Abstract
Objectives
On the basis of a large sample size and a long follow-up period, the objectives of this study were to evaluate the outcomes of direct pulp capping (DPC) in mature permanent teeth with carious pulp exposure using a kind of bioaggregate putty (BP) which commercially named iRoot BP Plus (Innovative Bioceramix, Inc., Vancouver, Canada) and to analyze the potential prognostic factors.
Materials and methods
The design of this research was retrospective regarding treatment procedures and prospective regarding the assessment of outcomes. The preoperative diagnosis of the teeth was either normal pulp or reversible pulpitis. Results were assessed based on clinical and radiographic examinations with at least 12 months of follow-up after DPC. No symptoms or signs, a positive response to electric pulp testing, a normal response to cold pulp testing and radiographs showing no abnormalities were considered to indicate success. Kaplan–Meier survival analysis was used to calculate the cumulative survival of teeth after DPC. Univariate and multivariate Cox proportional hazard regressions were used to analyze potential prognostic factors.
Results
Three hundred thirty-four patients, including a total of 354 teeth, were available for the final clinical examination. The follow-up period ranged from 12 to 85 months, with an average of 27.0 ± 0.8 months. The total success rate was 85% (302/354), and the cumulative survival rates at 1, 2, 3, 4, and 5 years were 92%, 87%, 83%, 76%, and 72%, respectively. Univariate analysis indicated a significantly increased risk of failure in patients aged above 40 years and those treated by resident operators (P ≤ 0.01), with hazard ratios of 2.18 and 2.27, respectively.
Conclusions
Under appropriate indication selection and treatment procedures, long-term success is possible in mature permanent teeth with carious pulp exposure by DPC using iRoot BP Plus. Patient age and operator experience are potential prognostic factors.
Clinical relevance
Clinical data on iRoot BP Plus as a pulp capping medicament in mature permanent teeth with carious pulp exposure is lacking. This study indicated the efficacy of BP in DPC. Younger patient and sophisticated operator are beneficial for the outcome of DPC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aim of vital pulp therapy (VPT) is to preserve healthy pulp tissue, which is conducive to promoting the development of tooth roots and the formation of reparative dentin, thereby preserving teeth with normal physiological function [1]. There is no consensus on the most effective method for managing cariously exposed pulp, but once exposure occurs, a conservative method is recommended [2]. Direct pulp capping (DPC) is one method of VPT that is mainly used to treat immature teeth in adolescents. With increased understanding of pulp biology, application of bioactive pulp capping materials, and improvement of clinical techniques, the clinical application of VPT in mature permanent teeth has been increasing recently [3]. As clinical studies have shown, the success rate of DPC in mature permanent teeth with carious pulp exposure using bioactive materials such as mineral trioxide aggregate (MTA) [4, 5], Biodentine [4, 6, 7], and iRoot BP Plus (Innovative Bioceramix, Inc., Vancouver, Canada) [8] ranges from 83 to 93%.
Although many studies have reported a certain level of clinical efficacy for DPC in mature permanent teeth, there is still ambiguity and inconsistency in clinical practice. First, there is still no consensus regarding the selection of cases. For example, many previous studies have indicated a significantly increased risk of failure in patients over 40 years of age [6, 9], while others have indicated no significant effect of age on the prognosis after DPC [7, 10]. Second, although the commercially available calcium silicate cements (CSCs) such as MTA and Biodentine showed good biocompatibility [11] and achieved higher long-term success rates compared with calcium hydroxide [12], it is still unclear that which CSC was superior to alternatives. iRoot BP Plus, a ready-to-use, premixed calcium silicate-based, and aluminum-free bioaggregate putty (BP), is mainly composed of tricalcium silicate, bicalcium silicate, calcium phosphate, tantalum oxide, and zirconium oxide. It is easy to handle, has a shorter setting time compared with MTA and has lower risk of tooth discoloration [13]. In vivo and vitro, BP has shown good biocompatibility with pulp tissue and induced the proliferation of dental pulp cells and the formation of reparative dentin bridges [14]. BP has also been found to be a suitable alternative pulp capping medicament for managing complicated crown fractures [13]. In contrast to MTA and Biodentine, which are the most commonly used materials in DPC [12], there have been few clinical studies on the use of BP in DPC in mature permanent teeth with carious pulp exposure. Thus, as mentioned above, further reviews and analyses of the effects of prognostic factors in DPC on indication selection, pulp status assessment, pulp capping materials, among others, are still needed.
