Abstract
Objectives
This study aims to investigate different treatments on new bone formation around immediate implants in the canine posterior mandible with varying sized mesial-distal gap.
Materials and methods
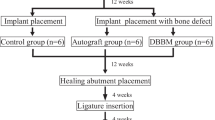
The 4th premolar and the 1st molar of six Labrador dogs were extracted from the mandible, and 4 dental implants were placed 1 mm below the level of the buccal bone crest. Moderate/large mesial-distal gaps between the implants and the sockets were treated with one of four methods and divided into the following groups: (1) the blank group, (2) the collagen membrane (CM) group, (3) the deproteinized bovine bone mineral (DBBM) group, and (4) the DBBM + CM group. Sequential fluorescent labeling was performed at 4, 8, and 10 weeks after the operation. After 12 weeks, the dogs were euthanized, and specimens were collected for micro-CT scanning and histological analysis.
Results
The survival rate of immediate implant was 100%. Micro-CT showed significant differences in bone mineral density (BMD) and bone volume fraction (BVF) among groups (P = 0.040, P = 0.009); other indicators were not significantly different among groups. Histological analysis showed the proportion of new bone formation and bone-to-implant contact were not significantly different among groups. No significant difference in bone reduction height around dental implant among four groups and varied mesial-distal gap size.
Conclusion
Owing to the restricted sample size, this pilot study lacks conclusive findings. Within the limitation, this study demonstrated that although DBBM significantly increase BMD and BVF, the use of DBBM/CM didn’t significantly improve bone formation and healing in extraction sockets around the implants in both moderate and large mesial-distal gap.
Clinical relevance
The use of deproteinized bovine bone in conjunction with collagen is a common practice in immediate implantation procedures in the posterior mandible. However, there is a lack of conclusive evidence regarding the timing and circumstances under which they should be employed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, dental implants have been widely used to restore the function and appearance of missing teeth. Conventionally, teeth are extracted beforehand and the extraction sites are allowed to heal for 3–6 months before implantation [1]. With the development of technology and the accumulation of experience, clinicians have begun to perform immediate implant placement (IIP) at both anterior and molar sites right after extraction [2]. The use of IIP has been increasingly preferred due to its advantages including reduced surgical procedures, shorter waiting times, and preservation of the alveolar ridge [3]. Besides, previous studies have demonstrated that success rates and alveolar bone loss were not significantly different between IIP and delayed implant placement in the molar region [4, 5]. These findings suggest that IIP can be a viable alternative to delayed implant placement.
However, there are various contentious issues pertaining to the procedure of IIP, particularly in molar sites. One of the most important considerations is the management of the gap between dental implants and the walls of the extraction socket, which can be also called jumping gap. Large jumping gaps can potentially lead to bone resorption and alterations in alveolar ridge contour, which was extremely important in the anterior area for aesthetic purposes. Clinicians agree that using DBBM and CM is suitable for aesthetic purposes in the anterior area. These materials have shown effectiveness in bone regeneration and repair, as well as improving the cosmetic result of dental procedures in the area [3, 6,7,8]. Collagen membranes (CM) and deproteinized bovine bone mineral (DBBM) are the most commonly used barrier membranes and bone graft materials in clinical practice. Studies have indicated that utilizing CM and DBBM alone or in combination can promote mineralized tissue formation [9]; reduce horizontal bone resorption [10]; and, particularly, preserve the contour of the alveolar ridge and the tissue thickness [11,12,13]. While numerous pre-clinical and clinical studies have delved into the parameters of jumping distances concerning dimensions, biomaterials, and treatments, the predominant focus remains on the anterior region’s jumping gap, primarily distributed bucco-lingually [14,15,16]. Conversely, there exists a dearth of scientific evidence regarding jumping gaps in the posterior area, despite notable anatomical distinctions from the anterior region. In the posterior region, the jumping gap is often substantially larger, characterized by a consistently thick and intact buccal plate that extends mesio-distally. These variations result in different sizes of gaps between the implant and the alveolar bone walls after IIP. Currently, it is common to utilize bone graft materials and/or barrier membranes to stimulate new bone growth around an implant in the posterior area. However, the use of these materials is linked to a complex surgical process, an extended healing period, and relatively high costs [10, 17]. The current literature suggests that there is insufficient data to determine whether bone graft materials and/or barrier membranes should be used to improve the success rate and patient satisfaction of IIP in the molar region. Moreover, it indicates that the presence or absence of grafting materials does not significantly affect the survival rate, success rate, or marginal bone loss [18,19,20]. ; Several recent clinical studies have reported high survival and success rates in the posterior area when no graft was used [21, 22]. These findings suggest that the use of grafting material does not enhance the clinical outcomes of IIP in the posterior area, despite its continued recommendation for this procedure.
There is ongoing debate among experts regarding the requirement of simultaneous bone grafting and barrier membrane placement for treating the jumping gap during IIP in the posterior region. This is due to a lack of rigorously designed controlled experiments that could demonstrate the effect of using bone graft materials or barrier membranes on osteogenesis in the molar region. The existing studies on IIP in the molar area are mainly based on some case reports or retrospective studies, which are more focused on the choice of operation [23], wide implants [24, 25], special surface treatment implants [26], or buccal bone wall defects [27]. Therefore, this issue remains clinically controversial and unresolved to date.
Hence, this study aimed to investigate the impact of various approaches for managing different-sized gaps and promoting osteogenesis in the extraction socket after implantation in the posterior mandible through a controlled animal experiment. The objective is to propose a viable solution to the problem mentioned above and provide guidance for clinical practice.
