Abstract
Objectives
Conducting a scoping review (SR) to assess scientific evidence for topical simvastatin’s impact on alveolar bone regeneration and determine its level of support for clinical applications.
Materials and methods
This SR followed the PRISMA-ScR and OSF registries protocol; systematic searching was conducted on MEDLINE/PubMed, Cochrane, Embase, Scopus, Web of Science, and LILACS, to identify relevant articles until June 2023. Inclusion criteria covered clinical trials, case series, prospective and retrospective studies, along with in vivo investigations, involving participants of any sex and age.
Results
Out of 1312 identified studies, 20 (9 in vivo, 11 RCTs) met inclusion criteria. RCTs focused on third molar extraction, in vivo on mandibular incisor surgery. The majority of RCTs employed a collagen sponge and a simvastatin concentration of 10mg; conversely, most in vivo studies favored polylactide-co-glycolide and a 2 mg simvastatin concentration. RCTs had 3-month follow-ups; in vivo, studies extended to 8 weeks. Seven RCTs assessed pain outcomes, simvastatin did not significantly affect pain in six studies. Among four RCTs on postoperative swelling, only two observed a significant increase in the simvastatin group. In general, positive bone formation and the absence of adverse effects directly linked to topical simvastatin were observed across the study models.
Conclusions
Intra-alveolar simvastatin post-tooth extraction has been to be shown to be effective and safe for preserving alveolar bone, with varied concentrations and carriers, with no significant adverse effects.
Clinical relevance
This review provides critical insights into the effects of simvastatin on alveolar bone regeneration, informing potential benefits and possible challenges associated with its post-extraction application.
OSF Registry protocol
osf.io/q3bnf
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After tooth loss, alveolar bone healing begins, which triggers a series of cellular events that can lead to tissue atrophy. Alveolar bone remodeling, also known as residual ridge resorption (RRR), is considered chronic, cumulative, progressive, and irreversible [1]. The dimensional reduction resulting from RRR affects both the height and thickness, and its degree, extent, and severity may be related to specific individual factors. The lack of adequate bone structure can result in various functional and esthetic consequences, as well as limit the possibility of dental rehabilitation, whether through prostheses or osseointegrated implants [2, 3]. Although the techniques and materials used to correct or prevent bone defects in the alveolar region are constantly evolving, they may still be associated with unpredictable and challenging outcomes, high costs, and morbidity [3, 4].
Bone preservation can be achieved through the stimulation of specific and organized biological events through the mechanisms of osteogenesis, osteoconduction, and osteoinduction [5]. There are various materials available to be applied directly into the socket after tooth extraction, ranging from the use of autogenous bone, bone substitutes, growth factors, and stem cells to the use of drugs with osteoinductive properties, such as statins [6]. Statins are a class of drugs used to combat hypercholesterolemia and prevent cardiovascular diseases, acting by reducing levels of cholesterol and lipoproteins in the blood and liver through the inhibition of an enzyme that produces mevalonate and isoprenoid compounds. Cholesterol is present in the composition of cell plasma membranes and participates in the formation of steroid hormones, bile production, and vitamin D synthesis. Additionally, it is an essential component of animal cell signaling pathways, making the action of statins pluripotent or pleiotropic [2, 7].
Simvastatin is the most studied statin and is a semi-synthetic analog of lovastatin, which is obtained from the fermentation of an ascomycete fungus called Aspergillus terreus [7]. Studies conducted by Mundy et al. in the mid-1990s, both in vitro and in vivo, suggested that statins could influence bone metabolism through the increase in genetic expression of Bone Morphogenetic Protein (BMP-2) [8]. The action of simvastatin on osteoinduction is attributed to its ability to increase the production of BMP-2 and vascular endothelial growth factor (VEGF), suppress osteoclasts, promote the differentiation of undifferentiated mesenchymal cells into osteoblasts, reduce inflammation, and stimulate neoangiogenesis [9, 10]. Simvastatin has the potential to influence bone regeneration both through oral and topical administration. However, positive results with systemic administration were associated with high doses for long periods of follow-up [11, 12]. Due to high hepatoselectivity in first-pass metabolism, and low affinity to bone, oral administration has been shown to be ineffective and potentially toxic. Simvastatin appears to be 50 times more effective in promoting bone regeneration when used topically [2, 13].
Different interceptive therapies to attenuate post-extraction alveolar ridge resorption have been proposed. Although the effectiveness of alveolar ridge preservation has been demonstrated in comparison to unassisted healing, a specific alveolar ridge preservation approach that patently and predictably renders superior outcomes has yet to be identified. Advances in biotechnology in the field of bone regeneration have sought viable alternatives to minimize the costs and morbidity of alveolar reconstruction procedures [14, 15]. In this context, simvastatin has been studied as an alternative for the reconstruction and maintenance of the dimensional characteristics of the alveolar bone. The objective of this work is to conduct a comprehensive review of the existing evidence on the effect of simvastatin, applied topically, on the process of alveolar bone regeneration in different animal models and in humans after tooth extraction. This SR synthesizes the existing literature on the impact of simvastatin topical use on alveolar bone after tooth extraction (study design). Here, we summarize the current evidence on the effect of topical application of simvastatin in extraction sockets (intervention) on alveolar bone preservation and reduction in bone loss (outcome) from animals or humans undergoing tooth extraction (population), compared to standard post-extraction care without simvastatin application (comparison).
Material and methods
Protocol and registration
This scoping review (SR) adopted the procedures described in the EQUATOR Network website and followed the PRISMA-ScR (PRISMA Extension for Scoping Reviews) [16]. This section was structured according to the “five steps” methodology proposed by Arksey and O’Malley [17] and enhanced by Levac et al. [18] ensuring methodological rigor and transparency in the review process. The SR protocol was registered in the OSF (Open Science Framework) database (osf.io/q3bnf).
-
Step 1: Identifying the research question
Focused question
Based on the PICOS framework:
-
Population (P): animals or humans undergoing tooth extraction
-
Intervention (I): topical application of simvastatin in extraction sockets
-
Comparator (C): standard post-extraction care without simvastatin application
-
Outcome of interest (O): alveolar bone preservation and reduction in bone loss
-
Study Design (S): a scoping review synthesizing existing literature on the impact of simvastatin topical use on alveolar bone after tooth extraction, this SR aimed to answer the focused questions: What effects of local application of simvastatin in alveolar repair after dental extraction have been reported in the literature?
