Abstract
Background and objective
The resorption of alveolar ridge bone and maxillary sinus pneumatization are challenges to implant-supported prosthetic rehabilitation. Bone regeneration using bone substitutes and growth factors are alternatives for maxillary sinus augmentation (MSA). Therefore, we sought to evaluate the effects of the association between leukocyte and platelet–rich fibrin (L-PRF) and deproteinized bovine bone mineral (DBBM) in MSA procedures.
Materials and methods
Thirty-six maxillary sinuses from 24 individuals were included in this randomized clinical trial. The maxillary sinuses were randomly grafted with LPRF and DBBM (test group) or grafted only with DBBM (positive control). Dental implants were installed in the test group following two periods of evaluation: after 4 (DBBM+LPRF4) and 8 (DBBM+LPFR8) months of sinus graft healing, while the control group received implants only after 8 months. Cone beam computed tomography (CBCT) was taken 1 week after surgery (T1) and before implant placement (T2). Bone samples were collected during implant placement for histomorphometric and immunohistochemical (IHC) analysis. The primary implant stability was assessed by resonance frequency analysis.
Results
CBCT analysis demonstrated a significant decrease in bone volume from T1 to T2 in all groups without differences among them. Histologically, the test group showed significantly increase in bone neoformation in both periods of evaluation (LPRF+DBBM4: 44.70±14.01%; LPRF+DBBM8: 46.56±12.25%) compared to the control group (32.34±9.49%). The control group showed the highest percentage of residual graft. IHC analysis showed increased staining intensity of osteocalcin (OCN), vascular endothelial growth factor (VEGF), and runt related transcription factor 2 (RUNX-2) in LPRF+DBBM4 group, and osteopontin (OPN) in the L-PRF+DBBM8. Primary implant stability was successfully achieved (above 60 in implant stability quotient) in all the evaluated groups.
Conclusion
Combination of L-PRF and DBBM increased and accelerated new bone formation allowing early implant placement probably due to the higher protein expression of RUNX2, VEGF, OCN, and OPN. These data suggest that the use of L-PRF might be an interesting alternative to use in combination with DBBM for augment the maxillary sinuses allowing the installation of appropriate length implants in shorter period of time.
Clinical relevance
This study showed improvement in bone neoformation and accelerated healing when associating L-PRF and DBBM for maxillary sinus augmentation procedures.
Trial registration
This study was registered before participant recruitment in Brazilian Registry of Clinical Trials (ReBEC - RBR-95m73t).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone graft procedures in maxillary sinuses are a common approach to rehabilitate patients with atrophic jaws with dental implants. This is especially true in the posterior region of the maxilla in consequence of pneumatization of the maxillary sinus and to the progressive resorption of the alveolar crest ridge [1]. In 1986, Tatum et al. [2] developed a technique for accessing the maxillary sinus lateral wall, enabling the elevation of the sinus floor followed by implant placement concomitantly or not with bone substitutes or autogenous bone. The technique involves the creation of a bone window in the anterior wall of the sinus to allow the elevation of the Schneider membrane. The achieved space is filled with a bone graft or growth factors, which leads to an increase in bone height.
The maxillary sinus is considered a favorable cavity for bone neoformation due to the osteogenic potential of the Schneiderian membrane [3,4,5,6]. Despite that, some authors claim that the blood clots alone in the maxillary sinus do not guarantee favorable bone neoformation, maintaining the implant surrounded by connective tissue, without direct bone-to-implant contact [5, 7]. Therefore, bone grafts have been associated with the sinus lifting procedures [8,9,10]. Autogenous bone has been considered the gold standard for bone regeneration in maxillary sinus due to its osteogenic properties. However, autogenous bone possesses some disadvantages, such as the need for a second surgical site, increased treatment time, limited amount of bone, and more patient discomfort. Consequently, the use of bone substitutes is warranted. Among them, DBBM has been extensively used in clinical practice and in pre-clinical studies due to its slow resorption rate and osteoconductive properties [8, 11,12,13,14,15]. Additionally, the use of DBBM overcomes some of the limitations of autogenous graft, such as postoperative morbidity and the reduced amount of bone availability [8, 9, 16]. On the other hand, the lack of osteoinductive and osteogenic properties [17] and increased repair period constitute major disadvantages of DBBM [18].
Therefore, growth factors have been investigated in association with DBBM to accelerate bone graft maturation and enhance bone neoformation [10, 19,20,21,22]. L-PRF is a platelet derivate that contains innumerous cytokines and growth factors. It is obtained by centrifuging the collected blood from the patient without adding anticoagulants [23]. The use of L-PRF in surgical procedures directly influences tissue healing and decreases the risk of infectious and hemorrhagic complications [19]. However, despite these favorable results, the literature is still divergent regarding the influence of L-PRF on bone tissue healing [10, 22, 24].
A recent systematic review of the regenerative potential of L-PRF concluded that there is moderate evidence that L-PRF acts beneficially in the preservation of the bone crest and the initial phase of osseointegration [25]. Regarding the use of L-PRF in maxillary sinus floor elevation, there are conflicting data in the literature due to the small number of existing randomized clinical studies and the different methodologies applied among the studies [24, 26, 27]. While some studies report positive effects of L-PRF on new bone formation and bone graft healing [10, 19, 21, 22, 24, 28, 29], other studies have shown no additional benefits of L-PRF in combination with bone biomaterials [26, 27]. In order to clarify such inconsistences found in the literature, we have designed this study with the main objective of investigating the potential of L-PRF combined with DBBM to allow early implant placement due to the possible favorable regenerative potential of the growth factor. To achieve this goal, the maxillary sinuses were rehabilitated with dental implants 4 months after maxillary sinus augmentation, which is half of the proposed normal healing period for this procedure. This approach combined with the proposed analysis (immunohistochemistry of bone markers), allowed investigating the regenerative capacity of this growth factor based on the expression of bone formation markers, which is the differential of our studies. Therefore, this randomized controlled clinical trial aimed to evaluate the beneficial effect of adding L-PRF to DBBM for improving bone regeneration after maxillary sinus augmentation. Our hypothesis is that L-PRF could reduce the healing time for implant placement and increase the amount of newly formed bone when used in combination with DBBM.