Materials and methods
This study was designed according to the modified Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines for preclinical in vivo experiments [28]. The research was carried out in Shanghai after receiving approval from the Medical Animal Care & Welfare Committee of Shanghai Ninth People’s Hospital affiliated with Shanghai Jiao Tong University, School of Medicine (HKDL2018225).
Animals and facilities
Six female Labrador dogs aged between 1.5 and 2 years, weighing approximately 20 kg, were used. All dogs were healthy, housed in kennels and fed a soft diet by qualified staff onsite during the entire procedure.
Experimental materials
A total of 22 cylindrical dental implants (OsseoSpeed™, Astra Tech®, Dentsply Sirona, USA; 4 mm in diameter and 10 mm long) were used. A bone substitute consisting of deproteinized bovine bone mineral (DBBM, Bio-Oss®, Geistlich, Wolhusen, Switzerland) and a non-crosslinked porcine collagen membrane (CM, Bio-Gide®, Geistlich, Wolhusen, Switzerland) was used.
Experimental design
In each hemimandible, the fourth premolar (P4) and the first molar (M1) were extracted, and implants were immediately inserted into the fresh socket. After immediate implant placement (IIP), the extraction sockets were allocated to one of the following groups. Every two sites of one hemimandible were allocated to one treatment group, which means that P4 and M1 were distributed among groups in a balanced way. As P4 and M1 in the dog is rather different in terms of volume and mesiodistal size, the groups were further divided into group with moderate gap (gap size = 2.61 ± 0.29 mm, from 2 to 2.9) and large gap (gap size = 8.91 ± 0.63 mm, from 8 to 9.7), detailed data can be obtained in Table 1. The large gap was over 8 mm, which represents the extreme circumstances occurred in immediate implantation. The sample size was determined by referring to existing similar studies [29]. G*Power version 3.1.9.7 (University Kiel, Germany. 1992–2014) was used for calculation of the sample size. The effect size d was 0.500, when the alpha (α) level was 0.05 and power was 80%, the estimated total sample size (n) should be at least 20. Considering samples dropping out, we decided to involve 6 dogs in our study (4 teeth per dog * 6 = 24).
In spite of the limited sample size in this preliminary study, various treatments were undertaken, employing the following modalities that were randomly assigned the following groups:
-
1.
Group Blank (n = 5, P4 = 3, M1 = 2), blank group.
-
2.
Group CM (n = 5, P4 = 3, M1 = 2), with CM (Bio-Gide®) only.
-
3.
Group DBBM (n = 6, P4 = 3, M1 = 3), with DBBM (Bio-Oss®) only.
-
4.
Group DBBM + CM (n = 6, P4 = 3, M1 = 3), with DBBM (Bio-Oss®) and CM (Bio-Gide®).
One of the dogs lost its both M1 in mandibular, and therefore, there were 5 implants in Group Blank and Group CM.
The order of treatments and measurements were determined randomly to minimize potential confounders.
Surgical procedures
Before commencing any surgical procedures, general anesthesia was induced in accordance with a previously established protocol [30]. In essence, this involved the intravenous administration of propofol (10 mg/ml, 0.6 ml/kg, Jiabo Pharmaceutical, Guangdong, China), followed by maintenance through a combination of N2O:O2 (1:1.5–2) and isoflurane, with endotracheal intubation serving as a facilitating measure. Local anesthesia comprising 2–4 ml of 2% lidocaine with epinephrine (Tianjin Pharmaceutical Co. Ltd, Tianjin, China) was administered at the surgical sites. The P4 and the M1 were extracted. Every tooth was extracted with minimally invasive technology, namely, separation with a cutting drill and removal with extracting forceps. The dimensions of the socket were carefully measured using a periodontal probe (Hu-Friedy®, Chicago, IL, USA) and confirmed that the walls of the socket were intact. Sharp edges of alveolar bone were trimmed away so that the septal bone was suitable for implantation. The site was prepared according to the manufacturer’s instructions. Two Ф4.0 × 11 mm cylindrical implants (OsseoSpeed™, Astra Tech®, Dentsply Sirona, USA) was placed separately into extraction sites of P4 and M1 with a torque of more than 35 Ncm. The implantation sites were located slightly lingually in the inter-septal bone, which allows an intact buccal bone plate. The average thickness of the buccal bone plate was 2.04 ± 0.27 mm (from 1.5 to 2.4 mm), which was measured at the shoulder level, detailed data can be obtained in Table S1. The implants were inserted within the interradicular septa, and the defects were positioned in a mesio-distal orientation. The shoulder of the implant was placed 1 mm below the level of the buccal bone crest, and a cover screw was installed. Each implant was placed at the septal bone. The gap size was measured between implant surface and edge of bone after implantation.
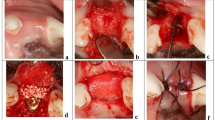
In Group Blank, after the implants were installed, no materials or CMs were placed, and blood filled the jumping gap naturally; that is, natural healing began. In Group CM, the jumping gap was not filled with any material. The mucoperiosteal flap on the lingual side was separated, and one side of a 30 mm × 40 mm Bio-Gide® membrane was inserted to cover the socket. Then, the other side of the membrane was covered with a buccal mucoperiosteal flap. In Group DBBM, an appropriate amount of Bio-Oss® was selected according to the size of the jumping gap. Large particles and the small particles were mixed at a ratio of 1:1 and moistened with saline in advance. The jumping gap was filled with Bio-Oss®, which was compacted to ensure that the jumping gap was filled with particles. Then, the mucoperiosteal flap was reset and sutured. In Group DBBM + CM, the jumping gap was filled with Bio-Oss® particles, followed by Bio-Gide®, and finally, the flaps were repositioned and sutured. Surgical photos of the different groups are shown in Fig. 1.