-
Step 2: Identifying relevant studies (Table 1)
Information sources
An extensive literature search was performed among six electronic databases, namely MEDLINE through PubMed (http://www.ncbi.nlm.nih.gov/sites/pubmed), Web of Science—WoS (https://www.webofknowledge.com) accessed through the Clarivate Analytics (https://clarivate.com), Cochrane Central Register of Controlled Trials (CENTRAL) (https://www.cochranelibrary.com), Embase (https://www.embase.com) and Scopus (http://www.scopus.com) through Elsevier (https://www.elsevier.com), and LILACS via VHL (https://bvsalud.org). Other sources (grey literature) were consulted through Google Scholar (https://scholar.google.com.br) and System for Information on Grey Literature in Europe (SIGLE) through OpenGrey (https://easy.dans.knaw.nl/ui/datasets/id/easy-dataset:200362/tab/2) databases. The protocol registration databases ClinicalTrials.gov, the Brazilian Registry of Clinical Trials (ReBEC) (https://ensaiosclinicos.gov.br), PROSPERO (https://www.crd.york.ac.uk/prospero/), and OSF (https://osf.io/wbfde/) were also assessed. Handsearch was performed in specialized periodicals (International Journal of Oral and Maxillofacial Surgery; Journal of Oral and Maxillofacial Surgery; Journal of Cranio-Maxillofacial Surgery; and Clinical Oral Investigations) and in reference lists of selected articles. Experts were identified using expertscape.com (https://expertscape.com) and contacted for other data sources.
Search strategy
Database search strategies included MeSH terms, entry terms, and keywords to query in PubMed, Web of Science, Cochrane Library, other sources (grey literature), and protocol registries. The search strategies for Embase, Scopus, and LILACS databases added Emtree, Index, and DeCS/MeSH terms, respectively. All terms were combined by the Boolean operators “OR” and “AND” connecting the key concepts in a “building blocks” strategy (Table 2). The electronic searches were performed in June 2023. Database alerts are set to identify studies published after the time of the searches, until the manuscript submission process.
No restrictions were placed on the language or date of publication when searching the electronic databases.
-
Step 3: Study selection
Selection of sources of evidence
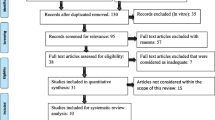
The retrieved articles were exported to Endnote® Web (www.myendnoteweb.com), and duplicates were removed by the program. A 2-phase selection process was conducted by two reviewers independently of each other (conventional double-screening): Phase 1, reviewers JAD and KGS examined the titles and abstracts of all references, applying the inclusion criteria; and in Phase 2, both reviewers applied the exclusion criteria in the full-text screening—full texts were evaluated and judged in the entire document. Inter-reviewer reliability in the study selection process was determined by the Cohen κ test, assuming an acceptable threshold value of 0.8 [19]. The disagreement at any stage was resolved by discussion and mutual decision with a third reviewer (DSB). The final decision was always based on the full-text reading. For more details on reasons for exclusion, see Fig. 1.
-
Step 4: Charting the data
Data charting process
The article screening process is depicted in the PRISMA flow diagram (Fig. 1). Any discrepancies between the researchers were discussed and resolved by consensus during team meetings. The main conclusions were extracted from the data and presented in the form of a narrative synthesis, with proper reference to the original studies.
-
Step 5: Collating, summarizing, and reporting the results
Data items
Data were independently extracted by reviewers JAD and KGS in a consensus meeting using a standardized form. After collecting and analyzing the data, the information was organized and presented as follows:
-
Descriptive data—numerical description of the general characteristics of the participants in the included studies and the dental groups undergoing exodontia; details about the type of vehicle used for topical application of the drug, along with the concentration used; and description of the instruments used for bone evaluation
-
Primary outcomes—pain; edema; bone measurement; and postoperative analgesics
-
Secondary outcomes—follow-up and adverse effects; risk of bias; and evidence level. Literature data were classified by study design and level of evidence according to the OCEBM (Oxford Center for Evidence-Based Medicine) 2011 [20], as presented in the text and tables
The qualitative data synthesis followed the synthesis without meta-analysis (SWiM) reporting guideline [21].
Study risk of bias assessment
Two reviewers (JAD and KGS) independently evaluated the quality of primary studies using the SYRCLE’s risk of bias tool for animal studies [22]. This tool is based on the Cochrane RoB tool and has been adjusted for aspects of bias that play a specific role in animal intervention studies. SYRCLE’s is structured into a fixed set of 10 domains of bias: sequence generation, baseline characteristics, allocation concealment, random housing––blinding, random outcome assessment––blinding incomplete outcome data, selective outcome reporting, and other sources of bias. These entries are related to six types of bias: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. A “yes” judgment indicates a low risk of bias; a “no” judgment indicates a high risk of bias; the judgment will be “unclear” if insufficient details have been reported to assess the risk of bias properly (Fig. 2).
Two reviewers (JAD and KGS) independently evaluated the quality of the included clinical trial studies by using the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) [23]. RoB 2 is structured into a fixed set of domains of bias, focussing on different aspects of trial design, conduct, and reporting, evaluating parameters that may be related to effect sizes and constrain internal validity. Two reviewers blindly classified the risk of bias according to the RoB 2 algorithm into low risk of bias, some concerns, or high risk of bias (Fig. 3).
When necessary, the risk of bias was reached in a consensus meeting with a third reviewer (DSB).
Results
Study selection
At the end of all searches, a total of 1312 studies were obtained (MEDLINE|PubMed, 326; Web of Science, 270; CENTRAL Cochrane, 55; Embase, 131; Scopus, 522; LILACS, 3; and other sources, 5). After removing 561 duplicates, we screened 754 articles through titles and abstracts (Phase 1). The full-text reading (Phase 2, n=28) confirmed the inclusion of 20 studies (9 experimental studies [in vivo] [1, 24,25,26,27,28,29,30,31] and 11 randomized controlled trials [RCTs] [2,3,4, 7, 32,33,34,35,36,37,38]. The selected publications ranged from the year 2005 to 2022. The inter-reviewer reliability was 0.91. For more details on the selection process, see Fig. 1.
Experimental (in vivo) studies
A total of 431 animals were included in the experimental studies, of which 413 were Wistar rats [1, 24, 25, 27,28,29,30,31] (393 males [95.1%] and in 20 rats [31]; the gender was not specified), and 18 were rabbits [26] (gender was not specified). Eight studies were designed as parallel-arm [1, 24,25,26,27,28,29,30] and only one a single-arm (split-mouth) [31] method. Most studies were published by Japanese researchers (N, 4) [24,25,26,27], followed by Chinese (N=3) [1, 28, 29], Iranian (N=1) [30], and Egyptian (N=1) [31]. The average age of Wistar rats ranged from 7 to 8 weeks (N=2) [28, 29] and up to 10 weeks (N=3) [24, 25, 27]. Three studies did not report the age of rats [1, 30, 31]. The average age of the rabbits was 20 weeks [26].
RCTs
A total of 226 patients were included in the RCTs [2, 4, 7, 32, 33, 35, 36] (69 males [30.5%] and 94 females [41.6%]; male-to-female ratio of 1:1.36). Three RCTs [3, 37, 38] did not report the gender of another 63 patients. The age range of the patients varied from 18 to 40 years. Seven RCTs [2,3,4, 7, 32, 33, 37] adopted the split-mouth design, while four [34,35,36, 38] used parallel groups. Most studies were published by Indian researchers (N=4) [4, 7, 32, 37], followed by Brazilian (N=3) [2, 3, 34], Egyptian (N=2) [36, 38], Iranian (N=1) [33], and Iraqi (N=1) [35].