Materials and methods
Study design
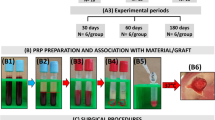
The participants of this randomized clinical trial were recruited in the Implantology clinic at the School of Dentistry at Araraquara, Brazil. Twenty-four patients who sought oral rehabilitation using implant-supported prostheses, in need for maxillary sinus augmentation to install the implants were selected. Patients included were partially or totally edentulous with residual height of the alveolar ridge less than or equal to 4 mm (confirmed using CBCT) and were older than 18 years. Patients with some health impairment, uncontrolled diabetic patients, smokers or ex-smokers, alcohol and/or drug users, pregnant women, patients with hematological disorders, patients undergoing radiotherapy treatment in the head and neck region, and bisphosphonate users and patients with pathologies in the maxillary sinus were not included.
A total of 36 maxillary sinuses, referring to 24 patients, were randomly allocated into 3 groups (n = 12/group). In the positive control group, the elevated sinus cavities were grafted only with DBBM (Bio-Oss®, Geistlich Pharma AG®, Switzerland) and the healing period until the implant placement was 8 months. The other groups were grafted with a mixture of L-PRF and DBBM and the healing periods until the implant placement were 4 (LPRF+DBBM4) and 8 (LPRF+ DBBM8) months after graft procedure (supplemental fig. 1). This study was approved by the Human Research Ethics Committee of the School of Dentistry at Araraquara (CAAE #41357514.5.0000.5416) and was performed in accordance with the principles stated in the Declaration of Helsinki in 1964. Furthermore, this clinical trial was registered before participant recruitment in Brazilian Registry of Clinical Trials (ReBEC - RBR-95m73t). All patients were informed about the objectives of the study and spontaneously agreed to participate by signing the Free and Informed Consent Form.
The number of samples for each group was determined using a statistical software (Bioestat 5.3®, Instituto Mamirauá, Belém, PA, Brazil) and was based on data from previous studies [22]. The mean difference in the percentage of bone neoformation (primary outcome) was set as 15% with a standard deviation of 11%. Considering a power test of 80%, and a significance level of 5%, a number of 12 samples per group were considered.
Technique to obtain the L-PRF
Before the surgical procedure, peripheral blood samples were collected from all the patients. Blood withdrawn was performed using the vacuum collection system - vacutainer (BD Vacutainer® Systems - NJ, USA). Four glass tubes with a capacity of 10 mL were collected. The tubes were immediately centrifuged with a 400G force (Kasvi K14-0815, Curitiba, PR, Brazil) according to the protocol proposed by Dohan et al. [24]. The fibrin clots were then obtained and deposited in a specific metallic box (Xpression®, Intra-lock System, São Paulo, Brazil) that allowed the elimination of exudate to obtain L-PRF membranes.
Maxillary sinus augmentation procedure
The maxillary sinuses were assigned to one of the treatment groups using a computer-generated randomization list. An individual blinded to the study design sealed all the number into an envelope allowing the randomization of the groups. The patients were not informed about the materials to be used.
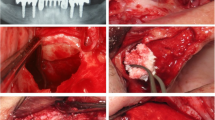
Surgical procedures were then performed after extra-oral antisepsis with 0.2% chlorhexidine digluconate and intra-oral antisepsis with 0.12% chlorhexidine digluconate. After local anesthesia (articaine hydrochloride with epinephrine 1:100,000 DFL, Brazil), a crestal incision over the edentulous area of the alveolar ridge and two vertical incisions in the vestibular region were made. The mucoperiosteal flap was detached, and the osteotomy for access to the lateral wall of the maxillary sinus was performed using a spherical burr. The maxillary sinus membrane was then detached and elevated using appropriate instruments, as described [30]. Subsequently, the envelope containing the treatment indication was opened and the assigned treatment was then implemented. The L-PRF membranes were cut into small fragments and mixed with DBBM. For each L-PRF membrane (4–5 mL) used, 0.5g of DBBM was added. The size of the DBBM particles used in all groups was 0.25–1 mm (Bio-Oss®, Geistlich Pharma AG). After augmentation of the maxillary sinus, a resorbable collagen membrane (Bio-Gide®, Geistlich Pharma AG) was used to cover the external window. The surgical areas were then sutured with 5-0 nylon thread (Ethicon®, Jonhson & Jonhson, New Brunswick, NJ, USA) using interrupted stitches. Patients received postoperative guidance and received amoxicillin 500 mg, nimesulide 100 mg, and dipyrone 500 mg. The suture was removed after 10 days. The operated region of all patients remained without direct occlusal load during the entire bone regeneration phase.
Implants placement and biopsy collection
After the proposed healing periods (4 and 8 months), a second surgical procedure was performed for implant placement. Prior to implant placement, a bone biopsy was harvested from each augmented sinus using a standard trephine drill (3i Implant Innovations®, FL, USA) with 3.0 mm in external diameter (2.0 mm in internal diameter) and 15 mm in length. The drill was positioned on the same axis of drilling and insertion of the implants and the harvested bone was carefully removed from the inside of the trephine. After that, the implants were inserted according to the manufacturer’s protocol. The implants had a diameter of 4 mm and a length of 11 mm (TitamaxTi EX ACQUA®, Neodent®, Curitiba, Brazil). During the osseointegration time, the patients did not use any temporary removable prostheses. All participants were followed up for 1 year to determine implant survival rate in each group.