The surgical operation for immediate implant placement depicting (a) the fresh extraction sockets of the fourth premolar (P4) and the first molar (M1), (b) The implants were implanted slightly lingually in the septal bone. (c) Gap size between implant and bone in P4 and M1. (d) The shoulder of the implant was placed 1 mm below the level of the buccal bone crest. (e) Group Blank: the implants were implanted and left to heal spontaneously, (f) Group CM: the sockets were covered with CM alone, (g) Group DBBM: the sockets were grafted with DBBM alone, and (h) Group DBBM + CM: the sockets were grafted with DBBM and covered with CM. (i) Wounds closure with sutures
Antibiotics (penicillin sodium, 400,000 IU/day, North China Pharmaceutical, Hebei, China) were injected into the animals and anti-inflammatory drugs (Prednisolone, 0.5 mg/kg, Sine, Shanghai, China) were taken orally for 7 days after the surgery, and over the next 14 days, the dogs were fed water-softened food.
Sequential fluorescence labeling
Triple fluorescence labeling of the alveolar bone was conducted according to previous research [31]. For the next four, eight and ten weeks, animals under anesthesia were injected with three kinds of fluorochromes: tetracycline hydrochloride (TE, 25 mg/kg), calcein (CA, 20 mg/kg), and alizarin red S (AL, 30 mg/kg).
Euthanasia
After 12 weeks, the dogs were euthanized with an overdose of anesthetics, and their bone blocks were harvested for histologic analysis. Undecalcified specimens were fixed in 10% buffered formalin.
Micro-CT analysis
The specimens were scanned by a micro-CT machine (Skyscan1076, Bruker, Belgium), and the settings were as follows: source voltage (kV) = 70, source current (uA) = 141, image pixel size (µm) = 36.5200, filter = Al 1.0 mm, exposure (ms) = 110, rotation step (deg) = 0.700, and frame averaging = On (1). The data were processed with software (Microview, Scanco Medical AG). The 35-µm thick 2D slices were reconstructed into a 3D model. The region of interest (ROI) of each implant was defined following previous studies [32]. Briefly, the ROI was demarcated as having a width of 1 mm from the implant platform and a height of 3 mm along the implant thread. The ROI included the partial region of regenerated tissue in extraction socket. The biopsies were approximately aligned with the implant axes. The main characteristics used to evaluate the newly formed bone were as follows: bone volume (BV), total volume (TV), bone surface (BS), bone mineral density (BMD, representing the density of bone mineral in the bone tissue), bone volume fraction (BV/TV, representing the ratio of bone tissue volume to tissue volume, which can directly reflect the changes of bone volume), bone surface volume ratio (BS/BV, representing the area of bone tissue per unit volume), trabecular thickness (Tb.Th, representing the average trabecular bone thickness), trabecular number (Tb.N, representing the mean number of bone and nonbone tissue intersections per millimeter), and trabecular separation (Tb.Sp, representing the mean width of the cavity between bone trabeculae). The data is shown in Fig. 2.
3D reconstruction and bone reduction height measurement
The data obtained from the micro-CT scans were reconstructed using Mimics 21.0 (Materialise, USA) software. The height of the new bone in the mesiodistal sites of the implants was obtained, and is shown in Fig. 3.
Histological analysis
After fixation in formaldehyde solution, the specimen was dehydrated with a series of alcohol of increasing concentrations. Then, the specimens were placed into the embedding solution (methyl methacrylate: dibutyl phthalate = 4:1). When the resin was completely cured, the specimen was obtained. Each mesiodistal section representing the central area of the implant was prepared for biopsy. This cutting direction was determined to observe bone formation in the mesiodistal sockets around the implants. After the sections were reduced to a thickness of 20–25 μm by grinding and polishing, they were subjected to fluorescence microscopy analysis (confocal laser scanning microscopy, TCS SP8 STED 3X, Leica, Wetzlar, Germany). The number of pixels labeled with different colors in each image was used to calculate the percentage of mineralized bone in ImageJ software (National Institutes of Health, USA). After that, when Stevenel’s blue and van Gieson’s picrofuchsin staining was complete, the sections were observed under a microscope (Olympus, Tokyo, Japan). The region of regenerated area was a rectangle with a length of 40 mm and a width of 10 mm located at the edge of the implant, below the shoulder. Bone-to-implant contact (BIC) was defined as the ratio of the length of contact between the implant and bone in the area to the full length of the implant thread. The percentage of the new bone area around the implants was determined by the ratio of the new bone area to the total regenerated area.
Data analysis
The data are shown as the mean value ± standard deviation. The primary variable of this research was mesiodistal bone reduction height values. The normality of the distribution was tested by the Shapiro–Wilk test, and then, the homogeneity of variance was analyzed. For the normally distributed data that met homogeneity of variance, one-way ANOVA was further performed to assess the differences among groups. If P < 0.05, then further post hoc Tukey tests were performed to evaluate the significance. For data that did not meet the normal distribution or homogeneity of variance, the Kruskal-Wallis test was used to assess the difference among groups, and Bonferroni correction was used to correct p-values. The above statistics were calculated with SPSS 25.0 software (Chicago, IL, USA).