Extracted tooth cluster
Regarding the experimental (in vivo) studies, the most reported experimental surgeries were as follows: (i) Wistar rats—right mandibular incisor extraction (N=5 studies and 225 teeth) [1, 24, 27,28,29], removal of both right and left mandibular incisors (N=1 study and 96 teeth) [25], bilateral mandibular first molar extraction (N=1 study and 40 teeth) [31] and left maxillary incisor extraction (n=1 study and 72 teeth) [30]—and (ii) rabbits—removal of both right and left mandibular incisors (N=1 study and 36 teeth) [26] (Supplementary Files 1).
The majority of RCTs (N=5) [2, 4, 7, 35, 37] used lower third molar extraction as the study model, totaling 258 surgeries, followed by lower first premolar surgeries (N=3 studies and 55 extractions) [32, 34, 38], lower first molar (N=2 studies and 41 extractions) [34, 36], upper first premolar extractions (N=1 study and 30 extractions) [32], and upper third molars (N=1 study and 26 extractions) [3] (Supplementary Files 2).
Carrier vehicles (delivery system) used for simvastatin
A variety of carrier vehicles was used in experimental (in vivo) studies, of which polylactide-coglycolide (PLGA) was the most used (N=3) [25, 28, 29]. Biomaterials such as freeze-dried collagen/calcium sulfate (FDC/CS) (N=1) [24], α-tricalcium phosphate (α-TCP) (N=1) [27], chitosan gel (N=1) [31], microsphere hydrogel (N=1) [1], gelatin hydrogel (N=1) [26], and freeze-dried bone allograft (FDBA) (N=1) [30] were also used (Supplementary Files 1).
Collagen sponge was the most commonly carried vehicle reported in the majority of RCTs (N= 8) [2, 4, 7, 32, 33, 35,36,37]. Other vehicles included poly (D,L-lactide-co-glycolide) (N=1) [3], methylcellulose (N=1) [34], and Nanobone® (Artoss Co, Germany) (N=1). Nanobone® consisted of synthetic nanocrystalline hydroxyapatite and silica fabricated in a sol/gel process [38] (Supplementary Files 2) (Fig. 4).
Simvastatin concentration
Simvastatin concentrations varied significantly among experimental (in vivo) studies. Regarding experiments using rats, Sato et al. used 2 mg in three out of six experimental groups [24]. Nishimura et al. employed concentrations of 0.1, 0.25, 0.5, 1, 2, and 4 mg simvastatin in six out of eight groups [25]. In another study, concentrations of 0.25, 0.5, and 1 mg simvastatin were used in three out of five rat groups [27]. Sherif et al. used 2.5% simvastatin in all groups [31]. In Li et al. [1], a concentration of 0.01g simvastatin was used in two out of three groups, while Abdi et al. [30] employed a concentration of 0.5% in two out of four groups. In only two studies, the concentration of simvastatin used was not clearly reported [28, 29]. In the rabbit study, simvastatin concentrations varied among 1, 10, and 67 μg in three out of six groups [26] (Supplementary Files 1).
The clinical trials reported different concentrations of simvastatin such as 10 mg (N=6) [4, 7, 32, 35,36,37], 20 mg (N=2) [2, 33], 80 mg (N=1) [38], 1.2% (N=1) [34], and 2% (N=1) [3] (Supplementary Files 2).
Methods of evaluation and major effects on bone formation
Experimental (in vivo) studies
Bone formation evaluation was assessed through soft X-ray examination plus histological analysis (N= 2) [1, 28]. Two studies used only histological analysis [29, 30]. Another two studies used soft X-ray radiography and bone mineral content (BMC) [24, 25]. Only one study used bone mineral content (BMC) and histological analyses [27]. Another one study used height and width measured using a bone caliper [31]. In the single study involving rabbits, soft X-ray radiography plus histological analysis were utilized [26].
The results varied according to the groups, evaluation times, concentration of simvastatin, and the carrier vehicle used. In the study by Sato et al. (2005) [24], a positive effect on bone formation was observed when simvastatin was associated with calcium sulfate, but no significant effect was found in groups that used simvastatin alone or in combination with freeze-dried collagen. Wu et al. (2008) [28] demonstrated significant bone formation in groups that received simvastatin after 4, 8, and 12 weeks, as evidenced by both imaging examinations and histomorphometric findings. Nishimura et al. [25] found that all groups treated with simvastatin showed significant bone formation compared to the control group or the group that received only PLGA. Liu et al. [27] observed a positive influence of simvastatin on bone formation, and the authors suggested that this occurred due to increased expression of growth factors such as TGF-β1, BMP-2 mRNA, and VEGF mRNA. Maruo et al. [27] reported statistically significant results in groups that used simvastatin at concentrations of 0.5 and 1 mg, both after 4 weeks and 8 weeks postoperatively, evaluating bone formation through histomorphometric examinations and micro-computed tomography. Sherif et al. [31] reported positive results in terms of width and height of bone formation after 4 weeks, but negative results after 1, 2, and 3 weeks. Li et al. [1] found significant results in the follow-up groups at 5 and 8 weeks postoperatively. In Abdi et al. [30], significant differences in the rate of osteogenesis were observed between the intervention groups after 5 and 8 weeks. In the rabbit study conducted by Tanigo et al. [26], the only group that showed a significant increase in bone formation (5 weeks of follow-up) was the one that used hydrogel gelatin with simvastatin micelles at a concentration of 10 μg.
RCTs
Cone-beam computed tomography (CBCT) was the primary tool chosen to evaluate bone formation (N=6) [2,3,4, 34,35,36]. Generally, bone density was assessed through software tools. In four studies [7, 32, 37, 38], bone formation was evaluated using the grayscale generated by software from intraoral radiograph histograms (IOPA). One study used histomorphometric analysis to determine the presence of live and dead bone, as well as trabecular, amorphous, and non-osteoblastic types [33]. Multiple evaluation methods for bone formation were reported among two RCTs. One of them used IOPA and CBCT [7], while the other used IOPA and histomorphometry [38].
Regarding the effects on bone formation, the majority of RCTs suggest favorable positive effects of topical use of simvastatin post-extraction (N=9) [2, 4, 7, 32, 34,35,36,37,38]. However, the other two RCTs did not find evidence of a positive influence of simvastatin on bone formation—one of these studies employed CBCT examinations for analysis [3], while the other used the histomorphometric method [33].
Methods of evaluation and effects on pain and swelling among RCTs
Seven RCTs evaluated the pain outcome [2, 4, 7, 34,35,36,37]. The 10-point visual analog scale (VAS) was the most widely used (N=6) [2, 4, 7, 34,35,36], while the 5-point VAS was employed in only one study [37]. The rescue analgesic varied considerably among the studies, with the most common ones being potassium diclofenac (N=1) [37], ibuprofen 400mg + paracetamol (N=1) [32], paracetamol 500 mg + codeine 30 mg (N=1) [3], ibuprofen 400 mg (N=1) [34], and paracetamol 750 mg (N=1) [2]. Only one study did not specify which NSAID was used as rescue medication [7] (Supplementary Files 2).