Large-sample, retrospective studies based with long-term follow-up periods are beneficial for analyzing the prognostic factors and long-term success rate of a treatment. In this study, historical clinical data were reviewed to assess the long-term success rate of DPC using BP in mature permanent teeth with carious pulp exposure, which was the first objective. The second objective was to analyze potential prognostic factors of DPC and make it clear for selection of cases in clinical practice.
Materials and methods
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist was followed in this study. The research was conducted with approval from the Biomedical Institutional Review Board of Peking University School and Hospital of Stomatology (PKUSSIRB-202163044). The study protocol was registered on chictr.org (ChiCTR2200057881).
Study participants
This study included all patients who underwent DPC at the Department of Cariology and Endodontology, Peking University School and Hospital of Stomatology, from January 2015 to February 2021 for whom complete preoperative information and treatment details were available. Patients were contacted by phone and invited to take part in this study. At a follow-up visit, they were given a participant information sheet and were asked to sign a declaration of informed consent. The inclusion and exclusion criteria for case selection in this study were as follows:
Inclusion criteria
-
1.
Mature permanent teeth with apical closure whose preoperative diagnosis was either normal pulp or reversible pulpitis.
-
2.
Preoperative radiographs showing intact and continuous periradicular ligaments, absence of periradicular translucency, no root resorption, and no root fracture.
-
3.
Pulp exposure due to decay removal.
-
4.
Teeth treated following the protocol [8] described below.
-
5.
BP as the pulp capping material.
-
6.
A follow-up period of at least 1 year.
Exclusion criteria
-
1.
Inability to attend clinical reviews due to systemic diseases or pregnancy.
-
2.
Incomplete preoperative information or treatment details.
-
3.
Lack of attendance to the clinic for permanent restoration with resin composite.
The diagnostic criteria for normal pulp included ① no history of spontaneous pain; ② no symptoms or sensitivity to cold, heat, or sweet stimuli; ③ pulp sensitivity (cold) testing showing the same response as a normal tooth. The diagnostic criteria for reversible pulpitis had difference in ② no symptoms or mild sensitivity to cold, heat, or sweet stimuli; ③ pulp sensitivity (cold) testing showing mildly “transient sensitivity” that disappeared upon removal of the stimulus.
Treatment procedure
Treatments were performed by faculty members or residents. Local anesthesia (articaine 4%, adrenaline 1:100,000, Pierre Rolland, France) and rubber dam isolation were performed. The caries was removed initially with a sterile round diamond bur at high speed, followed by a sterile low-speed tungsten carbide bur nearing the pulp chamber. Once the pulp was exposed (Fig. 1a), the caries was removed completely, and hemorrhage was controlled using a cotton pellet soaked with 1.25% or 2.5% sodium hypochlorite for 1 to 5 min. After controlling hemorrhage and confirming that the exposed pulp tissue was continuous blood filled (Fig. 1a), DPC was performed with BP (Fig. 1b). The material was placed over the exposed site and 1–2 mm of the surrounding dentin; the thickness of the BP was 1.5–2 mm. Then, a thin layer of self-adhering flowable resin (Dyad Flow, Kerr, German) was placed over the BP (Fig. 1c), and cavity restoration was performed with composite resin (Filtek Z350 XT or Filtek P60, 3 M ESPE, USA) (Fig. 1d) or glass ionomer cement (GIC, Changshu Shangchi Dental Materials Co., Ltd., China). GIC was replaced with a composite resin at the recall visit 1 to 2 weeks later.
A representative case of DPC. a Pulp exposure after caries removal. b Direct pulp capping with BP. c Placement of a thin layer of self-adhesive flowable resin over the BP. d Immediate restoration with resin composite. e Preoperative radiograph showing deep caries. f Immediate postoperative radiograph
Data collection
Two researchers conducted the follow-up examination and data collection. Preoperative information and treatment details were collected from patients’ records to explore potential prognostic factors. Preoperative data included age, sex, tooth type, tooth location, presence of previous restoration (yes or no), preoperative symptoms (no symptoms or mild sensitivity to heat or sweet stimulus), results of percussion, and pulp sensitivity testing (cold testing; with a response in normal limits or indicating transient sensitivity) as well as preoperative radiographs.
Details of treatment consisted of bleeding circumstance, pulp exposure size, type of cavity restoration (classes I, II, III, IV, or V), and operator (faculty member or resident). The bleeding circumstance was defined as oozing or normal; oozing accounted for no obvious bleeding or slightly oozing blood flow, while normal indicated that apparent blood flow from the exposed pulp that could be stopped within 5 min [15]. The pulp exposure size was categorized as having a diameter < 1 mm or ≥ 1 mm.