Results
All the sites healed uneventfully after surgery. No adverse events occurred during recovery.
3D reconstruction and bone reduction height
The mesiodistal bone reduction height values are shown in Fig. 3. The bone reduction height observed here was actually the distance from the top of the implant to the most coronal point of the alveolar ridge near the implant. This index is often measured by X-ray in clinical practice and is usually defined as marginal bone loss (MBL). The average bone reduction height value of Group Blank is -1.66 ± 0. 95 mm and − 2.18 ± 0.82 mm in moderate gap and large gap, respectively. Group CM has an average bone reduction height value of -1.38 ± 0.49 mm for cases with moderate gap, and − 1.16 ± 0.57 mm for cases with large gap. In Group DBBM, the mean bone reduction height measurement is -1.52 ± 0.97 mm and − 1.51 ± 1.25 mm for cases with moderate and large gaps, respectively. Group DBBM + CM shows an average bone reduction height measurement of -1.47 ± 0.68 mm for moderate gap cases and − 1.66 ± 1.22 mm for large gap cases. The statistical analysis showed no significant differences among the treatment groups and moderate/large gaps. More descriptive data was listed in Table S2.
Micro-CT measurements
It could be observed from the sagittal slides that in the two groups using DBBM, Group DBBM and Group DBBM + CM, the density of DBBM around the implant was relatively higher than that in the two groups without DBBM. In terms of quantitative analysis, there was a significant difference in BMD (mg/cm3) among these groups (P = 0.040). Further pairwise comparisons showed that there was a significant difference between Group CM and other groups. Although there was no significant difference between Group DBBM and Group Blank, the average BMD value of Group DBBM was comparable to that of Group DBBM + CM and higher than those of Group Blank and Group CM. The BMD value of Group Blank was equivalent to the average value of Group CM.
Regarding the BV fraction (BV/TV), Group Blank was significantly different from Group DBBM (P = 0.043) and Group DBBM + CM (P = 0.029). At the same time, Group CM was significantly different from Group DBBM (P = 0.043) and Group DBBM + CM (P = 0.029). Similar to BMD, the BVF values of Blank and C were equivalent, and the values of D and DBBM + CM were equivalent.
For the BS area to BV ratio (BS/BV), Tb.Th, Tb.N and Tb.Sp, there was no significant difference among the four groups (P > 0.05). Micro-CT measurement results are shown in Fig. 2. More descriptive data was listed in Table S3.
.
Fluorescence labeling
Sequential fluorescence staining indicated bone formation during different periods (Fig. 4). The ratio of areas with sequential fluorescent labels can represent the speed of bone formation in different periods. The fluorochrome-stained area proportion was calculated at 2, 6 and 10 weeks after surgery, and yellow, green and red represented weeks 2, 6 and 10, respectively. There was no statistically significant difference in the ratio of fluorescent staining area among the four groups at different periods, which means that during the observation period (12 weeks), different treatments had no significant effect on the rate of new bone formation. More descriptive data was listed in Table S4.
Fluorescence labeling showed newly formed bone around the implant of four groups: (a) Fluorescence labeling of Group Blank, Group CM, Group DBBM, and Group DBBM + CM. Subgroup 1 was tetracycline hydrochloride, subgroup 2 was calcein, and subgroup 3 was alizarin red. (b) The fluorochrome stained area proportion was calculated at 2, 6 and 10 weeks after surgery. Original magnification, ×40. Mean values ± standard deviation. The outline of the implant was indicated with white dotted line
Histological observations and histomorphometry
Newly formed bone was observed around all implants in the four groups (Fig. 5). In Group Blank, new bone accumulated around the coronal level of the implant, and the proportion of new bone was relatively low. The implant was supported mainly by woven bone. The outcome was similar in Group CM; woven bone accounted for a large proportion of the bone present, and the amount of lamellar bone was small. The patterns in Group DBBM and Group DBBM + CM were slightly different from those in the other two groups. The DBBM was mostly surrounded by a large amount of woven bone and accumulated apically, resulting in a dramatic increase in the stained area. It is worth noting that despite the abundance of new bone near the DBBM, the BV in the ROI seemed similar to that in the other two groups. Another noteworthy phenomenon was that a large number of DBBM particles occupied some of the space around the implants, which affected the combination of new bone and implants.
Histological analysis of the implants 12 weeks after surgery. (a) Histological section of different groups. (b) The percentage of newly formed bone area in the regenerated region. (F) The percentage of bone-to-implant contact the regenerated region. (g) The proportion of DBBM in the new bone area. Stevenel’s blue and van Gieson’s picrofuchsin staining; original magnification, ×40. Mean values ± standard deviation
The proportion of new bone was the lowest in Group Blank, 22 ± 9%, followed by 24 ± 12% in Group CM. The area of new bone in Group DBBM and Group DBBM + CM increased compared with that in the other two groups, as the proportion of new bone was 27 ± 6% (Group DBBM) and 25 ± 7% (Group DBBM + CM). However, there was no significant difference in the proportion of new bone area among the four groups (P = 0.588). For the BIC area, Group CM had the highest value at 43 ± 13%, followed by 35 ± 16% in Group DBBM; Group Blank had the lowest value at 27 ± 16%. There was no significant difference in BIC area. The residual rates of DBBM in Group DBBM and Group DBBM + CM were similar, with no significant difference. More descriptive data was listed in Table S5.