Resonance frequency analysis (RFA)
The primary implant stability was recorded immediately after implant placement by RFA (Osstell® device, Osstell AB, Göteborg, Sweden). SmartPegs were attached to the implants, and the implant stability quotient (ISQ) was measured for each implant (from 1 to 100). The measurements were performed in two directions, buccal-lingual and mesio-distal, and the mean values were used, as described [31]. The implant stability was assessed by one examiner who was blinded to the treatment protocol.
Volumetric analysis
CBCT was taken in three different moments: initial (TO), 1 week after bone graft surgery (T1) and before the implant placement (T2). The SCANORA® 3Dx tomography (Soredex, Tuusula, Finland) was used applying a resolution of 512 pixels, 14 bits per pixels, with a chromatic scale of 16,384 shades of gray. The image acquisition was obtained with scan set at 10 mAs current, and 90 kVp voltage and a scan time of 20 s using the 9-inch field of view (FOV). Raw data were reconstructed and exported in a DICOM file format (Planmeca Romexis 3D®, Planmeca Oy, Helsinki, Finland) for further analysis. The volumetric measurements were performed using sagittal sections of 1 mm, assessing the color of the differential hyperdensity of the images. The analysis was performed by a single, trained and blinded radiologist. Pearson’s correlation coefficient (r) was calculated to evaluate the reproducibility of two measurements performed by the same evaluator on five samples before starting the analysis. An r-value of 0.98 was obtained comparing measurements 1 and 2, indicating intra-examiner reproducibility.
Biopsy processing
Immediately after bone biopsies collection, the samples were fixed in a 10% neutral-buffered formaldehyde solution for 3 days followed by immersion on EDTA for sample decalcification. After sample processing, serial sections with 6-μm thickness were obtained along the entire length of the bone for histological analysis [8]. The slides were stained with both hematoxylin and eosin (H&E) for descriptive histology or with specific antibodies for immunohistochemical analysis.
Histomorphometric analysis
The images of the slides stained with H&E were captured and digitized using an optical light microscope (Diastar - Leica eichert & Jung products, Germany), with a digital camera (DFC-300-FX, Leica Microsystems, Germany) attached and connected to a microcomputer with digital image analysis software. The 5.3 mm2 region of interest (ROI) was determined immediately after the end of the patient’s residual alveolar bone and the beginning of newly formed bone so that there was no bias between the patients. The analysis was performed by an experienced blinded examiner using the Image J 1.45 software (National Institutes of Health, USA). The following parameters were evaluated: the percentage of newly formed bone, percentage of soft tissue, and percentage of residual graft [10, 22].
Immunohistochemical (IHC) analysis
The IHC labeling was performed using the immunoperoxidase detection method with the following primary goat polyclonal antibodies: vascular endothelial growth factor (VEGF), osteocalcin (OCN), runt-related transcription factor 2 (RUNX2), and osteopontin (OPN) (Santa Cruz Biotechnology®, Dallas, TX, USA). As a secondary antibody, anti-IgG biotinylate antibody (Pierce Biotechnology, Waltham, MA, USA) was used. The reaction was revealed using diaminobenzidine (Dakocytomation, Carpinteria, CA, USA). At the end of the reactions, Harris hematoxylin counter-staining was performed. Data analysis was performed using a semi-quantitative method using scores from 1 to 4 (0= absence or discrete labeling; 1= mild labeling; 3= moderate labeling; and 4= intense labeling). The immunostaining intensity scores were based on previously published studies [32, 33] and were performed by a blinded and calibrated examiner. Moreover, a planimetry technique using the Image J was also performed to quantify the DAB staining for all the biomarkers evaluated. Briefly, the region of interest (ROI) was delimitated on the tiff images from all groups and these digital images were used to measure the surface area of the bone samples using the Image J software (National Institute of Health, Baltimore, USA). The analysis was performed by a blinded and experienced examiner (RO).
Statistical analysis
Statistical analyses were performed using GraphPad Prism (version 6.0, GraphPad Software, Inc., La Jolla, CA, USA). The data were described using measures of central tendency (mean and median) and dispersion (standard deviation and 95% confidence interval). The Shapiro-Wilk test was used to assess the normality of data distribution. The maxillary sinus was considered the sample unit (n=12/group). The age parameter did not present a normal distribution and was analyzed by the Kruskal-Wallis test. The implant stability values and histomorphometric measurements, with the exception of the percentage of residual biomaterial, showed normal distribution and were analyzed using the ANOVA test followed by the Tukey test for multiple comparisons. Significant differences among groups in percentage of residual biomaterial were assessed by the Kruskal-Wallis test followed by the Dunn test for multiple comparisons. The differences were considered significant at P < 0.05. In addition, correlations between the bone formation markers and newly formed bone and between bone formation markers and volumetric measurements were investigated for the two groups (DBBM+L-PRF4 and DBBM+L-PRF8) using Pearson’s rank correlation coefficient. A further non-parametric model (linear regression) was used to explore the association between bone formation markers and any single variable of interest. Differences were considered significant at P < 0.05.
Results
The study sample consisted of 24 patients totalizing 36 maxillary sinuses. Among these patients, 14 were women, and 10 were men, with an average age of 54.08±10.07 years, with no significant differences between ages (Table 1). No complications were observed during or after surgical procedures, neither post-operative infection. No perforations were detected in the sinus membranes, and no participant was lost during the survey. All the surgical procedures (maxillary sinus augmentation and implant placement) were performed by the same experienced and qualified surgeon (ECP). Bilateral maxillary sinus lifting was performed in 12 patients. The allocation of the patients was as follows: 7 patients received L-PRF + DBBM4 in one side and only DBBM in the other side; 5 patients received only L-PRF + DBBM4; 5 patients received L-PRF + DBBM8 in one side and DBBM in the other side; and finally 7 patients received only L-PRF + DBBM8. After the proposed healing times, 46 implants were installed (18 in control group, 19 in LPRF+DBBM4 and 19 in LPRF+DBBM8). After 12 months of implant loading, the implant survival rate in the augmented maxillary sinus was 100% for all groups.
