Abstract
Objectives
Aim of this clinical study was to evaluate the effects on gingival thickness of three surgical techniques for root coverage: the coronally advanced flap (CAF) alone, with a sub-epithelial connective tissue graft (SCTG) or with leukocyte- and platelet-rich fibrin (L-PRF) membranes.
Methods
Sixty patients with RT1 single maxillary gingival recession were treated with CAF + L-PRF (20 patients), CAF + SCTG (20 patients) or CAF alone (20 patients). At baseline and 6-month after treatment, gingival thickness (GT), keratinized tissue width (KT), gingival recession (GR), clinical attachment level (CAL), probing depth (PD), PROMs, and the aesthetic outcome were recorded.
Results
CAF + SCTG and CAF + L-PRF groups showed a significantly greater mean GT increase than CAF alone (0.31 ± 0.10 mm) with no significant differences between CAF + SCTG (0.99 ± 0.02 mm) and CAF + L-PRF (0.92 ± 0.52 mm) groups (p = 0.55). CAF + SCTG was associated with a significantly greater KT gain (3.85 ± 1.04 mm), while in CAF + L-PRF (2.03 ± 0.53 mm) and CAF (1.50 ± 0.69 mm) groups, KT was not significantly increased. Both GR and CAL showed a significant within groups’ improvement, without among-groups differences. No significant among-groups difference for the aesthetic outcome but greater discomfort and pain-killer consumption in CAF + SCTG group was detected.
Conclusion
All investigated surgical techniques produced significant GR reduction and CAL gain. GT was similarly augmented by CAF + L-PRF and CAF + SCTG techniques; however, the CAF + SCTG technique produced a more predictable KT and GT increase.
Clinical relevance
The results of our study suggest that the CAF + SCTG technique represents the most predictable method for the clinician to improve the gingival phenotype, an important factor for long term gingival margin stability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 2017 Consensus Report of the World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions recommends the use of “periodontal phenotype” to indicate the combination of gingival phenotype and buccal bone morphotype [1]. Gingival phenotype refers to the three-dimensional gingival volume covering the buccal bone plate; it may be affected by environmental factors and modified by clinical interventions [1]. Gingival phenotype is determined by gingival thickness (GT) and keratinized tissue width (KT); these anatomic features allow us to classify the “phenotypes” in three categories: thin scalloped, thick flat, and thick scalloped [2]. The influence of soft tissue thickness on both the etiology of gingival recessions (GRs) and the clinical outcomes after root coverage procedures has been highlighted by several authors [3, 4]. It is commonly accepted that the thin phenotype is more prone to develop GRs [5]; similarly, worsening GRs are often associated with a thin phenotype [6]. Therefore, when covering exposed roots, it is desirable to obtain a thick gingival tissue to reduce the risk of recurrence [7], especially when the initial flap thickness is less than 0.8mm [8]. A review conducted by Hwang and Wang concluded that complete root coverage (CRC) was directly related to flap thickness [4]. Currently, the coronally advanced flap (CAF) alone or with the addition of a sub-epithelial connective tissue graft (SCTG), are the two main approaches for treating GRs [9]. CAF + SCTG demonstrated to be a predictable treatment of GRs, particularly in a long-term scenario [10], but the resulting palatal wound unavoidably increases patient’s morbidity [11].
On the other hand, CAF technique, while avoiding a second surgical site, reduces the surgical time and shows a better post-operative course, but is less effective in preventing GRs recurrence [12].
Leukocyte- and platelet-rich fibrin (L-PRF) may represent a valuable alternative to autogenous soft tissue grafts. L-PRF belongs to a group of second-generation blood autologous products, obtained from peripheral blood centrifugation without any anticlotting agent, to obtain a dense three-dimensional clot architecture concentrating platelets, fibrin, leukocytes, cytokines, and growth factor [13]. It promotes stabilization and revascularization of the flaps [14], contributes to soft tissue wound healing, and reduces post-operative discomfort [15]. L-PRF is used in various fields of regenerative medicine as well as in periodontal plastic surgery [16, 17].
The main aim of surgical procedures for the treatment of gingival recessions is to achieve a predictable root coverage [1]. This outcome has been extensively investigated by previous literature, while only few studies assessed the role of GT in obtaining and maintaining the complete root coverage over time [18].
Little is known about the occurrence of significant changes of the gingival thickness following CAF + L-PRF treatment [19,20,21]; GT increase seems to occur when a L-PRF membrane is used in association with CAF [20], while KT augmentation is still controversial [19, 22].
The purpose of this study was to investigate in what extent gingival thickness changes may affect the root coverage after three surgical techniques, CAF, CAF + SCTG, and CAF + L-PRF.