Discussion
This experiment aimed to compare the effects of using DBBM, CM, or a combination of both in the extraction sockets surrounding implants placed immediately. The present study showed that while DBBM can significantly enhance BMD and BVF, neither DBBM nor CM provided significant improvement in bone formation within the jumping gap.
Even though the proportion of new bone was not significantly different among the groups, the groups that used bone graft materials showed slightly better results than Group Blank and Group CM. This suggests that DBBM particles have the ability to maintain space and promote bone formation, which was consistent with the results of Sanz M et al [9]. The authors believed that, whether combined with CM or not, DBBM particles showed a significant ability to promote bone formation only in areas with large bone defects. It’s worth mentioning that the proportion of new bone in the Group DBBM was higher compared to the Group DBBM + CM, whereas the DBBM ratio in the Group DBBM was lower than that in Group DBBM + CM. The disparity can be attributed to the presence of barrier membrane, which elevated the proportion of DBBM and, conversely, lowered the proportion of new bone. This finding was confirmed in the study of Carmagnola D et al. [33], in which the DBBM was found to occupy a large amount of space, filling the area of potential new bone growth. This could explain why there was no statistically significant difference in the proportion of new bone among the different groups in the study. The lack of a significant difference between Group CM and Group Blank suggests that CM alone was not effective in promoting new bone formation in this type of defect. Our study findings contradict some literature on the topic. It is worth noting, however, that our study differed from previous research in terms of the research model utilized. Specifically, our study was conducted on extraction sockets with four intact bone walls, whereas prior research focused on non-contained defects with a single bone wall. This difference in methodology may have contributed to the differing results observed between our study and previous research. These findings suggest that CM may have a crucial role in single-wall defects, but in the case of fresh extraction sockets with intact bone walls, our study showed limited benefits of CM. In terms of the BIC ratio, Group CM had a relatively higher value than the others despite no significant difference. The results suggested that CM alone may be able to promote osseointegration. When the results of bone reduction height were taken into account, Group CM showed the best outcome. The relationship between BIC and marginal bone reduction height was also observed in another study by Catros et al. [34], where they found that as BIC increased, MBL decreased accordingly. Several studies have evaluated the positive role of CM in implant osseointegration [31, 35, 36], as CM can protect tooth extraction sockets and facilitate bone formation by providing a relatively stable environment for osteogenesis. Hence, CM played an irreplaceable role in the reconstruction of the extraction sockets.
However, our study found that the combination of CM with DBBM did not improve the outcome, even in cases where there were extremely large gaps between the implant and alveolar bone. Literature suggests that there is a direct relationship between the size of the gap and the distance from the bone margin to the point at which bone-implant contact (BIC) begins [37]. We have chosen a moderate gap size of approximately 2 mm based on the research of Naji BM et al. According to their findings, no bone graft is needed if the buccal bone plate remains intact [38]. Osseointegration was observed to be less effective when the gap size exceeded 2 mm, compared to smaller gaps where osseointegration occurred to a greater degree [39]. However, researches have shown comparable outcomes when treating gaps larger than 2 mm with or without bone grafts [38, 40, 41]. Our results showed that there was no significance among four treatment groups in both moderate and large gaps, even if the gap was larger than 9 mm.
Two possible reasons can be used to explain these results, one of them was that DBBM particles moved coronally, which may undermine the barrier effect of CM. This observation was also reported [13]. Due to the inherent limitations of CM, such as its susceptibility to deformation and absorption, the movement of the DBBM could deform the CM, rendering it incapable of retaining space. This speculation was also confirmed in the study of Jung UW et al. [12], where a crosslinked CM was compared with a non-crosslinked CM. The results showed that the crosslinked CM maintained its structure after 16 weeks, whereas the non-crosslinked CM had lost its integrity. Moreover, the crosslinked CM significantly promoted the formation of periosteum-like tissue. Another reason for the limited effect of DBBM and CM on osseointegration could be the considerable number of DBBM particles in the socket space, adversely affecting the blood supply around the implant and the natural healing process of the extraction socket [42], thus obstructing osseointegration. This hypothesis was based on the results of histological observation. The light pink DBBM particles accumulated around the implant, taking up space and hindering the new bone from integrating with the implant. In the three-dimensional reconstruction of the specimens, it was found that there was an annular gap around the shoulder of some implants in the groups where DBBM was used, which was confirmed in the study of Sanz M et al. [10]. The authors filled the gap with 90% DBBM and found that the bone graft failed to further promote the healing of the gap. It was found that the implant was surrounded by a soft tissue band, with a large number of DBBM particles wrapped in it. This finding was also consistent with that of Araújo M et al. [43], indicating that DBBM outside the area of new bone may be wrapped by connective tissue, hampering the gap from healing. This outcome further suggests that the presence of DBBM may impede the integration of new bone and implants, highlighting the protective role of CM. This finding may explain why the survival rate of the implant was unrelated to the grafting material and suggest that a stable space and sufficient blood supply may play a vital role in implant osseointegration.
MBL can provide an intuitive understanding of the influence of different methods. Group CM showed a subtle positive trend in accordance with the BIC results, but the difference still did not reach statistical significance, suggesting that DBBM and CM failed to improve MBL. This result was similar to that of a randomized clinical trial (RCT) study [7] and the same as that of the aforementioned systematic review [19]. According to the research of Mastrangelo F et al., inclusion of DBBM and CM did not improve MBL or probing depth but only improved the clinical aesthetic outcome and patient satisfaction. The difference between that research and this study was that the former focused on the maxillary anterior area. The systematic review by Ragucci et al. showed that bone graft materials had no significant impact on the survival rate, success rate, or MBL of implants, similar to the findings of the current study. Therefore, when the aesthetic outcome is not the primary concern, if DBBM and CM do not significantly improve bone formation in the sockets, there is no sufficient reason to recommend the use of these materials in the molar area.