Histomorphometric analysis
In all collected biopsies, the newly formed bone was in direct contact with the residual graft material. However, histomorphometric data (Fig. 1A) showed higher percentage of bone neoformation in the L-PRF groups (LPRF+DBBM4: 44.70±14.01% and LPRF+DBBM8: 46.56± 12.28%) than in the control group (32.34±9.49%). The percentage of bone neoformation between test groups was not statistically significant. The control group presented the highest percentage of residual graft (12.58±9.19%) compared to LPRF+DBBM4 (3.59±4.22%) and LPRF+DBBM8 (7.01±8.49%). There was statistical difference between control group and LPRF+DBBM4 group (Fig. 1B). The percentage of fibrous tissue was not significantly different between control (28.29±10.07%) and LPRF+DBBM4 (26.59±11.13%) and LPRF+DBBM8 (17.76±12.03%) groups (Fig. 1C).
Graphs indicating the results of histomorphometry, ISQ and volumetric reduction of the grafts in the different study groups. (A) New bone formation percentage in the different study groups. *Statistically significant difference in relation to the other groups (p = 0.0135). (B) Residual graft percentage in the different study groups. *Statistically significant difference (p = 0.0227). (C) Soft tissue percentage in the different study groups. *Statistically significant difference (p = 0.0579)
Figure 2A–C is the H&E-stained slides representing the L-PRF+DBBM4, L-PRF+DBBM8, and control group, respectively. It is possible to observe the increased amount of bone neoformation in the test groups compared to the control group. On the other hand, the test group presented with more residual bone graft compared to the test group probably due to the higher amount of biomaterial used to fill the maxillary sinus, when compared to the test groups. There was no difference in the amount of soft tissue in the bone biopsies among the different groups.
Histological images of control, L-PRF + DBBM4 and L-PRF + DBBM8 groups. NFB corresponds to the newly formed bone; B is residual graft; and ST corresponds to the soft tissue. (A) Histological images of L-PRF + DBBM 4 group with 50× magnification. In greater magnification (100×), a large amount of newly formed bone tissue can be seen. In addition, less biomaterial is observed, which means that graft maturation in this group was faster when compared to the control group. (B) Histological images of L-PRF + DBBM 8 group with 50× magnification. At higher magnification (100×), there is also a large amount of newly formed bone tissue and less biomaterial, demonstrating that the addition of L-PRF influences bone neoformation. (C) Histological images of control group with 50× magnification. In greater magnification (100×), new bone tissue is observed, but a large amount of residual graft is still detected after 8 months of repair
Immunohistochemical analysis
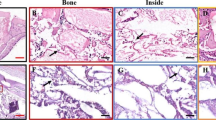
The IHC intensity scores showed higher RUNX2 expression on LPRF+DBBM4 group compared to the control and LPRF+DBBM8. The VEGF immunostaining was similar between test groups and lower for the control group. The immunostaining intensity of OPN and OCN was increased in LPRF+DBBM4 group compared to the other groups (Fig. 3). Figure 4 shows representative images from all groups highlighting the stained intensity of the bone formation markers in the region of interest.
Immunohistochemical images for proteins evaluated in the control group (A, D, G, J), L-PRF + DBBM 4 group (B, E, H, K) and L-PRF + DBBM 8 group (C, F, I, L). The images indicate the new bone formation next to the biomaterial and the red arrows represent the positive immunostaining. In A, B and C, positive immunostaining for RUNX2 is observed, showing positive cells for this transcription factor. Note the presence of cells next to the remnants of the biomaterial. In D, E, F, positive immunostaining for VEGF, a vascular endothelial growth factor, is observed, presenting itself marked in cells of the osteoblastic lineage, close to the biomaterial. In G, H, I, the immunostaining positive for osteocalcin is observed, present mainly in the mineralized matrix as well as in osteoblastic cells. In J, K, and L, positive markings for osteopontin are observed, characterizing the areas where mineral precipitation is observed next to the collagen matrix. Cells of the osteoblastic lineage positively labeled for this protein are also observed
The quantification of the DAB staining was also performed by means of planimetry technique using the images exported to the Image J software. According to the IHC intensity scores, the DBBM+L-PRF4 group showed significant increase in the staining for OCN compared to the other groups. Moreover, although without statistically significance, the VEGF and RUNX2 markers were also increased in the DBBM+L-PRF4 compared to the control group and DBBM+L-PRF8 (Fig. 5).
We have performed correlation analysis between the bone formation markers and the newly formed bone and also between the bone formation markers and the bone resorption (in the radiographical data). Our data showed that the only statistically significant correlation was found for newly formed bone and VEGF for the DBBM+L-PRF4 group (supplemental figure 2).
Resonance frequency analysis
Adequate primary implant stability was achieved in all groups. The ISQ values in the LPRF+DBBM4 (60.9±9.35) group were significantly lower in comparison to LPRF+DBBM8 (72.19±5.43) and control (75.56±4.60) groups (Fig. 6A). There was no difference in ISQ values between control and LPRF+DBBM8 groups.