Materials and methods
Experimental design
This is a prospective, randomized, and controlled single-blind clinical trial comparing the effects of three surgical treatment modalities for RT1 [23] single gingival recessions on gingival thickness: CAF with L-PRF (CAF + L-PRF group), CAF with SCTG (CAF + SCTG group), and CAF alone (CAF group). Clinical parameters were evaluated at baseline and 6 months after surgery. The study protocol was registered at ClinicalTrials.gov as NCT03712852 after the approval obtained by the G. D’Annunzio University ethic committee and the first participant was enrolled in November 2018. Figure 1 shows the CONSORT [24] flow diagram.
Patients’ inclusion criteria
(1) To be systemically healthy; (2) no medications affecting the periodontal status in the previous 6 months; (3) no pregnancy/lactating; (4) never-smoker/former-smoker ≥ 10 years; (5) full-mouth plaque score (FMPS) [25] and full-mouth bleeding score (FMBS) [26] < 20% at surgery, (6) ≥ 20 teeth without dental mobility, (7) no periapical lesions at experimental sites, (8) one maxillary RT1 buccal gingival recession. Each patient with a single maxillary recession was screened among patients diagnosed with gingival recession after having visited at the Unit of Periodontology of the G. D’Annunzio University of Chieti-Pescara, Italy.
Patient’s concern for GR progression, aesthetics, and dentinal hypersensitivity was the main indications for the surgical procedure. The patients signed a consent form approved by the ethical committee of the G. D’Annunzio University after having received comprehensive information. The study is in accordance with the declaration of Helsinki of 1975, as revised in 2013.
Sample size
The sample size was determined using a multi-arm clinical trial model. An expected standard deviation of 0.3 mm in GT was set according to a previous study [27]. A sample size of 18 patients per group was calculated using three pairwise test (familywise adjusted by the Bonferroni methods) to detect at the 6-month follow-up a minimum difference of 0.3 mm in GT among the three groups with an α set at 0.05 and a power of 0.80. The number of patients was increased of 10% (20 patients per each arm) considering possible dropouts.
Randomization and blinding protocol
Random allocation of defects to groups was obtained by a computer-generated table (R Core Team 2020), opaque envelopes were used to conceal group allocation and opened during surgery after defect’s debridement. Matching between group and treatment was known only to the study coordinator, not involved in the trial, responsible even for keeping and breaking the blinding.
Two blinding levels were planned. The first related to one examiner and data collector (LR), trained with a previous intra-examiner calibration exercise (20 patients measured for all periodontal parameters twice, 24 h apart to assure a Fleiss-Cohen’s \({\mathrm{Kappa}}_{w}\ge 0.6\)).
At the second level was the surgeon (PS), blinded until envelopes opening. The statistician analyzed letter-labeled data groups which were re-matched with the treatment labels by the coordinator after blinding breaking.
Pre-surgical treatment
Participants were instructed with adequate oral hygiene methods. It was suggested the use of an electric toothbrush with pressure control (Oral-B Pro 6000 Cross Action; Procter & Gamble Italia SPA Gattatico (RE)) and extra-soft head (Oral B Sensitive EBS17; Procter & Gamble Italia SPA Gattatico (RE). Instructions on the optimal use of dental floss, and/or interdental brush were given. All patients underwent professional supragingival scaling, and they were strictly monitored about the maintenance of periodontal health. All patients received an upper arch impression to obtain a cast model. Subsequently, a single measurement point was measured at the mid-buccal site of each study tooth on the study model. Then, an occlusal customized resin acrylic stent was prepared for each participant and stored for all the study duration [28].
Clinical measurement
All clinical parameters were measured by the same investigator (LR) at baseline and 6 months later. After the customized stent was applied, GT was measured by probing with a #15 endodontic reamer [29] (Mani, Reamer length 25 mm, size 15, Utsonomiya shi, Japan) 1 mm apical to the sulcus depth at the reference point (mid-buccal) marked on the stent. The reamer was inserted perpendicularly to the gingival surface until the hard tissue was reached. A silicon disk stop was moved to contact the soft tissue surface and fixed by a cyanoacrylate adhesive drop. Once the reamer was gently removed, the GT was calculated measuring the distances from the reamer’s tip to the silicon disk with a digital caliper (Mitutoyo, Model CD-6″ B, Andover, UK) accurate to the nearest 0.01 mm. Other measurements were accurately recorded to the nearest millimeter with a periodontal probe (XP 23/UNC15, Hu-Friedy MFG-Co, Inc., Chicago, IL, USA). GR was measured from the cement-enamel junction (CEJ) to the mid-buccal point of the gingival margin. KT resulted from the distance of the mid-buccal site of the gingival margin to the mucogingival junction. PD and CAL were measured as the distance between the bottom of the pocket and the gingival margin and CEJ, respectively. Complete root coverage (CRC) was evaluated for each experimental site. The percentage of obtained root coverage was also calculated for each GR.