At the follow-up examination, patients were subjected to clinical and radiographic examinations for outcome assessment. The collected information included the presence or absence of clinical symptoms after treatment, quality of restoration (intact or fractured, presence of recurrent or secondary caries), and results of pulp vitality and sensitivity testing (electric and cold testing), periodontal examination, and percussion testing. Periapical radiographs were taken to examine the depth of caries lesion, the periradicular condition, root resorption, pulp obliteration, and dentin bridge formation.
Radiographic calibration
The follow-up periapical radiographs were evaluated by two examiners (one endodontist and one radiographic specialist). Apical radiographic data were randomly selected from 20% of the study samples (70 cases), and measurements were repeated twice independently and at a 2-week interval to test the consistency of the examiners themselves and between the examiners. Discussion was required to reach a consensus if two examiners’ evaluation results were inconsistent.
Outcome assessment
As this study was retrospective and some cases were not regularly reviewed after treatment, the occurrence of success was defined as the date of the last review, and the occurrence of failure was defined as the date when the patient voluntarily sought medical treatment due to discomfort or the date when the review found failure. Evaluation of the treatment outcome was based on the results of clinical and radiographic examinations.
-
(1)
Success criteria: ① no clinical signs or symptoms such as spontaneous pain, percussion pain, excessive mobility, sinus tract, and swelling; and ② positive response to pulp vitality testing and a response to cold testing within normal limits (the same as a normal tooth); and ③ no root resorption or periapical rarefaction on radiographs.
-
(2)
Failure criteria: a case with any deviation from the success criteria was considered a failure. The case was considered an endodontic failure when there was pain, swelling, or a new periapical lesion; the case was considered a restorative failure when extraction or pulpectomy was required due to secondary or recurrent caries, loss of restoration, or tooth fracture.
Statistical analysis
Data were analyzed using SPSS version 26.0. Cohen’s kappa was used to assess interexaminer and intraexaminer agreement. The chi-square test was used to analyze the correlation of clinical factors between the reviewed and dropout cases. Kaplan‒Meier survival analysis was used to calculate the cumulative survival of teeth treated with DPC. The outcome rates were presented as percentages (%), and the survival time was defined as described above. Univariate and multivariate Cox proportional hazards regressions were used to analyze potential prognostic factors. Missing data were defined as unknown (see Table 3). The rate of dentin bridge formation was defined as follows: dentin bridge formation (%) = (number of teeth forming a dentin bridge/total number of teeth) × 100%. The level of significance was set at 2-tailed P < 0.05.
Results
Study cohort
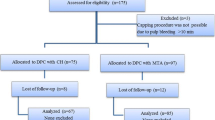
The process of case inclusion and exclusion is illustrated in Fig. 2. The data of 655 patients who underwent DPC were collected from the medical record database, and after review, 523 patients with a total of 551 teeth were included. One hundred four cases were excluded due to pulp capping with calcium hydroxide (CH) (75), absence of permanent restoration with resin composite (10), incomplete data records (13), pregnancy (4), deciduous teeth (1), and systemic disease (1). Three hundred thirty-four patients, including a total of 354 teeth, were available for the final clinical examination, and the recall rate was 64%. The reasons for dropout were as follows: 29 patients were uncontactable, 37 patients moved away from Beijing, and 131 patients refused to be reviewed (Fig. 2). There was no significant difference between the reviewed and dropout cases in age, sex, type of tooth, location of tooth, or class of cavity (Table 1). The age of the cases ranged from 12 to 82 years, with an average age of 32.3 ± 0.6 years. Among the reviewed cases, 112 were male and 242 were female. The follow-up period ranged from 12 to 85 months, with an average of 27.0 ± 0.8 months.
Survival outcomes
The total success rate of DPC was 85% (302/354). The cumulative survival rates at 1, 2, 3, 4, 5, and 6 years were 92%, 87%, 83%, 76%, 72%, and 62%, respectively, and the attended cases at each time point were, respectively, 331, 180, 86, 46, 32, and 13 teeth (Fig. 3 and Table 2). After analyzing the 52 cases of failed tooth treatment, 48 cases were defined as endodontic failures, and 4 cases were defined as restorative failures (Table 2).
Potential prognostic factors
Univariate analysis indicated a significantly increased risk of failure in patients aged above 40 years and those treated by resident operators (P ≤ 0.01), with hazard ratios of 2.18 and 2.27, respectively (Table 3 and Fig. 3).