This study found that using DBBM resulted in a significant increase in bone mineral density (BMD) and bone volume fraction (BVF) due to the high amount of hydroxyapatite in DBBM. However, this increase did not lead to a substantial increase in the amount or quality of new bone through histomorphometry, indicating that the increase in BMD and BVF may not improve osteogenesis. Also, DBBM and CM did not significantly improve the process and quality of trabecular bone reconstruction, as indicated by the values of Tb.N, Tb.Th, and Tb.Sp. Fluorescence labeling area, which can reflect the mineralization of new bone, did not differ significantly among the groups, suggesting that the grafting material did not accelerate bone formation around the implant.
In the context of extraction sockets of IIP in the posterior mandible, the use of DBBM was found to significantly increase the BMD and BVF around the implant. However, DBBM and CM had minimal effects on new bone formation, osseointegration, and bone quality, as concluded from the latest systematic review [19]. This is consistent with the findings of a similar study conducted by Urban T et al. [44] on indicators such as MBL and probing depth. Therefore, it is difficult to recommend a specific treatment method based on these results.
According to the current animal experiment, DBBM and CM may not have a significant impact on bone formation in the extraction socket of IIP in the posterior mandible, suggesting that there was no sufficient reason to recommend the application of these two kinds of materials in mesiodistal extraction sockets around IIP in mandibular molar sites. The presented findings offer carefully conducted experimental data, contributing to the understanding of debated issues and providing a foundation for further research in this area.
Due to the limitations of this study, it remains unknown whether loading and restoration can influence the stability of the implants. Furthermore, long-term follow-up and RCTs are still required to confirm this conclusion.
Conclusion
Owing to the restricted sample size, this pilot study lacks conclusive findings. Within the limitations of the current study, we demonstrated that although DBBM can significantly increase BMD and BVF, the use of DBBM and CM failed to show any significantly improve bone formation in the extraction sockets around the implant with both moderate and large mesial-distal gap.
References
Branemark PI (1983) Osseointegration and its experimental background. J Prosthet Dent 50:399–410
Schwartz-Arad D, Chaushu G (1997) The ways and wherefores of immediate placement of implants into fresh extraction sites: a literature review. J Periodontol 68:915–923
AlKudmani H, Al Jasser R, Andreana S (2017) Is bone graft or guided bone regeneration needed when placing Immediate Dental implants? A systematic review. Implant Dent 26:936–944. https://doi.org/10.1097/ID.0000000000000689
Ketabi M, Deporter D, Atenafu EG (2016) A systematic review of outcomes following Immediate Molar Implant Placement based on recently published studies. Clin Implant Dentistry Relat Res 18:1084–1094. https://doi.org/10.1111/cid.12390
Peñarrocha-Oltra D, Demarchi CL, Maestre-Ferrín L, Peñarrocha-Diago M (2012) Comparison of immediate and delayed implants in the maxillary molar region: a retrospective study of 123 implants. Int J Oral Maxillofac Implants 27:604–610. https://doi.org/10.4012/dmj.2011-216-e
Elaskary A, Abdelrahman H, Elsabagh HH, El-Kimary GI (2022) Does grafting the jumping gap in immediately placed anterior implants using vestibular socket therapy influence the labial bone thickness? J oral Maxillofacial Surgery: Official J Am Association Oral Maxillofacial Surg 80:1398–1407. https://doi.org/10.1016/j.joms.2022.05.001
Mastrangelo F, Gastaldi G, Vinci R, Troiano G, Tettamanti L, Gherlone E, Lo Muzio L (2018) Immediate Postextractive implants with and without bone graft: 3-year follow-up results from a Multicenter Controlled Randomized Trial. Implant Dent 27:638–645. https://doi.org/10.1097/id.0000000000000816
Gher M, Quintero G, Assad D, Monaco E, Richardson A (1994) Bone grafting and guided bone regeneration for immediate dental implants in humans. J Periodontol 65:881–891. https://doi.org/10.1902/jop.1994.65.9.881
Sanz M, Ferrantino L, Vignoletti F, de Sanctis M, Berglundh T (2017) Guided bone regeneration of non-contained mandibular buccal bone defects using deproteinized bovine bone mineral and a collagen membrane: an experimental in vivo investigation. Clin Oral Implants Res 28:1466–1476. https://doi.org/10.1111/clr.13014
Sanz M, Lindhe J, Alcaraz J, Sanz-Sanchez I, Cecchinato D (2017) The effect of placing a bone replacement graft in the gap at immediately placed implants: a randomized clinical trial. Clin Oral Implants Res 28:902–910. https://doi.org/10.1111/clr.12896
Benic GI, Thoma DS, Muñoz F, Sanz Martin I, Jung RE, Hämmerle CH (2016) Guided bone regeneration of peri-implant defects with particulated and block xenogenic bone substitutes. Clin Oral Implants Res 27:567–576. https://doi.org/10.1111/clr.12625