A total of six RCTs demonstrated no significant differences in pain when comparing the use of simvastatin to the control group [4, 7, 34,35,36,37]. Only one study reported statistically significant pain in the simvastatin group, observed at almost all evaluated time points in the study [2].
Four RCTs assessed postoperative swelling as the measurement of the horizontal distance between the corner of the mouth and the ear lobe, along with the vertical distance between the outer corner of the eye and the angle of the lower jaw [2, 4, 7, 37]. Data were primarily obtained preoperatively (baseline) and on the first, third, and seventh days after surgery.
Significant occurrence of postoperative swelling was reported in two studies [2, 4]. One of them reported a statistically greater increase in swelling in the intervention group on the third postoperative day, but this difference was not significant on the seventh postoperative day [2]. In the other study, no differences were found between the groups that received simvastatin and the control groups [4].
Follow-up
Regarding the experimental (in vivo) studies, the maximum follow-up period was 12 weeks (N=1) [28], followed by 8 weeks (N=4) [1, 25, 27, 30], 5 weeks (N=1 [rabbit study]) [26], and 4 weeks (N=3) [24, 29, 31] (Supplementary Files 1).
The longest follow-up period among RCTs was 6 months (N=1) [4]. In general, the most commonly employed period was 3 months (N= 7) [2, 3, 7, 34, 35, 37, 38], followed by 4 months (N=2) [32, 36], 2 months (N=1) [33], and 6 months (N=1) [4] (Supplementary Files 2).
Adverse effects and costs
No adverse effects were reported and directly attributed to the topical use of simvastatin in any of the laboratory (in vivo) or in the RCTs included in this review. However, only two RCTs [2, 34] conducted an analysis of the alveolar healing index (Landry Index), assessed 7 days postoperatively. One of the studies [2] signaled significant alterations in this clinical parameter, highlighting the need for further investigations into safety and potential undesirable reactions, especially those that may directly impact post-extraction alveolar healing.
In the realm of expenses, a recent study investigated the cost-effectiveness relationship of conventional grafts employed in alveolar bone preservation. The results of this study suggested that approaches incorporating allograft and xenograft are linked to elevated expenses [15]. In this context, the administration of simvastatin, recognized for its osteoinductive potential, emerges as a cost-effective alternative without compromising the overall efficacy of the treatment. However, a meticulous economic analysis is essential to evaluate the financial viability of intra-alveolar simvastatin application compared to conventional approaches. This encompasses not only the direct cost of the medication but also factors such as administration materials, additional office visits, and potential long-term savings associated with optimizing bone healing.
Evidence level
Based on the classification provided by the Oxford Center for Evidence-Based Medicine (OCEBM, 2011) [20], the data summarized in this scoping review was categorized in all included studies (Supplementary files 1 and 2) (Fig. 5).
Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence [20]. The studies were classified into two tiers: Level 2, comprising randomized trials or observational studies with a substantial effect, and Level 5, based on mechanism-based reasoning
Discussion
Evidence level
To the best of our knowledge, this represents the first SR regarding the influence of topical application of simvastatin on alveolar bone formation following tooth extraction. Among the 20 articles [1,2,3,4, 7, 24,25,26,27,28,29,30,31,32,33,34,35,36,37,38] selected for this review, diverse methodological frameworks were evident, consequently resulting in varying levels of evidence. The in vivo studies stood out as the most heterogeneous, as they encompassed different animal species, extraction of distinct dental groupings, varying concentrations, and carriers for simvastatin, in addition to employing distinct tools for assessing bone formation. Although the RCTs exhibited less heterogeneity, discrepancies arose in terms of the approach to evaluating bone formation, the specific dental groupings subject to extraction, as well as the concentration and vehicle used to deliver simvastatin.
The preclinical nature and challenges in the methodological design of in vivo experimental studies limit their level of evidence compared to RCTs. However, they are fundamental in understanding mechanistic insights and testing the efficacy, toxicity, and viability of new substances for therapeutic purposes. The preclinical studies (N=9) [1, 24,25,26,27,28,29,30,31] included in this review highlighted crucial parameters that attest to promising effects regarding tissue tolerance, safety, low toxicity, and positive impacts on bone formation through topical application of simvastatin.
RCTs provide a higher level of evidence due to their clinical nature, easiness in methodological design, variable control, and the potential to extrapolate results to other human beings. Nonetheless, the 11 selected RCTs [2,3,4, 7, 32,33,34,35,36,37,38] exhibit substantial heterogeneity, rendering a more comprehensive compilation and interpretation of data unfeasible within a systematic review with meta-analysis aimed at reliably addressing a clinical question.
Surgery on the right mandibular incisors was the most frequent procedure among the in vivo studies [1, 24, 27,28,29]. Experimental studies predominantly utilized a parallel-group design [1, 24,25,26,27,28,29,30], with the exception of a single split-mouth study [31]. Among the RCTs, the primary surgical procedure was the extraction of third molars [2,3,4, 7, 35, 37], and the split-mouth study design was more commonly employed [2,3,4, 7, 32, 33, 37]. Split-mouth studies provide a model with lower interindividual variability for evaluating bone formation and other associated clinical parameters, such as pain and swelling, as they allow for the comparison of two distinct interventions within the same individual. Furthermore, the choice of dental groupings in the various studies within this review took into account factors like convenience, procedural indications, recruitment speed, ease of reproducibility, and the ability to study bone formation parameters through predictable and consistent models.
Effect on bone regeneration
Autogenous grafts are still considered the gold standard in maxillofacial bone reconstructions. However, a singular therapeutic approach for the effective and predictable treatment of extraction sockets has not been identified yet [15]. The high morbidity, increased risk of postoperative complications, high costs, and unpredictability in the resorption rate of autogenous grafts have led to the need for the development of new materials with better characteristics [37]. In the initial phases of the healing process, the forming tissues are fragile and intricate structures which can be easily disturbed by external events. In this context, the use of simvastatin with the aim of speeding up the formation of mature tissues can be considered a minimally invasive approach. The osteoinductive potential of statins was first described by Mundy et al. in 1999 [8]. The focus of this study was simvastatin because it is the most commonly used and extensively studied statin in inducing bone formation. The literature suggests that lipophilic statins such as simvastatin, fluvastatin, lovastatin, and mevastatin are the ones that can exert the most anabolic effect on bone tissue. This is explained by the increased expression of markers for osteogenesis in osteoblasts (BMP-2, osteocalcin, osteopontin, alkaline phosphatase, mRNA for collagen type I and III) and in endothelial progenitor cells that produce vascular endothelial growth factor (VEGF) [9, 10]. The results from Liu et al. in 2009 corroborate the existing literature, demonstrating a notable elevation in the same markers through in situ hybridization in the tooth sockets of rats subjected to the topical application of PLGA + simvastatin at 1, 2, and 4 weeks post-extraction [29].
In this study, a significant portion of the evidence regarding the influence of simvastatin on bone formation was generated through imaging examinations and/or histomorphometric analysis. In the experimental studies, the primary methods for analyzing bone formation were soft X-ray radiography [1, 24, 25, 28], bone mineral content (BMC) [24, 25], and histological analysis [1, 27,28,29,30], while RCTs predominantly used IOPA [7, 32, 37, 38] and/or CBCT [2,3,4, 34,35,36]. The results of this review suggest a positive response to simvastatin in bone formation in the majority of the included studies. However, the results obtained in the in vivo studies were more heterogeneous and were influenced by variables such as evaluation time, simvastatin concentration, and the selected vehicle. In the study by Sato et al., simvastatin only showed a positive response when combined with calcium sulfate [24]. Wu et al. found positive effects on bone tissue with simvastatin combined with PLGA compared to PLGA alone at 4, 8, and 12 weeks [28]. Similarly, using histomorphometric analysis, Liu et al. found statistically significant differences in bone tissue formation in groups subjected to topical application of simvastatin combined with PLGA compared to the group subjected to PLGA alone [29]. In the study by Nishimura et al., both simvastatin combined with PLGA and PLGA alone showed positive results in bone formation [25]. The study by Maruo et al. reported positive results with the combination of 0.5 mg and 1 mg simvastatin with α-TCP compared to groups using α-TCP alone in post-extraction sockets [27]. Similarly, Sherif et al. combined 2.5% simvastatin with chitosan gel and compared it to rats that received only chitosan gel in post-extraction sockets, finding positive effects after 4 weeks [31]. In a similar vein, Li et al. used a microsphere formulation of simvastatin with hydrogel and compared it to hydrogel without simvastatin, obtaining statistically significant differences favoring the simvastatin group after 5 and 8 weeks of follow-up [1]. In the Abdi et al. study, simvastatin combined with FDBA graft was more effective in forming bone than FDBA alone after 8 weeks postoperatively [30].
Among the RCTs included in this SR, more methodologically homogeneous studies were found, with a concentration of 10 mg of simvastatin and collagen sponge as the most frequent [4, 7, 32, 35,36,37]. The results favored the simvastatin group in 9 RCTs regarding bone formation [2, 4, 7, 32, 34,35,36,37,38]. However, 2 RCTs did not find evidence of simvastatin’s influence on bone formation [3, 33]. The concentration of simvastatin and the vehicle used in the Sezavar et al. [33] study resembled that of Diniz et al. [2], but the results differed. Despite both studies using 20 mg of simvastatin combined with collagen sponge in post-extraction sockets, the differences in the results found may be related to the small sample size in Sezavar et al. [33] study, differences in methods of evaluating bone formation, the comparator group used, and the follow-up period.
Influence of the vehicle and simvastatin concentration on osteoinduction
The ability of simvastatin to influence bone metabolism appears to be dose-dependent and vehicle-dependent. Simvastatin concentration proves to be a fundamental and critical parameter to consider when the goal is to stimulate bone formation. High doses of simvastatin are associated with increased osteogenesis, osteoclastogenesis (osteolysis), and inflammation simultaneously, whereas low doses promote decreased bone formation and increased bone resorption [39]. The greatest variation in simvastatin concentration occurred in vivo studies, ranging from 0.1 mg to 0.01g in rat studies [1, 25], and from 1 to 67 μg in the rabbit study [26]. In clinical studies involving humans, simvastatin concentration ranged from 10 [4, 7, 32, 35,36,37] to 80 mg [38], with the 10 mg concentration being the most commonly used. Despite the wide range of simvastatin concentrations employed in the different study models included in this review, no negative effects regarding increased local inflammation or induction of osteolysis directly attributed to the presence of the drug were described at any of the concentrations used, even at concentrations as high as 80 mg. There is uncertainty as to whether the effects of simvastatin truly depend on the dosage or if the optimal effect is linked to a specific dosage [2, 7]. In this review, the majority of included studies showed evidence of bone formation across a diverse range of concentrations, spanning from the lowest to the highest [1, 2, 4, 7, 24,25,26,27,28,29,30,31,32, 34,35,36,37,38].
Simvastatin is not metabolized through proteolytic processes by tissue enzymes. It is a lipophilic drug and demonstrates good tolerance when used topically. However, to achieve a more effective clinical outcome and minimize the risk of inflammation, a vehicle that promotes slow and gradual deposition of the substance is necessary [7]. Prolonging the release period of simvastatin, facilitated by the carrier’s action, offers the advantage of enhancing the drug’s effect in different stages of bone formation. This includes modulating inflammation, inducing osteoblast differentiation, and controlling osteoclast action. Despite the wide variety of materials used as vehicles for simvastatin, most have biodegradation rates of at most 2 to 3 months [40]. Taking into account the biodegradation rate of the selected vehicle and the differences in bone turnover among the types of participants in the study models included in this review, the maximum follow-up periods varied significantly. The most commonly used were 12 weeks for rats [28], 5 weeks for rabbits [26], and 3 months for humans [2, 3, 7, 34, 35, 37, 38].
Tissue engineering has enabled the development of a variety of materials that can be used as carriers for simvastatin, such as scaffolds [24, 25, 27,28,29,30,31], microspheres [1], and hydrogels [26, 34], or even a combination of these [41]. Depending on the chosen type of carrier, the combination of carrier and simvastatin can either be directly injected at the site in liquid form or surgically implanted in solid form. Liquid carriers, like hydrogels, are cross-linked systems diluted in water, consisting of a continuous or outer phase made up of solid constituents, and a discontinuous or inner phase composed of liquid elements. The advantages of this type of carrier include good tissue tolerance, ease of manipulation, the potential to be mixed with bioactive substances and injected at the desired location, as well as its ability to spread and fill irregular spaces. Liquid carriers appear to have good applicability in situations involving small bone defects where less invasive procedures are needed [41]. Only four studies selected in this study used liquid carriers, three of which were in vivo studies [1, 26, 31] and one RCT [34]. Solid carriers have the advantage of promoting cell retention and migration, enabling the slow and gradual release of the associated bioactive substance, functioning as a framework that can exert biological and mechanical influences on osteoblast differentiation, and they may also promote the healing process when incorporated into newly formed bone tissue [40]. In this SR, ten clinical studies involving humans utilized solid carriers for simvastatin delivery [2,3,4, 7, 32, 33, 35,36,37,38].
Carriers can be manufactured from a variety of materials and, based on their composition, are categorized as either natural or synthetic, permanent, or biodegradable. Natural materials have the advantage of being more biocompatible but tend to be more readily absorbed [40]. While synthetic materials are less biocompatible and less biodegradable, they have greater commercial availability and facilitate more controlled rates of simvastatin release and matrix biodegradation [42]. The majority of materials used as carriers in the studies selected in this review, including those conducted in humans and in vivo, can be categorized as natural, such as freeze-dried collagen/calcium sulfate [24], tricalcium phosphate-α-TCP [27], chitosan gel [31], microspheres hydrogel [1], FDBA [30], gelatin hydrogel [26], collagen sponge [2, 4, 7, 32, 33, 35,36,37], Nanobone® [38], and methylcellulose [34]. In contrast, other materials can be considered synthetic, like poly [3] and PLGA [25, 28, 29]. However, efforts are underway to develop hybrid structures in order to minimize the drawbacks of both synthetic and natural materials.
Effects on pain and swelling
Pain and swelling were clinical parameters assessed in only 7 out of the 11 RCTs included in this review [2, 4, 7, 34,35,36,37]. The most performed surgery in articles evaluating pain was the extraction of mandibular third molars (N=5) [2, 4, 7, 35, 37], followed by extraction of first molars and first premolars (N=1) [34], and exclusively first molars extraction (N=1) [36]. The majority of studies assessed pain and edema using lower third molar surgery as a model, as these are symptoms commonly associated with the postoperative period of this type of surgery. Surgical trauma tends to range from moderate to intense, involving the compromise of both soft and hard tissues of the alveolus. The inflammatory response triggers the production and release of algogenic substances responsible for the characteristic pain and edema in these surgeries [43, 44]. Simvastatin may act by reducing inflammation due to its effects on inhibiting enzymes that degrade tissues, such as matrix metalloproteinases (MMPs) [45]. Evaluation of these signs and symptoms is crucial as a clinical indicator to assess tissue tolerance, safety, and acceptability of topical simvastatin use after tooth extraction by patients. The majority of studies did not find statistically significant effects of the simvastatin group compared to the control group (N=6) [4, 7, 34,35,36,37]. The only study in which there was a positive influence of the simvastatin group on pain was that of Diniz et al. The authors suggested that the absence of a collagen sponge in the control group could be a possible explanation for this difference in pain perception [2]. However, in the study by Chauhan et al., there was no positive influence of the simvastatin group, despite also not using a collagen sponge in the control group and having a similar sample size [37]. Nevertheless, it is important to note that the difference in findings of these studies may be related to the variation in the concentration of simvastatin used, as Diniz et al. employed twice the concentration [2].
Swelling was assessed in only 4 out of the 11 included RCTs in this study [2, 4, 7, 37]. Although the methods and assessment timings were quite similar across the articles, there was a discrepancy in the obtained results. Two RCTs identified significant signs of swelling increase in the group that received simvastatin [2, 4]. The study by Deepanjali et al. [4] employed a methodology very similar to that of Degala et al. [7] in terms of simvastatin concentration, vehicle, swelling assessment method, and dental group extracted. However, the results were distinct. The peak of post-operative edema related to third molar extraction can persist for up to 72 h, making this a crucial period for patient evaluation [46]. Deepanjali et al. observed a significant increase in swelling in the simvastatin group as early as the first post-operative day, but not on the third or seventh day [4]. Conversely, the study by Degala et al. assessed swelling on the first and seventh postoperative days, without identifying significant differences between the groups [7]. In the study by Diniz et al., the swelling was evaluated on the third and seventh postoperative days, with significance observed in the simvastatin group only on the third day [2].
Future perspectives
A singular therapeutic approach for the effective and predictable treatment of extraction sockets has not been identified yet. The methodological discrepancies led to few studies using the same protocol of simvastatin [15]. Although evidence points to promising clinical applications of topical simvastatin, many questions still need to be answered before its widespread use. Currently, there is no vehicle capable of providing controlled, uniform, and continuous release of simvastatin. A significant portion of the drug undergoes degradation by local enzymes or is diluted and incorporated into the circulatory system. Additionally, simvastatin exhibits low affinity and limited selectivity for target cells, which may reduce its effectiveness and increase toxicity. Therefore, the development of new technologies, such as antibody-drug conjugation, could enhance selectivity and optimize the osteogenic and anti-osteoclastogenic effects on the cells of interest, thereby reducing potential undesirable toxic effects [40].
A recent study developed polyacid and biphasic ceramic scaffolds embedding simvastatin (PLGA + HA/βTCP + SIM) with the aim of achieving a more controlled release of simvastatin. This approach also leverages the combined benefits of the individual characteristics of the materials used in the composition of the carrier, including the release of phosphate and calcium ions from the biphasic ceramics, along with enhanced compression resistance and improved degradation rates. The polymeric scaffolds within the carrier facilitate the incorporation of the bioactive substance for osteoinduction. Tests conducted in the study by Sordi et al. on stem cells from human exfoliated deciduous teeth using PLGA + HA/βTCP + SIM demonstrated promising results, as they stimulated alkaline phosphatase activity and increased the levels of calcium, osteocalcin, and osteonectin proteins [47].
In vivo studies suggest a promising use of topically applied simvastatin on the surface of titanium implants to enhance surface treatment and osseointegration. However, crucial questions persist, such as determining the optimal concentration and the need for more substantial evidence regarding the effects of implant osseointegration in humans [48]. Recently, a combination of a spongy xenogeneic scaffold loaded with simvastatin was employed in the reconstruction of severe alveolar horizontal defects, showing promising results. The authors compared this technique to conventional guided bone regeneration using xenogenic bone graft plus collagenous membrane and observed significantly greater bone production in the group with the spongy xenogeneic scaffold loaded with simvastatin [49].
Currently, grafts such as allogeneic and xenogeneic are widely employed in alveolar preservation, acting as osteoconductive structural supports [15, 37]. However, Barootchi et al.’s study suggests that despite a low complication rate, conventional grafts may not fully eliminate the need for additional grafting in dental implant surgeries, potentially leading to the presence of large amounts of non-integrated bone graft particles at the time of surgical reentry, lack of primary stability, and implant osseointegration failure.Additionally, allogeneic or xenogeneic alveolar ridge preservation is associated with higher costs, although it shows better performance compared to the use of alloplastic grafts or spontaneous healing [15]. Comparatively, the results of this review suggest that simvastatin also exhibits good tissue tolerance, and its action stands out for directly influencing the biological processes of bone formation, unlike conventional grafts that focus on creating a conducive environment for regeneration. Simvastatin, with its osteoinductive action, offers an innovative perspective, while conventional grafts remain a reliable choice for providing structural support in bone regeneration. New tissue engineering techniques, combining the effects of a vehicle with osteoconductive properties with the effects of a bioactive substance with osteoinductive properties, such as simvastatin, have the potential to significantly optimize treatments in the field of bone reconstruction associated or not with implant dentistry and maxillofacial surgery. While the use of simvastatin for bone reconstruction purposes may be on the horizon, it is important to consider that future research will face the challenge of establishing reliable parameters for practical application in a clinical setting. This includes determining the most effective concentration, the ideal vehicle, and the follow-up time for achieving optimal results without exposing patients to additional risks. In addition, no studies addressed aspects of the learning curve or the cost–benefit ratio of the minimally invasive approaches, such as simvastatin. We also emphasize the need for well-conducted randomized clinical studies, with larger sample sizes (adequately powered) and longer follow-up periods, as well as the possibility of multicenter studies to precisely define the effects of topical application in various indications in Dentistry.
Conclusions
Intra-alveolar simvastatin application after tooth extraction has demonstrated effectiveness and safety in alveolar bone preservation, utilizing various concentrations and carrier vehicles, with no notable adverse effects. However, there are significant limitations in the studies that still do not allow for conclusive and error-free recommendations for the topical use of simvastatin in dentistry. The results presented in this SR exhibit conflicting data regarding dosage, duration of drug delivery, and test subject species (human, rat, and rabbit); some studies have small sample sizes, limited post-operative follow-up time, and diverse study designs, and therefore, they need to be analyzed and interpreted with caution.
References
Li X, Liu X, Ni S, Liu Y, Sun H, Lin Q (2019) Enhanced osteogenic healing process of rat tooth sockets using a novel simvastatin-loaded injectable microsphere-hydrogel system. J Craniomaxillofac Surg 47:1147–1154. https://doi.org/10.1016/j.jcms.2019.04.011
Diniz JA, Barbirato DS, Nascimento EHL, Pontual AA, Dourado ACAG, Laureano Filho JR (2022) Tomographic evaluation of the effect of simvastatin topical use on alveolar bone microarchitecture, pain and swelling after mandibular third molar extraction: a randomized controlled trial. Clin Oral Investig 26:3533–3545. https://doi.org/10.1007/s00784-021-04322-8
Oliveira MN, Rau LH, Marodin A, Corrêa M, Corrêa LR, Aragones A, Magini RS (2017) Ridge preservation after maxillary third molar extraction using 30% porosity PLGA/HA/β-TCP scaffolds with and without simvastatin: a pilot randomized controlled clinical trial. Implant Dent 26:832–840. https://doi.org/10.1097/ID.0000000000000655
Deepanjali M, Prasad TS, Manodh P (2022) Efficacy of simvastatin in bone regeneration after surgical removal of mandibular third molars. Oral Maxillofac Surg. https://doi.org/10.1007/s10006-022-01081-y
Srinivas B, Das P, Rana MM, Qureshi AQ, Vaidya KC, Raziuddin SJA (2018) Wound healing and bone regeneration in postextraction sockets with and without platelet-rich fibrin. Ann Maxillofac Surg 8:28–34. https://doi.org/10.4103/ams.ams_153_17
Gupta S, Del Fabbro M, Chang J (2019) The impact of simvastatin intervention on the healing of bone, soft tissue, and TMJ cartilage in dentistry: a systematic review and meta-analysis. Int J Implant Dent 5:17–27. https://doi.org/10.1186/s40729-019-0168-4
Degala S, Bathija NA (2018) Evaluation of the efficacy of simvastatin in bone regeneration after surgical removal of bilaterally impacted third molars-a split-mouth randomized clinical trial. J Oral Maxillofac Surg 76:1847–1858. https://doi.org/10.1016/j.joms.2018.04.035
Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G (1999) Stimulation of bone formation in vitro and in rodents by statins. Science 286:1946–1949. https://doi.org/10.1126/science.286.5446.1946
Wang Z, Li Y, Zhou F, Piao Z, Hao J (2016) Effects of statins on bone mineral density and fracture risk. Medicine 95:1–8. https://doi.org/10.1097/MD.0000000000003042
Griffiths SL, Cartmell SH (2012) Use of statins for enhancing bone-tissue-engineered grafts. Eur J Plast Surg 35:151–157. https://doi.org/10.1007/s00238-007-0190-2
Tan J, Yang N, Fu X, Cui Y, Guo Q, Ma T, Yin X, Leng H, Song C (2015) Single-dose local simvastatin injection improves implant fixation via increased angiogenesis and bone formation in an ovariectomized rat model. Med Sci Monit 21:1428–1439. https://doi.org/10.12659/MSM.892247
Yin H, Li J, Yu X, Fu Z (2011) Effects of simvastatin on osseointegration in a canine total hip arthroplasty model: an experimental study. J Arthroplast 26:1534–1539. https://doi.org/10.1016/j.arth.2010.10.008
Gutierrez GE, Lalka D, Garrett IR, Rossini G, Mundy GR (2006) Transdermal application of lovastatin to rats causes profound increases in bone formation and plasma concentrations. Osteoporos Int 17:1033–1042. https://doi.org/10.1007/s00198-006-0079-0
Pradeep AR, Priyanka N, Kalra N, Naik SB, Singh SP, Martande S (2012) Clinical efficacy of subgingivally delivered 1.2-mg simvastatin in the treatment of individuals with class II furcation defects: a randomized controlled clinical trial. J Periodontol 83:1472–1479. https://doi.org/10.11607/prd.2936
Barootchi S, Tavelli L, Majzoub J, Stefanini M, Wang HL (2000) Avila-Ortiz G (2023) Alveolar ridge preservation: complications and cost-effectiveness. Periodontology 92:235–262. https://doi.org/10.1111/prd.12469
Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C et al (2018) PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 169:467–473. https://doi.org/10.7326/M18-0850
Arksey H, O’Malley L (2005) Scoping studies: towards a methodological framework. Int J Soc Res Methodol 1:19–32. https://doi.org/10.1080/1364557032000119616
Levac D, Colquhoun H, O'Brien KK (2010) Scoping studies: advancing the methodology. Implement Sci 5:69. https://doi.org/10.1186/1748-5908-5-69
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174. https://doi.org/10.2307/2529310
Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, Moschetti I, Phillips B, Thornton H (2011) Explanation of the 2011 oxford centre for evidence-based medicine (OCEBM) levels of evidence (background document). Oxford centre for evidence-based medicine. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/explanation-of-the-2011-ocebm-levels-of-evidence/. Accessed 26 Jun 2023
Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, Hartmann-Boyce J, Ryan R, Shepperd S, Thomas J, Welch V, Thomson H (2020) Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 368:l6890. https://doi.org/10.1136/bmj.l6890
Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14:43. https://doi.org/10.1186/1471-2288-14-43
Sterne J, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clin Res Eed) 366:l4898. https://doi.org/10.1136/bmj.l4898
Sato D, Nishimura K, Ishioka T, Kondo H, Kuroda S, Kasugai S (2005) Local application of simvastatin to rat iincisor socket: carrier-dependent effect on bone augmentation. J Oral Tissue Engin 2:81–85. https://doi.org/10.11223/jarde.2.81
Nishimura K (2008) Local application of simvastatin to rat incisor sockets augments bone. Kokubyo Gakkai Zasshi 75:49–54. https://doi.org/10.5357/koubyou.75.49
Tanigo T, Takaoka R, Tabata Y (2010) Sustained release of water-insoluble simvastatin from biodegradable hydrogel augments bone regeneration. J Control Release 143:201–206. https://doi.org/10.1016/j.jconrel.2009.12.027
Maruo K, Sato D, Machida T, Kasugai S (2010) Effects of alpha-tricalcium phosphate containing simvastatin on alveolar ridge augmentation. J Oral Tissue Engin 7:143–152. https://doi.org/10.11223/jarde.7.143
Wu Z, Liu C, Zang G, Sun H (2008) The effect of simvastatin on remodelling of the alveolar bone following tooth extraction. Int J Oral Maxillofac Surg 37:170–176. https://doi.org/10.1016/j.ijom.2007.06.018
Liu C, Wu Z, Sun HC (2009) The effect of simvastatin on mRNA expression of transforming growth factor-beta1, bone morphogenetic protein-2 and vascular endothelial growth factor in tooth extraction socket. Int J Oral Sci 1:90–98. https://doi.org/10.4248/ijos.08011
Abdi Z, Azizian M, Abbasi N, Darabi A, Abdal K (2021) Socket preservation using freeze-dried bone allograft with and without simvastatin in rats. J Mazandaran Univ Med Sci 31:1–10
Sherif Y, El Masry N, Karam S, Nasra M (2016) Evaluation of local administration of simvastatin on height and width of the healing extraction socket in rat mandible. Alex Dent J 41:283–286. https://doi.org/10.21608/adjalexu.2016.58040
Saifi AM, Giraddi GB, Ahmed N (2017) Healing of extraction socket following local application of simvastatin: a split mouth prospective study. J Oral Biol Craniofacial Res 7:106–112. https://doi.org/10.1016/j.jobcr.2017.04.001
Sezavar M, Bohlouli B, Farhadi S, Tabatabaee S, Latifi R (2018) Simvastatin effects on dental socket quality: a comparative study. Contemp Clin Dent 9:55–59. https://doi.org/10.4103/ccd.ccd_719_17
Cruz R, Moraschini V, Calasans-Maia MD, de Almeida DCF, Sartoretto SC, Granjeiro JM (2021) Clinical efficacy of simvastatin gel combined with polypropylene membrane on the healing of extraction sockets: a triple-blind, randomized clinical trial. Clin Oral Implants Res 32:711–720. https://doi.org/10.1111/clr.13740
Mahdi HS, Sahar SH, Al-Adili (2021) Efficacy of simvastatin and gel foam combination local application on bone density, after surgical removal of mandibular third molars. J Res Med Dent Sci 9:472–477
Abu sheehah H, Hosny A, El Mohandes W (2022) Evaluation of simvastatin efficacy on bone regeneration for socket preservation. Al-Azhar J Dent Sci 25:43–48. https://doi.org/10.21608/ajdsm.2020.44274.1119
Chauhan AS, Maria A, Managutti A (2015) Efficacy of simvastatin in bone regeneration after surgical removal of mandibular third molars: a clinical pilot study. J Maxillofac Oral Surg 14:578–585. https://doi.org/10.1007/s12663-014-0697-6
Soliman O, Hussein A, Gobran H (2020) Evaluation and comparison of histologic changes in extraction sites grafted with simvastatin mixed with nanobone. Egypt J Oral and Maxillofac Surg 11:111–117. https://doi.org/10.21608/omx.2020.39319.1081
Maritz FJ, Conradie MM, Hulley PA, Gopal R, Hough S (2001) Effect of statins on bone mineral density and bone histomorphometry in rodents. Arterioscler Thromb Vasc Biol 10:1636–1641. https://doi.org/10.1161/hq1001.097781
Oryan A, Alidadi S, Moshiri A, Maffulli N (2014) Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res 9:18. https://doi.org/10.1186/1749-799X-9-18
Shuid AN, Ibrahim N, Mohd Amin MC, Mohamed IN (2013) Drug delivery systems for prevention and treatment of osteoporotic fracture. Curr Drug Targets 14:1558–1564. https://doi.org/10.2174/1389450114666131108153905
Liu X, Holzwarth JM, Ma PX (2012) Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromol Biosci 12:911–919. https://doi.org/10.1002/mabi.201100466
Lima CAA, Favarini VT, Torres AM, da Silva RA, Sato RFL (2017) Oral dexamethasone decreases postoperative pain, swelling, and trismus more than diclofenac following third molar removal : a randomized controlled clinical trial. Oral Maxillofac Surg 21:321–326. https://doi.org/10.1007/s10006-017-0635-0
Oryan A, Kamali A, Moshiri A (2015) Potential mechanisms and applications of statins on osteogenesis: current modalities, conflicts and future directions. J Control Release 215:12–24. https://doi.org/10.1016/j.jconrel.2015.07.022
Leung BP, Sattar N, Crilly A, Prach M, Carey M, Payne DW, Madhok R, Campbell C, Gracie AJ, Liew FY, McInnes IB (2003) A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol 170:1524–1530. https://doi.org/10.4049/jimmunol.170.3.1524
Ritto FG, Pimentel T, Canellas JVS, Junger B, Cruz M, Medeiros PJ (2019) Randomized double-blind clinical trial evaluation of bone healing after third molar surgery with the use of leukocyte and platelet-rich fibrin. Int J Oral Maxillofac Surg 48:1088–1093. https://doi.org/10.1016/j.ijom.2019.01.020
Sordi MB, Curtarelli RB, Mantovani IF, Moreira AC, Fernandes CP, Cruz ACC, Magini RS (2021) Enhanced osteoinductive capacity of poly(lactic-co-glycolic) acid and biphasic ceramic scaffolds by embedding simvastatin. Clin Oral Investig 26:2693–2701. https://doi.org/10.1007/s00784-021-04240-9
Sendyk DI, Deboni MC, Pannuti CM, Naclério-Homem MG, Wennerberg A (2016) The influence of statins on osseointegration: a systematic review of animal model studies. J Oral Rehabil 43:873–882. https://doi.org/10.1111/joor.12438
Esmaeili V, Boostani H, Ahmadpour F (2023) Efficacy of spongy xenogeneic scaffold loaded with simvastatin in the treatment of severe alveolar horizontal defect: a clinical and histological study. Niger J Clin Pract 26:369–375. https://doi.org/10.4103/njcp.njcp_86_22
Acknowledgements
The authors would like to express their gratitude to the University of Pernambuco and the research team for their invaluable support.
Author information
Authors and Affiliations
Contributions
All authors contributed and have equal responsibility for the design and execution of the research, analysis, and interpretation of the results and writing of the article. JAD: conceptualization, research design, methodology development, data curation, compilation of tables and figures, initial draft composition, thorough review and editing. KGS: conceptualization, research design, data curation, initial draft composition, thorough review and editing. DSB: conceptualization, research design, methodology development, data curation, compilation of tables and figures, initial draft composition, thorough review and editing. RJHV: research design, methodology development, initial draft composition. ACAGD: conceptualization, research design, methodology development, data curation, initial draft composition. JRLF: conceptualization, research design, methodology development, data curation, compilation of tables and figures, initial draft composition, thorough review and editing. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Diniz, J.A., Dourado, A.C.A.G., Barbirato, D.d. et al. Effect of simvastatin topical use on alveolar bone after tooth extraction: a scoping review. Clin Oral Invest 28, 86 (2024). https://doi.org/10.1007/s00784-023-05482-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-023-05482-5