(A) Values of the primary stability (mean and standard deviation) of the implants in the different study groups. *Statistically significant difference in relation to the other groups (p <0.0001). (B) Values (mean and standard deviation) of the percentage of volumetric reduction of the grafts in the different study groups. No significant difference was observed between the groups (p = 0.4934; ANOVA and Tukey test)
Volumetric analysis
The mean graft volume observed in T1 was not statistically different among groups (control: 1.39±0.53 cm3; LPRF+DBBM4: 1.69±0.42 cm3; LPRF+DBBM8: 1.68 ± 1.05 cm3). After the healing period (T2), the mean graft volume decreased in all groups (control: 0.90±0.28 cm3; LPRF+DBBM4: 1.11±0.25 cm3; LPRF+DBBM8: 0.95 ± 0.48 cm3) (Tables 2, 3, and 4). The graft volume difference between the two periods (T1 and T2) within each group was statistically significant. A reduction in graft volume in T2 among groups was not significant as well as the percentage of graft resorption (Fig. 6B and Fig. 7). In all groups, the bone volume obtained was adequate for implant placement.
Volumetric analysis was performed using the segmentation function in Planmeca (Planmeca Romexis 3D, Planmeca Oy, Helsinki, Finland). The CT images were displayed simultaneously in four different views: sagittal (red, x-axis), coronal (green, y-axis), axial (blue, z-axis), and a three-dimensional rendering (3D-rendered view). (A) Central axial section of the grafted area 1 week after the surgical procedure (T1); (B) central axial section of the grafted area 8 months after sinus elevation and before implant placement (T2); (C) sagittal section demonstrating the segmentation process by which the graft perimeter was outlined according to different gray scales to produce a 2D region of interest (in red); (D) representative 3D view of the entire final segmented 3D reconstruction of the graft volume (in red) in the maxillary sinuses
Discussion
The results of the current study have demonstrated that the association of L-PRF with DBBM increased new bone formation in maxillary sinus augmentation. Moreover, L-PRF induced higher protein expression of RUNX2, VEGF, OCN, and OPN. Our data also indicated that there were no differences in the radiographic graft volume after the healing periods. The ISQ values in the LPRF+DBBM4, although lower when compared to the other groups, presented with great primary implant stability after 4 months of healing (above 60 implant stability quotient). These data suggest that the use of L-PRF might be an interesting alternative to use in combination with DBBM for augment the maxillary sinuses, and thus allowing the installation of appropriate length implants in a shorter period of time.
The histomorphometric analysis showed significant increase of new bone formation in the test groups. Even with half of the healing time, the LPRF+DBBM4 group provided more new bone formation than the control group, indicating faster bone graft healing. This finding is probably attributed to the localized and continuous release of growth factors and cytokines from the membranes that favor the tissue repair process [34]. This property is only possible due to the natural and slow polymerization of the fibrin network during centrifugation. This fact allows a high percentage of equilateral bonds, which promote the establishment of a flexible three-dimensional structure with multiple fibers, making it capable of transplanting and support cytokine activation and slower and longer release [35, 36]. This feature is the differential of L-PRF when compared to other platelet aggregates [19, 35, 37].
The ability of L-PRF in accelerate bone neoformation was previously analyzed by our group [10, 22] and others [19, 21, 27] in clinical studies. Briefly, Choukroun et al. [19] evaluated the effects of LPRF associated with DBBM in maxillary sinus augmentation. This study indicated that the addition of L-PRF to the DBBM reduced the healing period and allowed the implant placement after 4 months of healing without interfering on bone neoformation. This data was corroborated by previous study that found increased bone neoformation when L-PRF was combined with DBBM for maxillary sinus floor augmentation [38]. Zhang et al. [27] showed similar results regarding the use of L-PRF in maxillary sinus. Histomorphometric analysis found 1.4-fold increase in the percentage of new bone formation in the test group (L-PRF+DBBM) compared to the control group (DBBM) (18.35 ± 5.62% versus 12.95 ± 5.33%) and a 1.5-fold increase in the percentage of residual bone graft in the control group than in the test group (28.54 ± 12.01% versus 19.16 ± 6.89%). On the other hand, Nizam et al. [21] compared the new bone formation in maxillary sinus using the association of bone grafts with L-PRF after 6 months of repair; however, this study exhibited no significant difference on new bone formation between control (only biomaterial) and the test groups (biomaterial + L-PRF).
These heterogeneous results among the studies with L-PRF can be explained by the different techniques for obtaining the platelet concentrate, different relative centrifugal forces (RCF) applied, and the different times of healing analyzed. There is variation regarding the number of L-PRF membranes used, the amount of blood collected, the type of tube used, type of centrifuge, and the relative centrifugal force (RCF) assigned [25, 39]. However, it is indisputable that L-PRF is a favorable biomaterial for dental procedures, being a completely autogenous platelet derivative, which dispenses the use of anticoagulants and bovine thrombin [40], capable of incorporating all blood components favoring to the immune and inflammatory response [41].
IHC analysis of proteins related to bone metabolism (RUNX-2, VEGF, OCN, OPN) plays an important role to evaluate the molecular effects of L-PRF on bone tissue. The current study provides, for the first time, data in humans indicating that the association of L-PRF with DBBM contributes to bone neoformation, presenting increased immunostaining for VEGF protein, which might be accounted for the observed increased bone neoformation. In addition, the RUNX-2 immunostaining was higher in the LPRF+DBBM4 group. The RUNX-2 was used to mark the differentiation of pre-osteoblasts that is important for new bone deposition. Immunostaining of OCN, marker of bone formation, was also higher in LPRF+DBBM4 group implying increased bone mineralization. All those data regarding immunostaining was corroborated by quantification of DAB staining by means of planimetry technique. Collectively, these results suggest a favorable ambient for implant placement and represented the moment of bone healing with more metabolic reactions and osteoblast differentiation. In line with our results, a previous animal study [32] was conducted to evaluate the effect of different bone materials placed in the rat calvaria. Five different biomaterials were used: autogenous bone, DBBM, L-PRF, L-PRF + DBBM, and only blood clot. After 4 and 8 weeks, the L-PRF + DBBM group showed increased bone formation and improved expression of VEGF. Although promising results were achieved when using L-PRF, more studies are needed to corroborate these molecular findings.