Jung UW, Cha JK, Vignoletti F, Nunez J, Sanz J, Sanz M (2017) Simultaneous lateral bone augmentation and implant placement using a particulated synthetic bone substitute around chronic peri-implant dehiscence defects in dogs. J Clin Periodontol 44:1172–1180. https://doi.org/10.1111/jcpe.12802
Sanz-Martin I, Ferrantino L, Vignoletti F, Nunez J, Baldini N, Duvina M, Alcaraz J, Sanz M (2018) Contour changes after guided bone regeneration of large non-contained mandibular buccal bone defects using deproteinized bovine bone mineral and a porcine-derived collagen membrane: an experimental in vivo investigation. Clin Oral Investig 22:1273–1283. https://doi.org/10.1007/s00784-017-2214-z
Xu L, Zhang S, Chen Y, Yu F, Han C, Wu D, He D (2023) The relationship between labial soft tissue changes and jumping spaces after immediate implant placement and restoration in the anterior maxilla: a prospective study. Biomolecules and Biomedicine
Naiem SN, Al-Nawas B, Tawfik OK, El-Nahass H (2023) Jumping gap in immediate implant placement in the esthetic zone: a virtual implant planning using cone-beam computed tomography. J Prosthodontic Res. https://doi.org/10.2186/jpr.JPR_D_23_00033
El Ebiary SO, Atef M, Abdelaziz MS, Khashaba M (2023) Guided immediate implant with and without using a mixture of autogenous and xeno bone grafts in the dental esthetic zone. A randomized clinical trial. BMC Res Notes 16:331. https://doi.org/10.1186/s13104-023-06612-8
Araújo MG, Linder E, Lindhe J (2011) Bio-oss collagen in the buccal gap at immediate implants: a 6-month study in the dog. Clin Oral Implants Res 22:1–8. https://doi.org/10.1111/j.1600-0501.2010.01920.x
Mohamed H, Serag Eldien A, Zahran A (2018) Augmentation versus no augmentation for Immediate Postextraction implants. Int J Dent 2018:5209108. https://doi.org/10.1155/2018/5209108
Ragucci G, Elnayef B, Criado-Cámara E, Del Amo F, Hernández-Alfaro F (2020) Immediate implant placement in molar extraction sockets: a systematic review and meta-analysis. Int J Implant Dentistry 6:40. https://doi.org/10.1186/s40729-020-00235-5
Clementini M, Tiravia L, De Risi V, Vittorini Orgeas G, Mannocci A, de Sanctis M (2015) Dimensional changes after immediate implant placement with or without simultaneous regenerative procedures: a systematic review and meta-analysis. J Clin Periodontol 42:666–677. https://doi.org/10.1111/jcpe.12423
Amato F, Polara G (2018) Immediate Implant Placement in single-tooth molar extraction sockets: a 1- to 6-Year retrospective clinical study. Int J Periodontics Restor Dent 38:495–501. https://doi.org/10.11607/prd.3179
Hattingh A, Hommez G, De Bruyn H, Huyghe M, Vandeweghe S (2018) A prospective study on ultra-wide diameter dental implants for immediate molar replacement. Clin Implant Dent Relat Res 20:1009–1015. https://doi.org/10.1111/cid.12666
Chen Z, Li J, Wang H, Yu H (2019) Initial bone volume changes after Immediate Implant Placement Associated with filling the gap using bovine bone in Molar sites. Int J Oral Maxillofac Implants 34:521–528. https://doi.org/10.11607/jomi.6750
Hattingh A, De Bruyn H, Van Weehaeghe M, Hommez G, Vandeweghe S (2020) Contour changes following Immediate Placement of Ultra-wide implants in molar extraction sockets without bone grafting. Journal of clinical medicine 9. https://doi.org/10.3390/jcm9082504
Tallarico M, Xhanari E, Pisano M, Gatti F, Meloni S (2017) Molar replacement with 7 mm-wide diameter implants: to place the implant immediately or to wait 4 months after socket preservation? 1 year after loading results from a randomised controlled trial. Eur J Oral Implantol 10:169–178
Guarnieri R, Di Nardo D, Gaimari G, Miccoli G and L T (2018) One-stage laser-microtextured implants immediately placed in the inter-radicular septum of molar fresh extraction sockets associated with GBR technique. A case series study. J Clin Experimental Dentistry 10:e996–e1002
Hu C, Gong T, Lin W, Yuan Q, Man Y (2017) Immediate implant placement into posterior sockets with or without buccal bone dehiscence defects: a retrospective cohort study. J Dent 65:95–100. https://doi.org/10.1016/j.jdent.2017.07.010
Vignoletti F, Abrahamsson I (2012) Quality of reporting of experimental research in implant dentistry. Critical aspects in design, outcome assessment and model validation. J Clin Periodontology null 6–27. https://doi.org/10.1111/j.1600-051X.2011.01830.x
Jung UW, Cha JK, Vignoletti F, Nuñez J, Sanz J, Sanz M (2017) Simultaneous lateral bone augmentation and implant placement using a particulated synthetic bone substitute around chronic peri-implant dehiscence defects in dogs. J Clin Periodontol 44:1172–1180. https://doi.org/10.1111/jcpe.12802
Almohandes A, Abrahamsson I, Dionigi C, Berglundh T (2022) Surgical treatment of experimental peri-implantitis using mechanical and chemical decontamination procedures: a pre-clinical in vivo study. J Clin Periodontol 49:518–525. https://doi.org/10.1111/jcpe.13607
Lyu C, Shao Z, Zou D, Lu J (2020) Ridge alterations following Socket Preservation using a Collagen Membrane in Dogs. Biomed Res Int 2020:1487681. https://doi.org/10.1155/2020/1487681