Multivariate analysis also screened 2 risk factors, namely, patient age over 40 years and treatment by a resident operator (P < 0.05), with hazard ratios of 2.06 and 2.19, respectively.
There was no significant correlation between treatment outcome and sex, tooth type, tooth location, class of restoration, preoperative signs or symptoms, percussion or pulp sensitivity test result, bleeding circumstance, pulp exposure size, cavity restoration, postoperative discomfort, or depth of caries (Table 3).
Radiographic outcomes
For the assessment of dentin bridge, Cohen’s kappa analysis showed an interexaminer agreement of 0.956 and intraexaminer agreements of 0.927 and 0.981 for the two examiners, respectively.
No dentin bridge formation was observed in 64% of successfully treated teeth (192/302) (Fig. 4a, b). Dentin bridges were observed in 109 teeth on periapical radiographs, accounting for only 36% of successfully treated teeth (n = 302) (Fig. 4c–e). Pulp chamber narrowing was observed in 2 teeth (Fig. 4f, g). Diffuse pulp calcification and root resorption were not observed in any case.
Presentation of radiographs in 3 cases. a, b No dentin bridge formation was observed. a Preoperative radiograph showing deep caries. b Radiograph 39 months after DPC. c–e Dentin bridge formation was observed. c Preoperative radiograph showing deep caries. d Radiograph immediately after DPC. e Radiograph 24 months after DPC. f–h Pulp chamber narrowing was observed. f Preoperative radiograph showing a normal pulp chamber. g Pulp chamber narrowing was observed at 12 months after DPC. h Further pulp chamber narrowing at 24 months after DPC
Discussion
On the basis of a large sample size and a long follow-up period of over 4 years, the design of this research was retrospective regarding treatment procedures and prospective regarding the assessment of treatment outcomes. In this study, the total success rate of DPC was 85% (302/354), which was similar to the outcome of other researches using MTA [4, 5, 16, 17] and Biodentine [4, 6, 7]. The Kaplan–Meier analysis showed that the cumulative survival rates at 1, 2, 3, 4, 5, and 6 years were 92%, 87%, 83%, 76%, 72%, and 62%, respectively. A long-term retrospective analysis found that the cumulative survival rates after DPC with MTA were 93%, 89%, and 71% at 2, 4, and 6 years, respectively [16]. Kundzina et al. found that the cumulative survival rate in MTA group was 85% at 3 years [17]. Consequently, the cumulative survival rates for DPC with BP showed similar outcome to the researches with MTA. On the basis of a limited follow-up period and the decreasing of attended cases by year, it was insufficient to provide a reliable long-term survival rate for DPC with BP. As the attended cases decrease, the cumulative survival rate dropped sharply at approximately 6 years in the plot of total survival (Fig. 2a), which increased the uncertainty of the survival estimate [18].
Age is an important prognostic factor in VPT, and the reparative ability of pulpal tissue is considered better in younger patients. In this study, the success rate was significantly higher in patients under 40 years of age than in those over 40, which has also been confirmed in previous studies [6, 9, 19]. Forty years of age has been found to be a significant cutoff point regarding the outcome of DPC [6, 9], and an analysis has shown that DPC is more cost-effective in younger patients (≤ 40 years old), which accounted for choosing 40 as the cutoff point in this study [20]. In addition, as the average age of the included patients was 32 years, 30 and 35 years of age were also analyzed as cutoff points, and the outcomes indicated a significant difference between the younger and older age groups. However, Cho et al. found a significant effect of age on the outcome by univariate analysis but not by multivariate analysis [9]. Additionally, some other studies could not confirm the effect of age on the treatment outcome [7, 10]. Harms et al. found the younger cohort seemed to show higher cumulative survival rate than the older cohort although no significant difference was found [7]. These results suggest that the effect of age on prognosis is influenced by many factors. In this study, multivariate analysis also indicated that age over 40 years was a risk factor (P < 0.05), with a hazard ratio of 2.06. This could be explained by changes in homeostasis and functional regulation in the human body during aging. In vivo, the proliferation and differentiation potential of human dental pulp stem cells decrease with age [21].
Operator experience was found to be a potential prognostic factor, which is notable in this study because some of the operators were residents. The success rate was higher among cases treated by faculty than those treated by residents. The practice of all residents was conducted under the guidance of faculty and followed the same guidelines. One reason for the poorer outcomes may be that more operation time is needed, in general, by residents, which could lead to poorer results regarding infection and trauma control.