The achievement of optimal primary implant stability has been considered an important factor for implant success [42, 43]. All implants placed in this study showed adequate ISQ values, although the LPRF+DBBM4 group showed lower values when comparing with the others groups. This result was expected due the half time of bone repair. On the other hand, the ISQ values of L-PRF+DBBM4 group (60.90 ± 9.35) can be considered satisfactory and did not jeopardize the implant osseointegration [8, 9]. Previous studies verified that ISQ values ranging from 55 to 68 represent a safe level of stability, while an ISQ value below 55 should be considered a signal for caution [43, 44]. The primary implant stability was also evaluated in a clinical study where the implants were placed 6 months after sinus lifting using only L-PRF [45]. The mean ISQ value for seventeen implants was 66.5 ISQ (range from 57 to 75 ISQ). Our data suggest that an appropriate maturation of the bone graft was achieved in all groups, which allowed the insertion of implants with optimal primary stability.
We have also investigated the volumetric alterations in maxillary sinus after bone graft using CBCT. As expected, the results showed an increase in bone graft volume after the healing period in all groups (T0 to T1) followed by a subsequent decrease in the graft volume between T1 and T2 period without statistical differences among groups. A systematic review was conducted to evaluate bone graft volume changes in maxillary sinus augmentations with different biomaterials [46]. All studies indicated the reduction in bone volume in the follow-up period (ranging from 6 months to 6 years). The authors concluded that the bone graft resorption would always occur especially in the early healing periods; however, the reduction in volume graft does not hamper implant osseointegration and function.
It is important to bear in mind that this study has some caveats that need to be taken into account when interpreting the results. First, the healing time is short and increased follow-up periods are recommended. Secondly, there are different healing times comparing the test (LPRF+DBBM4) and the control. Lastly, the initial maxillary sinus volume was not measured, neither the exactly amount of bone graft used in each sinus. These data could offer important information about the resorption rates and new bone formation and should be confirmed by other studies in the near future.
In conclusion, this study showed that the association of L-PRF with DBBM enhanced new bone formation and accelerates bone maturation through the increased expression of VEGF, RUNX2, and OCN allowing early implant placement. The association of L-PRF with DBBM might reduce the amount of bone graft needed to fill the maxillary sinus, thus reducing the total costs for the patient. Taken together, our data suggested that the use of L-PRF combined with DBBM might be an interesting alternative to fill the maxillary sinus previous to implant placement allowing faster oral rehabilitation with dental implant–supported prosthesis.
Data availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
References
Boyne PJ, James RA (1980) Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg 38:613–616
Tatum H Jr (1986) Maxillary and sinus implant reconstructions. Dent Clin North Am 30:207–229
Ocak H, Kutuk N, Demetoglu U, Balcioglu E, Ozdamar S, Alkan A (2017) Comparison of bovine bone-autogenic bone mixture versus platelet-rich fibrin for maxillary sinus grafting: histologic and histomorphologic study. J Oral Implantol 43:194–201. https://doi.org/10.1563/aaid-joi-D-16-00104
Palma VC, Magro-Filho O, de Oliveria JA, Lundgren S, Salata LA, Sennerby L (2006) Bone reformation and implant integration following maxillary sinus membrane elevation: an experimental study in primates. Clin Implant Dent Relat Res 8:11–24. https://doi.org/10.2310/j.6480.2005.00026.x
Srouji S, Ben-David D, Lotan R, Riminucci M, Livne E, Bianco P (2010) The innate osteogenic potential of the maxillary sinus (Schneiderian) membrane: an ectopic tissue transplant model simulating sinus lifting. Int J Oral Maxillofac Surg 39:793–801. https://doi.org/10.1016/j.ijom.2010.03.009
Srouji S, Kizhner T, Ben David D, Riminucci M, Bianco P, Livne E (2009) The Schneiderian membrane contains osteoprogenitor cells: in vivo and in vitro study. Calcif Tissue Int 84:138–145. https://doi.org/10.1007/s00223-008-9202-x
Sul SH, Choi BH, Li J, Jeong SM, Xuan F (2008) Effects of sinus membrane elevation on bone formation around implants placed in the maxillary sinus cavity: an experimental study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105:684–687. https://doi.org/10.1016/j.tripleo.2007.09.024
de Molon RS, Magalhaes-Tunes FS, Semedo CV, Furlan RG, de Souza LGL, de Souza Faloni AP, Marcantonio E Jr, Faeda RS (2019) A randomized clinical trial evaluating maxillary sinus augmentation with different particle sizes of demineralized bovine bone mineral: histological and immunohistochemical analysis. Int J Oral Maxillofac Surg 48:810–823. https://doi.org/10.1016/j.ijom.2018.09.003
Dos Anjos TL, de Molon RS, Paim PR, Marcantonio E, Marcantonio E Jr, Faeda RS (2016) Implant stability after sinus floor augmentation with deproteinized bovine bone mineral particles of different sizes: a prospective, randomized and controlled split-mouth clinical trial. Int J Oral Maxillofac Surg 45:1556–1563. https://doi.org/10.1016/j.ijom.2016.09.004
Pichotano EC, de Molon RS, Freitas de Paula LG, de Souza RV, Marcantonio E Jr, Zandim-Barcelos DL (2018) Early placement of dental implants in maxillary sinus grafted with leukocyte and platelet-rich fibrin and deproteinized bovine bone mineral. J Oral Implantol 44:199–206. https://doi.org/10.1563/aaid-joi-D-17-00220