Paeng K-W, Cha J-K, Thoma DS, Jung RE, Jung U-W, Benic GI (2022) Effect of collagen membrane and of bone substitute on lateral bone augmentation with titanium mesh: an experimental in vivo study. Clin Oral Implants Res 33:413–423. https://doi.org/10.1111/clr.13901
Carmagnola D, Berglundh T, Araújo M, Albrektsson T, Lindhe J (2000) Bone healing around implants placed in a jaw defect augmented with Bio-oss. An experimental study in dogs. J Clin Periodontol 27:799–805. https://doi.org/10.1034/j.1600-051x.2000.027011799.x
Catros S, Sandgren R, Pippenger B, Fricain J, Herber V, El Chaar E (2020) A Novel Xenograft Bone Substitute supports stable bone formation in Circumferential defects around Dental implants in Minipigs. Int J Oral Maxillofac Implants 35:1122–1131. https://doi.org/10.11607/jomi.8265
Masaki C, Nakamoto T, Mukaibo T, Kondo Y, Hosokawa R (2015) Strategies for alveolar ridge reconstruction and preservation for implant therapy. J Prosthodontic Res 59:220–228. https://doi.org/10.1016/j.jpor.2015.04.005
Guarnieri R, Stefanelli L, De Angelis F, Mencio F, Pompa G, Di Carlo S (2017) Extraction socket preservation using porcine-derived collagen membrane alone or Associated with Porcine-derived bone. Clinical results of Randomized Controlled Study. J oral Maxillofacial Res 8:e5. https://doi.org/10.5037/jomr.2017.8305
Greenstein G, Cavallaro J (2013) Managing the buccal gap and plate of bone: immediate dental implant placement. Dent Today 32:70 72 – 7; quiz 78 – 9
Naji BM, Abdelsameaa SS, Alqutaibi AY, Said Ahmed WM (2021) Immediate dental implant placement with a horizontal gap more than two millimetres: a randomized clinical trial. Int J Oral Maxillofac Surg 50:683–690. https://doi.org/10.1016/j.ijom.2020.08.015
Wilson TG Jr., Schenk R, Buser D, Cochran D (1998) Implants placed in immediate extraction sites: a report of histologic and histometric analyses of human biopsies. Int J Oral Maxillofac Implants 13:333–341
Tarnow DP, Chu SJ (2011) Human histologic verification of osseointegration of an immediate implant placed into a fresh extraction socket with excessive gap distance without primary flap closure, graft, or membrane: a case report. Int J Periodontics Restor Dent 31:515–521
Wilson TG Jr., Carnio J, Schenk R, Cochran D (2003) Immediate implants covered with connective tissue membranes: human biopsies. J Periodontol 74:402–409. https://doi.org/10.1902/jop.2003.74.3.402
Morjaria K, Wilson R, Palmer R (2014) Bone healing after tooth extraction with or without an intervention: a systematic review of randomized controlled trials. Clin Implant Dent Relat Res 16:1–20. https://doi.org/10.1111/j.1708-8208.2012.00450.x
Araújo M, Linder E, Wennström J, Lindhe J (2008) The influence of Bio-oss Collagen on healing of an extraction socket: an experimental study in the dog. Int J Periodontics Restor Dent 28:123–135
Urban T, Kostopoulos L, Wenzel A (2012) Immediate implant placement in molar regions: a 12-month prospective, randomized follow-up study. Clin Oral Implants Res 23:1389–1397. https://doi.org/10.1111/j.1600-0501.2011.02319.x
Funding
This study was supported by the National Natural Science Foundation of China (32171347, 92368111), CAMS Innovation Fund for Medical Sciences (CIFMS) (2019-I2M-5-037), the Foundation of Leading Talents from Shanghai Health Commission (2022XD038), Training Programme for Research Physicians in Innovation and Transformation from shanghai hospital development center (SHDC2022CRD002), Institute of Biomaterials and Regenerative Medicine Joint Research Project from shanghai Jiao Tong University School of Medicine (2022LHA04), Crossover Research Fund Project from Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (JYJC202012), Clinical Research Booster Project from Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (JYLJ202102), Medicine Biological Sample Project from Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University School (YBKB202210), Original Exploration Project Funding of Shanghai Ninth People’s Hospital (JYYC007).
Author information
Authors and Affiliations
Contributions
Author contributions:Yiwen Zhang and Jing Wu were responsible for data collection and analysis; Yiwen Zhang, Jing Wu and Qiutong Yang guided the writing of the manuscript; Yong Zhou and Mohan Wang were responsible for the surgical approach; Zhiyuan Zhang and Duohong Zou were responsible for the critical revisions of the paper and conceived the concept for the study.
Corresponding author
Ethics declarations
Ethics approval
The research was carried out in Shanghai after receiving approval from the Medical Animal Care & Welfare Committee of Shanghai Ninth People’s Hospital affiliated with Shanghai Jiao Tong University, School of Medicine (HKDL2018225).
Consent to participate
Not Applicable.
Conflict of interest
The authors declare that they have no conflicts of interest regarding the present study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Wu, J., Yang, Q. et al. Bone formation in large/moderate gap after immediate implantation in response to different treatments: a pre-clinical study in the canine posterior mandible. Clin Oral Invest 28, 181 (2024). https://doi.org/10.1007/s00784-024-05559-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-024-05559-9