Patient-reported and aesthetic outcomes
Participants were required to record the number of painkillers assumed during the first post-operative week. At the 2-week follow-up the patients evaluated on a VAS (1–10) the level of discomfort suffered after the surgery. The final gingival aesthetic was evaluated using the root coverage esthetic score (RES) at the 6-month follow-up, according to Cairo et al. [30].
Surgical technique
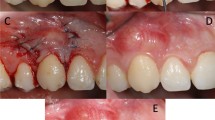
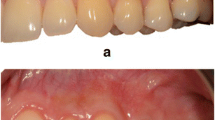
All surgical procedures (Fig. 2) were performed by the same experienced clinician (PS). Root debridement was accurately performed using sharp curettes. According to De Sanctis and Zucchelli [31], two horizontal incisions were performed mesial and distal to the GR at a distance from the tip of the anatomical papillae equal to the depth of the recession plus 1 mm. Secondly, two oblique incisions were made from the end of the two horizontal incisions to the alveolar mucosa. A trapezoidal-shaped flap was elevated using a split-full-split approach [31] until the CEJ of the tooth could be passively covered by the marginal portion of the flap. The anatomic papillae coronal to the horizontal incisions were diepithelialized to supply a connective tissue surface for flap’s surgical papillae suturing. In L-PRF + CAF and CAF + SCTG groups, the L-PRF grafts and the SCTGs were placed over the exposed root surface at the CEJ level. The grafts were positioned on the root defect, stabilized by sutures with 4–0 braided resorbable polylactic sutures (Ethicon, Johnson & Johnson, Pomezia, Italy), and covered by the flap that was coronally positioned. This coronal position was maintained through 4–0 silk sutures (Ethicon, Johnson & Johnson, Pomezia, Italy) about 1–2 mm over the CEJ in a tension-free position.
A CAF treatment. Clinical aspect of the control site at baseline. B The trapezoidal-shaped flap is elevated. C The flap is advanced and sutured coronally. D Clinical aspect of the site 6 months after surgery. E CAF + SCTG treatment. Clinical aspect of the control site at baseline. F The flap elevation with the split-full-split approach. G SCTG is placed over the exposed root surface. H The graft is covered by the flap that was coronally positioned and sutured. I Clinical aspect of the control site 6 months after surgery. L. CAF + L-PRF treatment. The test site before treatment. M The flap elevation with the split-full-split approach. N L-PRF membranes is positioned and fixed on the recession site. O Coronally advanced flap is sutured over the L-PRF membranes. P Clinical aspect of the gingival recession after 6 months
L-PRF preparation
The Dohan-Choukroun et al. [32] protocol was applied to produce L-PRF immediately before surgery. From each patient of the L-PRF groups, 30 ml of blood was collected in three 10-ml sterile tubes without anticoagulant, and it was quickly centrifuged (IntraSpin™, Intra-Lock System Europa SpA, Salerno, Italy) at 2700 revolutions/minute for 12 min (RCF-clot = 408 g; RCF-max = 653 g).
The fibrin clot (L-PRF) was collected and squeezed in the L-PRF box to obtain 2 membranes: each membrane was turned in on itself, and two membranes placed one over the other to obtain a 1.5-mm-thick L-PRF graft measured by a standard caliper (Mitutoyo, Model CD-6″ B, Andover, UK) [33].
Connective tissue graft preparation
The connective tissue graft was harvested from the palatal area on the opposite side of the gingival defect according to Zucchelli et al. [11]. Briefly, two horizontal and two vertical incisions delimitated the donor area. The graft was separated from the underlying tissues by the scalpel’s blade oriented parallel to the palatal surface to obtain an about 2-mm-thick graft. Then, the graft was de-epithelialized by a 15c blade, and the fatty tissue was eliminated until obtaining an about 1.5-mm-thick graft measured by a standard caliper (Mitutoyo, Model CD-6″ B, Andover, UK).
Postoperative care
Analgesic consumption was recommended in case of pain after the surgical procedure (400 mg of oral ibuprofen, Nurofen Express 400 mg, Reckitt Benckiser Group, Slough, Berkshire, UK); all participants received 2 g/day amoxicillin + clavulanic acid (Augmentin, SmithKline Beecham, Milan, Italy) for 6 days for post-operative infection control, and 0.12% chlorhexidine rinses (Dentosan 0.12 Trattamento Mese, Johnson & Johnson, Pomezia, Italy), twice daily for 3 weeks. Sutures were removed after 14 days, after that patient were instructed to cautious brushing by a soft toothbrush and interdental brushing; meantime, the patients used a 1% chlorhexidine gel (Corsodyl Dental gel, GlaxoSmithKline Consumer Healthcare S.p.A. — Baranzate, Italy) twice daily. Weekly supragingival professional hygiene and motivational reinforcement were administered to the patients for 6 weeks.