Dos Santos PL, de Molon RS, Queiroz TP, Okamoto R, de Souza Faloni AP, Gulinelli JL, Luvizuto ER, Garcia IR Jr (2016) Evaluation of bone substitutes for treatment of peri-implant bone defects: biomechanical, histological, and immunohistochemical analyses in the rabbit tibia. J Periodontal Implant Sci 46:176–196. https://doi.org/10.5051/jpis.2016.46.3.176
Kim YJ, de Molon RS, Horiguti FR, Contador GP, Coelho MA, Mascarenhas VI, de Souza Faloni AP, Cirelli JA, Sendyk WR (2018) Vertical bone augmentation using deproteinized bovine bone mineral, absorbable collagen sponge, and recombinant human bone morphogenetic protein-2: an in vivo study in rabbits. Int J Oral Maxillofac Implants 33:512–522. https://doi.org/10.11607/jomi.5959
Jensen T, Schou S, Stavropoulos A, Terheyden H, Holmstrup P (2012) Maxillary sinus floor augmentation with Bio-Oss or Bio-Oss mixed with autogenous bone as graft in animals: a systematic review. Int J Oral Maxillofac Surg 41:114–120. https://doi.org/10.1016/j.ijom.2011.08.010
Simonpieri A, Choukroun J, Del Corso M, Sammartino G, Dohan Ehrenfest DM (2011) Simultaneous sinus-lift and implantation using microthreaded implants and leukocyte- and platelet-rich fibrin as sole grafting material: a six-year experience. Implant Dent 20:2–12. https://doi.org/10.1097/ID.0b013e3181faa8af
Tajima N, Ohba S, Sawase T, Asahina I (2013) Evaluation of sinus floor augmentation with simultaneous implant placement using platelet-rich fibrin as sole grafting material. Int J Oral Maxillofac Implants 28:77–83. https://doi.org/10.11607/jomi.2613
Browaeys H, Bouvry P, De Bruyn H (2007) A literature review on biomaterials in sinus augmentation procedures. Clin Implant Dent Relat Res 9:166–177. https://doi.org/10.1111/j.1708-8208.2007.00050.x
Zerbo IR, de Lange GL, Joldersma M, Bronckers AL, Burger EH (2003) Fate of monocortical bone blocks grafted in the human maxilla: a histological and histomorphometric study. Clin Oral Implants Res 14:759–766. https://doi.org/10.1046/j.0905-7161.2003.00967.x
Pinchasov G, Juodzbalys G (2014) Graft-free sinus augmentation procedure: a literature review. J Oral Maxillofac Res 5:e1. https://doi.org/10.5037/jomr.2014.5101
Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J, Dohan DM (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101:299–303. https://doi.org/10.1016/j.tripleo.2005.07.012
Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101:e37–e44. https://doi.org/10.1016/j.tripleo.2005.07.008
Nizam N, Eren G, Akcali A, Donos N (2018) Maxillary sinus augmentation with leukocyte and platelet-rich fibrin and deproteinized bovine bone mineral: a split-mouth histological and histomorphometric study. Clin Oral Implants Res 29:67–75. https://doi.org/10.1111/clr.13044
Pichotano EC, de Molon RS, de Souza RV, Austin RS, Marcantonio E, Zandim-Barcelos DL (2019) Evaluation of L-PRF combined with deproteinized bovine bone mineral for early implant placement after maxillary sinus augmentation: a randomized clinical trial. Clin Implant Dent Relat Res 21:253–262. https://doi.org/10.1111/cid.12713
Tadjoedin ES, de Lange GL, Bronckers AL, Lyaruu DM, Burger EH (2003) Deproteinized cancellous bovine bone (Bio-Oss) as bone substitute for sinus floor elevation. A retrospective, histomorphometrical study of five cases. J Clin Periodontol 30:261–270. https://doi.org/10.1034/j.1600-051x.2003.01099.x
Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101:e51–e55. https://doi.org/10.1016/j.tripleo.2005.07.010
Liu R, Yan M, Chen S, Huang W, Wu D, Chen J (2019) Effectiveness of platelet-rich fibrin as an adjunctive material to bone graft in maxillary sinus augmentation: a meta-analysis of randomized controlled trails. Biomed Res Int 2019:7267062. https://doi.org/10.1155/2019/7267062
Strauss FJ, Stahli A, Gruber R (2018) The use of platelet-rich fibrin to enhance the outcomes of implant therapy: a systematic review. Clin Oral Implants Res 29(Suppl 18):6–19. https://doi.org/10.1111/clr.13275
Zhang Y, Tangl S, Huber CD, Lin Y, Qiu L, Rausch-Fan X (2012) Effects of Choukroun's platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: a histological and histomorphometric study. J Craniomaxillofac Surg 40:321–328. https://doi.org/10.1016/j.jcms.2011.04.020
Shiezadeh F, Taher M, Shooshtari Z, Arab H, Shafieian R (2023) Using platelet-rich fibrin in combination with allograft bone particles can induce bone formation in maxillary sinus augmentation. J Oral Maxillofac Surg. https://doi.org/10.1016/j.joms.2023.03.015
Powell CA, Casarez-Quintana A, Zellner J, Al-Bayati O, Font K (2022) The application of leukocyte- and platelet-rich fibrin (L-PRF) in maxillary sinus augmentation. Clin Adv Periodontics 12:277–286. https://doi.org/10.1002/cap.10216
de Molon RS, de Paula WN, Spin-Neto R, Verzola MH, Tosoni GM, Lia RC, Scaf G, Marcantonio E Jr (2015) Correlation of fractal dimension with histomorphometry in maxillary sinus lifting using autogenous bone graft. Braz Dent J 26:11–18. https://doi.org/10.1590/0103-6440201300290
Guastaldi FPS, Queiroz TP, Marques DO, Santos ABS, Molon RS, Margonar R, Guastaldi AC (2021) Comparative evaluation of implants with different surface treatments placed in human edentulous mandibles: a 1-year prospective study. J Maxillofac Oral Su. https://doi.org/10.1007/s12663-021-01600-6
do Lago ES, Ferreira S, Garcia IR Jr, Okamoto R, Mariano RC (2020) Improvement of bone repair with l-PRF and bovine bone in calvaria of rats. histometric and immunohistochemical study. Clin Oral Investig 24:1637–1650. https://doi.org/10.1007/s00784-019-03018-4