The effect of the depth of caries on periapical radiographs on the prognosis after DPC was also analyzed in this study. The ESE proposed the concepts of deep caries and extremely deep caries in 2019 [2]. Demant et al. found that bacterial invasion into the pulp tissue and partial coronal pulp tissue necrosis were usually observed in teeth diagnosed with extremely deep caries, while in teeth diagnosed with deep caries, bacteria usually invaded the primary dentin, and bacterial infection was not observed in the pulp tissue [22]. However, a statistically significant effect of the depth of caries on the prognosis after DPC was not found in this study, and this method could not be used for teeth with buccal or lingual caries. This result may be due to the diagnosis of the included cases being normal pulp or reversible pulpitis, which likely involve greater odontoblast survival.
It is necessary to focus on the discomfort of patients before and after treatment in clinical research. The incidence of short-term postoperative discomfort, which was defined as discomfort occurring within 2 weeks after treatment, was 28.0% and 34.2% in cases of successful and failed treatment, respectively, with no significant difference between the two groups (Table 3). Most of the complaints of discomfort consisted of mild sensitivity to cold and heat stimulation, and there were a few reports of slight spontaneous pain. The postoperative discomfort lasted for a short time and resolved spontaneously. Some studies have found significant pain relief after VPT similar to [23] or even better [24] than the pain relief achieved with root canal treatment. However, few studies have described the incidence of short-term postoperative discomfort.
Although a high rate of dentin bridge formation has been observed after DPC with BP in animal studies and in vivo [14, 25], it is difficult to identify all dentin bridges due to the limited resolution of periapical radiographs. Dentin bridge formation was observed in 109 of 302 successfully treated teeth (36%) in this study. Interestingly, in the two teeth with a normal pulp status, dentin bridge formation was observed under the microscope after loss of the fillings, while it could not be observed on the periapical radiographs. The relationship between the outcome of VPT and the observation of dentin bridge formation on radiographs is still uncertain [26]; thus, dentin bridge formation was not included in the outcome assessment criteria in this study. The mechanism and importance of dentin bridge formation, as well as the definition of and criteria for evaluating dentin bridges, require further exploration in the future.
Conclusions
This retrospective cohort study has shown that on the basis of appropriate indication selection and treatment procedures, long-term success is possible in mature permanent teeth with carious pulp exposure through DPC using BP. Patient age and operator experience are potential prognostic factors. In the future, more high-quality prospective studies should be conducted to further investigate the outcomes and prognostic factors of DPC in mature permanent teeth with carious pulp exposure.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
George B, Sergio K, Nicholas C (2016) Cohen’s pathway of the pulp, 11th ed. Chapter 23: Vital Pulp Therapy. Elsevier, Missouri, pp 849
Duncan HF, Galler KM, Tomson PL et al (2019) European Society of Endodontology position statement: management of deep caries and the exposed pulp. Int Endod J 52:923–934
Taha NA, About I, Sedgley CM et al (2021) Conservative management of mature permanent teeth with carious pulp exposure. Int Endod J 46:S33–S41
Linu S, Lekshmi MS, Varunkumar VS et al (2017) Treatment outcome following direct pulp capping using bioceramic materials in mature permanent teeth with carious exposure: a pilot retrospective Study. J Endod 43:1635–1639
Suhag K, Duhan J, Tewari S et al (2019) Success of direct pulp capping using mineral trioxide aggregate and calcium hydroxide in mature permanent molars with pulps exposed during carious tissue removal: 1-year follow-up. J Endod 45:840–847
Lipski M, Nowicka A, Kot K et al (2018) Factors affecting the outcomes of direct pulp capping using Biodentine. Clin Oral Investig 22:2021–2029
Harms CS, Schäfer E, Dammaschke T (2019) Clinical evaluation of direct pulp capping using a calcium silicate cement-treatment outcomes over an average period of 2.3 years. Clin Oral Investig 23:3491–3499
Liu SY, Gong WY, Liu MQ et al (2020) Clinical efficacy observation of direct pulp capping using iRoot BP Plus therapy in mature permanent teeth with carious pulp exposure. Zhonghua Kou Qiang Yi Xue Za Zhi 55:945–951
Cho SY, Seo DG, Lee SJ et al (2013) Prognostic factors for clinical outcomes according to time after direct pulp capping. J Endod 39:327–331