Statistical analysis
Descriptive statistics of GT, KT, CAL, REC, and PPD were planned, expressed as observed means ± SDs and 95% confidence intervals. Multiple univariate analyses for each variable were performed. All outcomes were first analyzed with linear models assuming residuals normality (ANOVA for GT, ANCOVAs for all the others but CAL, which was analyzed with a moderated regression followed by Johnson-Neyman technique) with nonparametric case-resampling bootstrap (Figure-1-Supplementary Material). GT was analyzed by pairwise Cliff’s delta tests, not assuming equal-shaped distributions, and by a heteroscedastic ANOVA with Games-Howell post hoc tests. A sensitivities analysis with several types of robust methods (M-estimators and high breakdown LTS estimators, both with Huber’s, Hampel’s, and Biweight’s loss functions) was conducted to get an effect-size estimate by means of bootstrap bias corrected and accelerated (BCa) 95% Bonferroni-Holm simultaneous confidence intervals.
All the outcomes were analyzed by post hoc distribution-free Nemenyi’s tests. Multiplicity adjustments used Bonferroni’s or Tukey’s method. Statistical software R 3.5.1 (R Core Team (2020). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.) was used.
Results
Sixty patients (twenty-seven women), between 18 and 47 years (mean 32.4 ± 5.0 years), were enrolled in this study. All patients completed the trial fitting with specifications, and no postoperative complications were detected. Experimental groups were balanced by age and gender (p > 0.05).
Out of 60 maxillary recessions examined, 17 (6CAF + L-PRF; 6CAF + SCTG; 5CAF) incisors, 23 canines (8CAF + L-PRF; 7CAF + SCTG; 8CAF), and 20 premolars (6CAF + L-PRF; 7CAF + SCTG; 7CAF) were included. The results obtained in this study are summarized in Tables 1 and 2, and in Figs. 3 and 4.
Pairwise Nemenj’s test group comparisons for all clinical parameters and the Johnson-Neyman technique probing the interaction between treatment and CAL baseline in the CAL gain regression for the CAF + L-PRF and CAF-SCTG group comparison. (Johnson-Neyman significant region is outside the CAL baseline range 4.76 to 6.31 p < 0.017)
A comparison between graphs about the confirmatory analysis (first column) and to two post-hoc analyses (second and third columns) to obtain the best possible fit. The exploratory analyses, described in the text, are a 2 way-ANOVA per sub-groups identified by a baseline threshold value of 1.2 mm in the second column, and an ANOVA for four groups (the L-PRF group was divided into high- and low-responders). The first row shows the boxplots, the second row reports the per-treatment regression lines of the GT increase on GT baseline values (with several attempts of modeling the two subgroups in column two), and the third row reports the residuals density plots, showing the treatments variance not explained by each model
GT significantly increased from baseline in all experimental groups. CAF + SCTG and CAF + L-PRF groups showed a significantly greater GT increase (0.99 ± 0.02 and 0.92 ± 0.52 respectively) as compared to the CAF group (0.31 ± 0.10) while their direct comparison was inconclusive (NS). KT showed a significant increase from baseline to the 6 months follow-up in the CAF + SCTG group, while the same result was not observed in CAF + L-PRF and CAF groups. In particular, KT has more than doubled in the CAF + SCTG group, and this increase was significantly greater when compared to CAF + L-PRF and CAF groups; KT increase was not significantly different between the latter two groups. GR significantly decreased and CAL significantly improved in each group from baseline to the 6 months follow-up, without significant differences. CRC was 74% in CAF + L-PRF group, 83% in CAF + SCTG group, and 73% in CAF group, while the mean root coverage was 80,15% with CAF + L-PRF, 86% with CAF + SCTG, and 75% with CAF technique. PD showed limited changes in the experimental period without significant differences among groups, the only exception being CAF + SCTG and CAF groups comparison (p = 0.012). Figure 3 shows pairwise group comparisons for all clinical parameters and the Johnson-Neyman technique probing the interaction between treatment and baseline CAL in the CAL gain regression for the CAF + PRF and CAF-SCTG group comparison. No significant difference was detected for RES among the three groups at 6 months but the CAF + SCTG group showed both a greater patient-reported discomfort VAS values (CAF + SCTG vs. CAF p = 0.011, CAF + SCTG vs. CAF + L-PRF p = 0.038) and painkillers consumption (CAF + SCTG-CAF p < 0.001, CAF + SCTG-CAF + L-PRF p < 0.001) than the other 2 groups.
Discussion
This study evaluated the results of 3 different surgical techniques CAF, CAF + SCTG, and CAF + L-PRF in changing GT at recession defects. To our knowledge, this was the first study to perform a multilevel analysis of three groups CAF + L-PRF, CAF + SCTG, and CAF, having GT as a primary outcome; although the main outcome of these surgical procedures is to offer a root coverage, GT was chosen as primary outcome considering the growing interest in gingival biotype as a promising potential factor of root coverage. The transgingival probing is the method chosen to measure GT. Although other new procedure have been proposed (ultrasound, CT), it is the first [29] and still the gold standard method according to the literature [34]. Our data reported a significant increase in GT when using L-PRF membranes; however, the CAF + L-PRF group showed a remarkable different distribution in GT increase as compared with the CAF + SCTG.
The GT increase distribution in the CAF + L-PRF group looked hardly Gaussian; it was more dispersed, right-skewed, and apparently bimodal. At least 7 cases (35%, with best performances) should be excluded to make it normal; hence, the CAF + L-PRF group estimate would result dramatically lower (Fig. 4). The records from these 7 patients were promptly checked out, but no gross errors appeared. Consequently, the confirmatory analysis required both distribution-free hypothesis tests and robust resampling methods to get confidence intervals in a usual metric.
Since the diagnostics suggested a possible misspecification of the parametric model, two further attempts to improve the fit were made. We must emphasize that due to the exiguous sample size these exploratory post hoc analyses aimed to understand the idiosyncratic features of this sample rather than to provide a generalizable model (Fig. 4).
The best-responding L-PRF cases were all showing GT baseline scores below a threshold, so baseline GT was included to split the cases into 2 subgroups. Although a cutoff of 0.8 mm was reported in the literature [8], the value selected for this specific sample was 1.2 mm.
The 2-way Welch ANOVA with treatment and subgroup factors showed a not-significant interaction treatments-by-subgroup (p = 0.13). As for subgroups, only those within the L-PRF treatment differed significantly (p = 0.002): patients treated with L-PRF having initial GT < 1.2 mm obtained a greater increase than patients with GT > 1.2 mm. This result observed in the CAF + L-PRF group, in line with previous authors [8], was not detected in the SCTG group. However, a second was required since this attempt fixed the residuals' asymmetry, but not the variances heterogeneity.
The second attempt was to divide into two the L-PRF group only, according to the outcome performance similarity, using the K-means algorithm. The resulting four-group “one-way ANOVA” test showed all significant group differences, with SCTG group in between the L-PRF-best and -worst groups. The residuals were finally showing a good behavior.
It might be supposed that L-PRF membranes offered a variable GT increase, with some L-PRF grafts that offered a better performance than others. This result could be ascribed to different biological characteristics of the L-PRF membranes in each patient. In fact, although the L-PRF protocol was strictly standardized, and identical among patients (same centrifuge, tubes, and centrifugation time), it was reported that mechanical vibrations might impact the final L-PRF architecture and composition [35]. Therefore, this range of vibrations could produce a slight variation in L-PRF clots, with different biological and clinical effects [35].
Our data agree with those reported by several authors, confirming a significant GT gain when comparing CAF + L-PRF with CAF treatments [19,20,21]. Aroca et al. [20] hypothesized that this result may be related to the space-making effect of L-PRF membrane and to the growth factors anabolic activity influencing the gingival and periodontal ligament fibroblasts. However, we reported a significant GT increase in the CAF group from the baseline [36], contrary to what reported in aforementioned studies [19,20,21]. Similar findings were detected in previous studies [37, 38], offering the hypothesis that this increase may be related to the split-full-split flap elevation. To our knowledge, a few studies compared GT increase between SCTG and L-PRF [22, 39] treatments, and their results are in good agreement with ours, reporting no statistical difference in GT between groups 6 months after surgery. A new finding from our study compared to the previous ones, is that we reported a relevant variability for GT increase in the CAF + L-PRF group, with some sites increasing more than 2 mm and others, instead, only 0.4 mm (Fig. 4). This result may be explained by the different vascularization process that occurs after CAF + SCTG in comparison with CAF + L-PRF procedures. In fact, histological analysis in gingival sites treated by L-PRF and SCTG, respectively, reported that, although L-PRF stimulate an earlier vessel formation, SCTG-treated sites show a greater number of blood vessels than L-PRF treated ones [40]. Furthermore, Hwang et al. [4] reported that flap survival depends on blood supply, and this vascular system is essential to minimize the tissue shrinkage while promoting wound healing. Hence, the pre-existence of a vascular network in SCTG could easily reactivate circulation in the graft [41], reducing tissue shrinkage and explaining the more predictable GT increase in the CAF + SCTG-treated sites.
Previous studies showed different effect size on GT increase after CAF + L-PRF technique, these values ranging from 0.3 to 0.5 mm [19,20,21,22, 42]. However, these results could be ascribed to different methods used to measure GT. This parameter was recorded at the middle of keratinized tissue (Kuka et al. [21]), at 3 mm (Aroca et al. [20] and Thamaraiselvan et al. [19]) and at 2 mm (Eren et al. [22]) from gingival margin. Furthermore, these results derives from studies carried-out with different CAF techniques (i.e. treatment of single/multiple recessions): in fact, multiple gingival defects treated in a single surgical session represent a further clinical challenge compared with the treatment of isolated GR [20]. Similarly, the different GT increase [19,20,21,22, 42] may be related to the thickness of the L-PRF membrane to cover the exposed root: some authors did not measure the L-PRF graft [19, 22, 42], while Aroca, et al. [20] used a thinner layer (0.5-mm) compared to the present study (1.5-mm).
In our study, KT did not increase significantly in both CAF group and CAF + L-PRF group at 6 month in line with previous result [20]. A significant KT increase in CAF + SCTG group compared with CAF and CAF + L-PRF groups was detected according to the literature [6]; this result could be related to the potential of the palatal graft to activate epithelial keratinization [43].
The present study shows no statistically significant differences for GR reduction among the 3 groups. Therefore, all the 3 techniques similarly improved root coverage after 6 months. These observations are congruent with those from literature [21, 22, 39, 44], the only exception being Aroca et al. [20] who concluded that L-PRF addition produces less root coverage. However, this study [20] shows some limitations: as observed by Del Corso et al [45], Aroca et al. [20] used a single L-PRF membrane (0.5-mm-thick) to cover multiple recessions, while it is suggested that at least two layers of L-PRF membranes should be applied in periodontal plastic surgery procedures [45]. Furthermore, Aroca et al. [20] stored the L-PRF membranes at low temperature until surgery, affecting the potential activity of this living biomaterial [46]. In addition, clinical measurements were recorded by two non-masked investigators [20], probably affecting the conclusive results.
In the present study a significant CAL gain in the 3 groups was obtained without differences among groups, confirming previous results [20, 42].
We reported significant differences in PD changes among the 3 groups; this result was mostly due to the high statistical power of the study: in fact, all values ranged between 0.1 and 0.5 mm with confidence interval < 1 mm, thus resulting not-clinically relevant. The role of the graft in offering a better aesthetic outcome is still controversial. Some authors [47] reported a significant higher RES when the exposed root is grafted with SCTG or L-PRF, while other authors [48, 49] reported that CAF alone showed an overall higher aesthetic outcome compared with CAF + SCTG. This study reported no differences in terms of overall aesthetic score between the three groups at 6 months. This result may be related to the largely sensitivity of overall RES for the amount root coverage achieved [48], the baseline GT [8] as parameter for adding or not a graft, and the relative short-term follow-up (6 months). Furthermore, both CAF and CAF + L-PRF groups needed less analgesic consumption and discomfort compared with CAF + SCTG treatment that required a second surgical wound on the palate and longer operative times [11].
A growing body of literature investigated the role of GT in surgical procedures for root coverage, reporting that thick gingival tissues show greater resistance to inflammatory damages [50], prevent GR relapse [6], and promote CRC [4]. These conclusions about thick soft tissues may be ascribed to the greater volume of collagen matrix [4], the higher tissue vascularization [4], and the multiple layers of keratinized epithelium [4]; these features favor a better wound healing and provide a mechanical barrier from traumatic injuries [4]. These findings suggest that thick gingival tissues should be considered as a major factor to improve long-term tissue stability [51]. In fact, it was demonstrated that CAF is similarly effective to CAF + SCTG in the treatment of GRs [52] in the short term; however, in the long-term follow-up,
GR recurred in 39% of the CAF-alone–treated sites [53]. On the other hand, the additional use of SCTG under the flap seems to be effective in improving gingival thickness, producing a predictable result in the long term [51, 54].
Although it is reported that 6-month follow-up period is enough to evaluate the gingival margin stability after CAF procedure [55] and all previous studies that compare the CAF alone or CAF + SCTG with the L-PRF membrane showed a 6-month follow-up, this aspect can be considered the main limitation of this study since it may be too-short to fully assess the changes of some clinical parameters as KT [36, 56]. This could explain the absence of a significant KT increase after CAF procedure in this study, contrary to what has been reported in studies with longer follow-up periods [31, 44]. The use of the silk sutures may be considered a limitation of our procedures. However, according to Tatakis et al. 2016 [57], there is no significant difference in the number of sites obtaining CRC based on the type of suture material used; furthermore, to minimize the potential inflammation risk of silk sutures, linked to the greater accumulation of bacterial plaque, the patients rinsed with a chlorhexidine mouthwash for a period of time that exceeded the time the sutures were in place.
Conclusion
Within its limitations, this study shows that, while CAF + L-PRF, CAF + SCTG, and CAF are similarly effective in reducing GR, both CAF + SCTG, and CAF + L-PRF produce positive increases of gingival thickness. CAF + L-PRF technique avoids palatal wound, implying a reduced operative time and less discomfort but offers less predictable results in terms of GT increase. CAF + SCTG technique produces better results for KT increase and most predictable results for GT gain, confirming this technique as the current gold standard for root coverage.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article, its supplementary materials, and from the corresponding author [author MP] on request.
References
Jepsen S, Caton JG, Albandar JM et al (2018) Periodontal manifestations of systemic diseases and developmental and acquired conditions: consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 89(Suppl 1):S237–S248. https://doi.org/10.1002/JPER.17-0733
Zweers J, Thomas RZ, Slot DE, Weisgold AS, Van der Weijden FGA (2014) Characteristics of periodontal biotype, its dimensions, associations and prevalence: a systematic review. J Clin Periodontol 41(10):958–971. https://doi.org/10.1111/jcpe.12275
Maroso FB, Gaio EJ, Rösing CK, Fernandes MI (2015) Correlation between gingival thickness and gingival recession in humans. Acta Odontol Latinoam AOL 28(2):162–166. https://doi.org/10.1590/S1852-48342015000200011
Hwang D, Wang HL (2006) Flap thickness as a predictor of root coverage: a systematic review. J Periodontol 77(10):1625–1634. https://doi.org/10.1902/jop.2006.060107
Kim DM, Neiva R (2015) Periodontal soft tissue non-root coverage procedures: a systematic review from the AAP Regeneration Workshop. J Periodontol 86(2 Suppl):S56-72. https://doi.org/10.1902/jop.2015.130684
Chambrone L, Tatakis DN (2016) Long-term outcomes of untreated buccal gingival recessions: a systematic review and meta-Analysis. J Periodontol 87(7):796–808. https://doi.org/10.1902/jop.2016.150625
Parameters of Care (2000) American Academy of Periodontology. J Periodontol 71(5 Suppl):i-ii, 847–883. https://doi.org/10.1902/jop.2000.71.5-S.i
Cairo F, Cortellini P, Pilloni A et al (2016) Clinical efficacy of coronally advanced flap with or without connective tissue graft for the treatment of multiple adjacent gingival recessions in the aesthetic area: a randomized controlled clinical trial. J Clin Periodontol 43(10):849–856. https://doi.org/10.1111/jcpe.12590
Cortellini P, Pini Prato G (2012) Coronally advanced flap and combination therapy for root coverage. Clinical strategies based on scientific evidence and clinical experience. Periodontol 2000 59(1):158–184. https://doi.org/10.1111/j.1600-0757.2011.00434.x
Bhatavadekar NB, Gharpure AS, Chambrone L (2019) Long-term outcomes of coronally advanced tunnel flap (CATF) and the envelope flap (mCAF) plus subepithelial connective tissue graft (SCTG) in the treatment of multiple recession-type defects: a 6-year retrospective analysis. Int J Periodontics Restorative Dent 39(5):623–630. https://doi.org/10.11607/prd.4026
Zucchelli G, Mele M, Stefanini M et al (2010) Patient morbidity and root coverage outcome after subepithelial connective tissue and de-epithelialized grafts: a comparative randomized-controlled clinical trial. J Clin Periodontol 37(8):728–738. https://doi.org/10.1111/j.1600-051X.2010.01550.x
Zucchelli G, Mounssif I, Mazzotti C et al (2014) Coronally advanced flap with and without connective tissue graft for the treatment of multiple gingival recessions: a comparative short- and long-term controlled randomized clinical trial. J Clin Periodontol 41(4):396–403. https://doi.org/10.1111/jcpe.12224
Del Corso M, Vervelle A, Simonpieri A et al (2012) Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: Periodontal and dentoalveolar surgery. Curr Pharm Biotechnol 13(7):1207–1230. https://doi.org/10.2174/138920112800624391
Bansal M, Kumar A, Puri K, Khatri M, Gupta G, Vij H (2016) Clinical and histologic evaluation of platelet-rich fibrin accelerated epithelization of gingival wound. J Cutan Aesthetic Surg 9(3):196–200. https://doi.org/10.4103/0974-2077.191647
Femminella B, Iaconi MC, Di Tullio M et al (2016) Clinical comparison of platelet-rich fibrin and a gelatin sponge in the management of palatal wounds after epithelialized free gingival graft harvest: a randomized clinical trial. J Periodontol 87(2):103–113. https://doi.org/10.1902/jop.2015.150198
DohanEhrenfest DM, Andia I, Zumstein MA, Zhang CQ, Pinto NR, Bielecki T (2014) Classification of platelet concentrates (platelet-rich plasma-PRP, platelet-rich fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J 4(1):3–9
Bielecki T, DohanEhrenfest DM (2012) Leukocyte- and platelet-rich plasma (L-PRP)/fibrin (L-PRF) in medicine - past, present, future. Curr Pharm Biotechnol 13(7):i–ii. https://doi.org/10.2174/138920112800624274
Barootchi S, Tavelli L, Di Gianfilippo R et al (2022) Soft tissue phenotype modification predicts gingival margin long-term (10-year) stability: longitudinal analysis of six randomized clinical trials. J Clin Periodontol 49(7):672–683. https://doi.org/10.1111/jcpe.13641
Thamaraiselvan M, Elavarasu S, Thangakumaran S, Gadagi JS, Arthie T (2015) Comparative clinical evaluation of coronally advanced flap with or without platelet rich fibrin membrane in the treatment of isolated gingival recession. J Indian Soc Periodontol 19(1):66–71. https://doi.org/10.4103/0972-124X.145790
Aroca S, Keglevich T, Barbieri B, Gera I, Etienne D (2009) Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: a 6-month study. J Periodontol 80(2):244–252. https://doi.org/10.1902/jop.2009.080253
Kuka S, Ipci SD, Cakar G, Yılmaz S (2018) Clinical evaluation of coronally advanced flap with or without platelet-rich fibrin for the treatment of multiple gingival recessions. Clin Oral Investig 22(3):1551–1558. https://doi.org/10.1007/s00784-017-2225-9
Eren G, Atilla G (2014) Platelet-rich fibrin in the treatment of localized gingival recessions: a split-mouth randomized clinical trial. Clin Oral Investig 18(8):1941–1948. https://doi.org/10.1007/s00784-013-1170-5
Cairo F, Nieri M, Cincinelli S, Mervelt J, Pagliaro U (2011) The interproximal clinical attachment level to classify gingival recessions and predict root coverage outcomes: an explorative and reliability study: Interproximal CAL for gingival recessions. J Clin Periodontol 38(7):661–666. https://doi.org/10.1111/j.1600-051X.2011.01732.x
The CONSORT Group. CONSORT. Available at http://www.consort-statement.org/. Accessed 2 May 2013
O’Leary TJ, Drake RB, Naylor JE (1972) The plaque control record. J Periodontol 43(1):38. https://doi.org/10.1902/jop.1972.43.1.38
Ainamo J, Bay I (1975) Problems and proposals for recording gingivitis and plaque. Int Dent J 25(4):229–235
Mufti S, Dadawala SM, Patel P, Shah M, Dave DH (2017) Comparative evaluation of platelet-rich fibrin with connective tissue grafts in the treatment of Miller’s class I gingival recessions. Contemp Clin Dent 8(4):531–537. https://doi.org/10.4103/ccd.ccd_325_17
Vandana KL, Gupta I (2016) The relation of gingival thickness to dynamics of gingival margin position pre- and post-surgically. J Indian Soc Periodontol 20(2):167–173. https://doi.org/10.4103/0972-124X.175173
Paolantonio1 M, Murro C di, Cattabriga A, Cattabriga M (1997) Subpedicle connective tissue graft versus free gingival graft in the coverage of exposed root surfaces a 5-year clinical study. J Clin Periodontol 24(1):51–56. https://doi.org/10.1111/j.1600-051X.1997.tb01184.x
Cairo F, Rotundo R, Miller PD, Pini Prato GP (2009) Root coverage esthetic score: a system to evaluate the esthetic outcome of the treatment of gingival recession through evaluation of clinical cases. J Periodontol 80(4):705–710. https://doi.org/10.1902/jop.2009.080565
de Sanctis M, Zucchelli G (2007) Coronally advanced flap: a modified surgical approach for isolated recession-type defects: three-year results. J Clin Periodontol 34(3):262–268. https://doi.org/10.1111/j.1600-051X.2006.01039.x
Dohan DM, Choukroun J, Diss A et al (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101(3):e45–50. https://doi.org/10.1016/j.tripleo.2005.07.009
Wei Y, Cheng Y, Wang Y, