover, in view of previous reports of a relationship between higher doses of antipsychotic treatment and smaller GM and WM volumes [36], we conducted a correlation analysis between all our significant results and cumulative doses of chlorpromazine (almost exclusively of second-generation antipsychotics). No association was observed in our samples. Therefore, this is an issue that should be further analyzed in future studies.
The present study has some limitations. The main one is the small sample size in the subgroups of BP and other psychotic disorders; this may have introduced type II errors and so further replication with larger samples is necessary. The group of BP patients all had psychotic symptoms with a first manic episode and the results are limited to this subgroup, (probably more severe) and cannot be extrapolated to all BP patients. The strengths of the study include the very short duration of disorder at baseline, the uniformity of patients regarding age and duration of disorder, its longitudinal design using a control group of similar age, the re-evaluation after 2 years in all cases to better establish the diagnosis, and the use of a longitudinal voxel-based method to map the progression of changes after follow-up both in patients and controls.
These results, which showed no differences at baseline when the disorder is just starting and a larger reduction in GM areas specifically in SCZ patients during the first 2 years of disease, indicate that the disorder has an important influence on GM abnormalities in these patients. In contrast, the impact in BP patients seems to be lower. It is also important to stress that in cases in which no definite diagnosis of SCZ or BP could be reached after 2 years, changes in GM were similar to those in control with a lower degree of severity both clinically and in neuroimaging studies. The prediction of GM decrease in cortical and subcortical areas by baseline IQ and working memory may help clinicians to detect SCZ patients who are going to have more neuroimaging abnormalities during the course of their disorder, and the intensification of strategies to counteract the progression of cognitive and brain abnormalities such as cognitive rehabilitation may improve prognosis in more severe young patients. Further studies with EO patients with longer follow-up periods are needed to determine whether these abnormalities progress over the years and whether in some patients they can be stabilized.
References
Andreasen NC, Nopoulos P, Magnotta V et al (2011) Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry 70:672–679
Haijma SV, Van Haren N, Cahn W et al (2013) Brain volumes in schizophrenia: a meta-analysis in over 18,000 subjects. Schizophr Bull 39:1129–1138
Vidal CN, Rapoport JL, Hayashi KM et al (2006) Dynamically spreading frontal and cingulate deficits mapped in adolescents with schizophrenia. Arch Gen Psychiatry 63:25–34
Gogtay N (2008) Cortical brain development in schizophrenia: insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr Bull 34:30–36
Pagsberg AK, Baare WF, Raabjerg Christensen AM et al (2007) Structural brain abnormalities in early onset first-episode psychosis. J Neural Transm 114:489–498
Janssen J, Reig S, Parellada M et al (2008) Regional gray matter volume deficits in adolescents with first-episode psychosis. J Am Acad Child Adolesc Psychiatry 47:1311–1320
Tang J, Liao Y, Zhou B et al (2012) Decrease in temporal gyrus gray matter volume in first-episode, early onset schizophrenia: an MRI study. PLoS ONE 7:e40247
Reig S, Moreno C, Moreno D et al (2009) Progression of brain volume changes in adolescent-onset psychosis. Schizophr Bull 35:233–243
Arango C, Rapado-Castro M, Reig S et al (2012) Progressive brain changes in children and adolescents with first-episode psychosis. Arch Gen Psychiatry 69:16–26. doi:10.1001/archgenpsychiatry.2011.150
Fraguas D, Díaz-Caneja CM, Pina-Camacho L et al (2014) Progressive brain changes in children and adolescents with early-onset psychosis: a meta-analysis of longitudinal MRI studies. Schizophr Res 173:132–139
Ayesa-Arriola R, Roiz-Santianez R, Perez-Iglesias R et al (2013) Neuroanatomical differences between first-episode psychosis patients with and without neurocognitive deficit: a 3-year longitudinal study. Front Psychiatry 4:134
Gutierrez-Galve L, Chu EM, Leeson VC et al (2015) A longitudinal study of cortical changes and their cognitive correlates in patients followed up after first-episode psychosis. Psychol Med 45:205–216
Gutierrez-Galve L, Wheeler-Kingshott CA, Altmann DR et al (2010) Changes in the frontotemporal cortex and cognitive correlates in first-episode psychosis. Biol Psychiatry 68:51–60
Castro-Fornieles J, Parellada M, Gonzalez-Pinto A et al (2007) The child and adolescent first-episode psychosis study (CAFEPS): design and baseline results. Schizophr Res 91:226–237
Kaufman J, Birmaher B, Brent D et al (1997) Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988
Peralta V, Cuesta MJ (1994) Psychometric properties of the positive and negative syndrome scale (PANSS) in schizophrenia. Psychiatry Res 53:31–40
Cannon-Spoor HE, Potkin SG, Wyatt RJ (1982) Measurement of premorbid adjustment in chronic schizophrenia. Schizophr Bull 8:470–484
Hollingshead A, Redlich F (1958) Social class and mental illness. Wiley, New York
Zabala A, Rapado M, Arango C et al (2010) Neuropsychological functioning in early-onset first-episode psychosis: comparison of diagnostic subgroups. Eur Arch Psychiatry Clin Neurosci 260:225–233
Tzourio-Mazoyer N, Landeau B, Papathanassiou D et al (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Arango C (2012) Progressive brain changes in children and adolescents with first-episode psychosis. Arch Gen Psychiatry 69:16. doi:10.1001/archgenpsychiatry.2011.150
Chua SE, Cheung C, Cheung V et al (2007) Cerebral grey, white matter and csf in never-medicated, first-episode schizophrenia. Schizophr Res 89:12–21
Molina V, Sanz J, Villa R et al (2010) Voxel-based morphometry comparison between first episodes of psychosis with and without evolution to schizophrenia. Psychiatry Res 181:204–210
Watson DR, Anderson JM, Bai F et al (2012) A voxel based morphometry study investigating brain structural changes in first episode psychosis. Behav Brain Res 227:91–99
Reig S, Sanchez-Gonzalez J, Arango C et al (2009) Assessment of the increase in variability when combining volumetric data from different scanners. Hum Brain Mapp 30:355–368
Desco M, Hernandez JA, Santos A, Brammer M (2001) Multiresolution analysis in fMRI: sensitivity and specificity in the detection of brain activation. Hum Brain Mapp 14:16–27
Gogtay N, Sporn A, Clasen LS et al (2004) Comparison of progressive cortical gray matter loss in childhood-onset schizophrenia with that in childhood-onset atypical psychoses. Arch Gen Psychiatry 61:17
Gogtay N, Thompson PM (2010) Mapping gray matter development: implications for typical development and vulnerability to psychopathology. Brain Cogn 72:6–15
Uda S, Matsui M, Tanaka C et al (2015) Normal development of human brain white matter from infancy to early adulthood: a diffusion tensor imaging study. Dev Neurosci 37:182–194
Mane A, Falcon C, Mateos JJ et al (2009) Progressive gray matter changes in first episode schizophrenia: a 4-year longitudinal magnetic resonance study using VBM. Schizophr Res 114:136–143
DeLisi LE (2007) The concept of progressive brain change in schizophrenia: implications for understanding schizophrenia. Schizophr Bull 34:312–321
McDonald C, Bullmore E, Sham P et al (2005) Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. Br J Psychiatry 186:369–377
Woodberry KA, Giuliano AJ, Seidman LJ (2008) Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry 165:579–587
Ho B-C, Andreasen NC, Nopoulos P et al (2003) Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry 60:585–594
Lappin JM, Morgan K, Morgan C et al (2006) Gray matter abnormalities associated with duration of untreated psychosis. Schizophr Res 83:145–153
Ho B-C, Andreasen NC, Ziebell S et al (2011) Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 68:128–137
Acknowledgements
This study was supported by a grant from the Carlos III Institute of Health, Cooperative Research Thematic Network (RETICS)-G03/032 and from the Spanish Ministry of Science and Innovation, CIBERSAM. Support was also given by SGR 489, and by the Alicia Koplowitz Foundation and S2010/BMD-2422 AGES.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Rapado Castro was supported by a Sara Borrell Health Research Fellowship from the Institute of Health Carlos III, Spanish Ministry of Economy and Competitiveness, an Alicia Koplowitz Research Grant, an Alicia Koplowitz Short-Term Visiting Fellowship from the Alicia Koplowitz Foundation and an IiSGM Fellowship Award for Short-Term Placements from the Health Research Institute from the Hospital Gregorio Marañon (IiSGM) (Madrid, Spain). Dr. Gonzalez-Pinto has received grants and served as consultant, advisor or CME speaker for the following entities: Almirall, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Glaxo-Smith-Kline, Janssen-Cilag, Jazz, Johnson & Johnson, Lundbeck, Merck, Otsuka, Pfizer, Sanofi-Aventis, Servier, Shering-Plough, Solvay, the Spanish Ministry of Science and Innovation (CIBERSAM), the Ministry of Science (Carlos III Institute), the Basque Government, the Stanley Medical Research Institute, and Wyeth. Dr. Graell was received honoraria or grants from Eli Lilly and Shire. Dr. Arango has been a consultant to or has received honoraria or grants from Abbot, AMGEN, AstraZeneca, Bristol-Myers Squibb, Caja Navarra, CIBERSAM, Fundación Alicia Koplowitz, Instituto de Salud Carlos III, Janssen-Cilag, Lundbeck, Merck, Ministerio de Ciencia e Innovación, Ministerio de Sanidad, Ministerio de Economía y Competitividad, Mutua Madrileña, Otsuka, Pfizer, Roche, Servier, Shire, Schering Plough and Takeda.
Ethical Standards
The study was approved by the institutional review board at each clinical center. All parents or legal guardians gave written informed consent before the study began and all patients agreed to participate.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Castro-Fornieles, J., Bargalló, N., Calvo, A. et al. Gray matter changes and cognitive predictors of 2-year follow-up abnormalities in early-onset first-episode psychosis. Eur Child Adolesc Psychiatry 27, 113–126 (2018). https://doi.org/10.1007/s00787-017-1013-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-017-1013-z