Pereira RS, Gorla LF, Boos F, Okamoto R, Garcia Junior IR, Hochuli-Vieira E (2017) Use of autogenous bone and beta-tricalcium phosphate in maxillary sinus lifting: histomorphometric study and immunohistochemical assessment of RUNX2 and VEGF. Int J Oral Maxillofac Surg 46:503–510. https://doi.org/10.1016/j.ijom.2017.01.002
Pedrosa WF Jr, Okamoto R, Faria PE, Arnez MF, Xavier SP, Salata LA (2009) Immunohistochemical, tomographic and histological study on onlay bone graft remodeling. Part II: calvarial bone. Clin Oral Implants Res 20:1254–1264. https://doi.org/10.1111/j.1600-0501.2009.01747.x
Anitua E (1999) Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants 14:529–535
Dohan Ehrenfest DM, Rasmusson L, Albrektsson T (2009) Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol 27:158–167. https://doi.org/10.1016/j.tibtech.2008.11.009
van Hinsbergh VW, Collen A, Koolwijk P (2001) Role of fibrin matrix in angiogenesis. Ann N Y Acad Sci 936:426–437. https://doi.org/10.1111/j.1749-6632.2001.tb03526.x
Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, Gogly B (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101:e45–e50. https://doi.org/10.1016/j.tripleo.2005.07.009
Everts PA, van Zundert A, Schonberger JP, Devilee RJ, Knape JT (2008) What do we use: platelet-rich plasma or platelet-leukocyte gel? J Biomed Mater Res A 85:1135–1136. https://doi.org/10.1002/jbm.a.31570
Tatullo M, Marrelli M, Cassetta M, Pacifici A, Stefanelli LV, Scacco S, Dipalma G, Pacifici L, Inchingolo F (2012) Platelet rich fibrin (P.R.F.) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations. Int J Med Sci 9:872–880. https://doi.org/10.7150/ijms.5119
Castro AB, Meschi N, Temmerman A, Pinto N, Lambrechts P, Teughels W, Quirynen M (2017) Regenerative potential of leucocyte- and platelet-rich fibrin. Part A: intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J Clin Periodontol 44:67–82. https://doi.org/10.1111/jcpe.12643
Degidi M, Daprile G, Piattelli A (2012) Primary stability determination by means of insertion torque and RFA in a sample of 4,135 implants. Clin Implant Dent Relat Res 14:501–507. https://doi.org/10.1111/j.1708-8208.2010.00302.x
Meredith N (1998) Assessment of implant stability as a prognostic determinant. Int J Prosthodont 11:491–501
Sennerby L, Meredith N (2000) (2008) Implant stability measurements using resonance frequency analysis: biological and biomechanical aspects and clinical implications. Periodontol 47:51–66. https://doi.org/10.1111/j.1600-0757.2008.00267.x
Nedir R, Bischof M, Szmukler-Moncler S, Bernard JP, Samson J (2004) Predicting osseointegration by means of implant primary stability. Clin Oral Implants Res 15:520–528. https://doi.org/10.1111/j.1600-0501.2004.01059.x
Shanbhag S, Shanbhag V, Stavropoulos A (2014) Volume changes of maxillary sinus augmentations over time: a systematic review. Int J Oral Maxillofac Implants 29:881–892. https://doi.org/10.11607/jomi.3472
Acknowledgements
The authors would like to thank Geistlich Pharma AG® (Wolhusen, Switzerland) for the free donation of Bio-Oss® and BioGide® membranes, and Neodent® (Curitiba, PR, Brazil) for the donation of the implants.
Funding
Carolina M. de Almeida Malzoni and Elton Carlos Pichotano were supported by grant provided by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Author information
Authors and Affiliations
Contributions
Carolina Mendonca de Almeida Malzoni: methodology, conceptualization, formal analysis, writing — original draft, writing — review and editing, visualization, final approval of the submitted version; Elton Carlos Pichotano: methodology, formal analysis, writing — review and editing, visualization, final approval of the submitted version; Luis Guilherme Freitas de Paula: methodology, conceptualization, supervision, formal analysis, writing — review and editing, visualization, final approval of the submitted version; Ricardo Violante de Souza: methodology, formal analysis, writing — review and editing, visualization, final approval of the submitted version; Roberta Okamoto: methodology, formal analysis, writing — review and editing, visualization, final approval of the submitted version; Elcio Marcantonio Jr: methodology, conceptualization, supervision, formal analysis, writing — review and editing, visualization, final approval of the submitted version; Rupert S. Austin: methodology, formal analysis, writing — review and editing, visualization, final approval of the submitted version; Rafael Scaf de Molon: methodology, formal analysis, writing — original draft, writing — review and editing, visualization, final approval of the submitted version; Daniela Leal Zandim-Barcelos: methodology, formal analysis, conceptualization, supervision, writing — review and editing, visualization, final approval of the submitted version
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This study was approved by the Human Research Ethics Committee of the School of Dentistry at Araraquara (CAAE #41357514.5.0000.5416) and was performed in accordance with the principles stated in the Declaration of Helsinki in 1964. Furthermore, this clinical trial was registered before participant recruitment in Brazilian Registry of Clinical Trials (ReBEC - RBR-95m73t).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Almeida Malzoni, C.M., Pichotano, E.C., Freitas de Paula, L.G. et al. Combination of leukocyte and platelet–rich fibrin and demineralized bovine bone graft enhanced bone formation and healing after maxillary sinus augmentation: a randomized clinical trial. Clin Oral Invest 27, 5485–5498 (2023). https://doi.org/10.1007/s00784-023-05167-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05167-z