Mente J, Hufnagel S, Leo M et al (2014) Treatment outcome of mineral trioxide aggregate or calcium hydroxide direct pulp capping: long-term results. J Endod 40:1746–1751
Emara R, Elhennawy K, Schwendicke F (2018) Effects of calcium silicate cements on dental pulp cells: a systematic review. J Dent 77:18–36
Cushley S, Duncan HF, Lappin MJ et al (2021) Efficacy of direct pulp capping for management of cariously exposed pulps in permanent teeth: a systematic review and meta-analysis. Int Endod J 54(4):556–571
Rao Q, Kuang J, Mao C et al (2020) Comparison of iRoot BP plus and calcium hydroxide as pulpotomy materials in permanent incisors with complicated crown fractures: a retrospective study. J Endod 46:352–357
Liu S, Wang S, Dong Y (2015) Evaluation of a bioceramic as a pulp capping agent in vitro and in vivo. J Endod 41:652–657
Asgary S, Eghbal MJ, Shahravan A et al (2022) Outcomes of root canal therapy or full pulpotomy using two endodontic biomaterials in mature permanent teeth: a randomized controlled trial. Clin Oral Investig 26(3):3287–3297
Çalışkan MK, Güneri P (2017) Prognostic factors in direct pulp capping with mineral trioxide aggregate or calcium hydroxide: 2- to 6-year follow-up. Clin Oral Investig 21(1):357–367
Kundzina R, Stangvaltaite L, Eriksen HM et al (2017) Capping carious exposures in adults: a randomized controlled trial investigating mineral trioxide aggregate versus calcium hydroxide. Int Endod J 50(10):924–932
Carter RE, Huang P (2009) Cautionary note regarding the use of CIs obtained from Kaplan-Meier survival curves. J Clin Oncol 27(2):174–175
Dammaschke T, Leidinger J, Schafer E (2010) Long-term evaluation of direct pulp capping–treatment outcomes over an average period of 6.1 years. Clin Oral Investig 14:559–567
Brodén J, Davidson T, Fransson H (2019) Cost-effectiveness of pulp capping and root canal treatment of young permanent teeth. Acta Odontol Scand 77:275–281
Ning T, Shao J, Zhang X et al (2020) Ageing affects the proliferation and mineralization of rat dental pulp stem cells under inflammatory conditions. Int Endod J 53:72–83
Demant S, Dabelsteen S, Bjørndal L (2021) A macroscopic and histological analysis of radiographically well-defined deep and extremely deep carious lesions: carious lesion characteristics as indicators of the level of bacterial penetration and pulp response. Int Endod J 54(3):319–330
Beauquis J, Setbon HM, Dassargues C et al (2022) Short-term pain evolution and treatment success of pulpotomy as irreversible pulpitis permanent treatment: a non-randomized clinical study. J Clin Med 11:787
Asgary S, Eghbal MJ (2010) The effect of pulpotomy using a calcium-enriched mixture cement versus one-visit root canal therapy on postoperative pain relief in irreversible pulpitis: a randomized clinical trial. Odontology 98:126–133
Okamoto M, Takahashi Y, Komichi S et al (2018) Dentinogenic effects of extracted dentin matrix components digested with matrix metalloproteinases. Sci Rep 8(1):10690
Duncan HF (2022) Present status and future directions-vital pulp treatment and pulp preservation strategies. Int Endod J 55:497–511
Acknowledgements
The authors would like to thank Ms. Xueying Li for her assistance and advice on the statistical analysis.
Funding
The research was supported by the National Natural Science Foundation of China (81870753), Beijing Municipal Science & Technology Commission (Z211100002921042), and the Program for New Clinical Techniques and Therapies of Peking University School and Hospital of Stomatology (PKUSSNCT-21G03).
Author information
Authors and Affiliations
Contributions
Jiaqi Chen: conceptualization, data curation, formal analysis, investigation, methodology, and writing—original draft. Siyi Liu: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, and writing—review and editing. Muqing Liu: formal analysis—radiographic data. Yanmei Dong: conceptualization, methodology supervision, and writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The research was conducted with approval from the Biomedical Institutional Review Board of Peking University School and Hospital of Stomatology (PKUSSIRB-202163044).
Consent to participate
A written informed consent was obtained from all participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiaqi Chen and Siyi Liu have contributed equally and share the first authorship.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, J., Liu, S., Liu, M. et al. Multivariate prognostic analysis of direct pulp capping using a bioceramic material in mature permanent teeth with carious pulp exposure: a retrospective cohort study. Clin Oral Invest 27, 5287–5296 (2023). https://doi.org/10.1007/s00784-023-05148-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05148-2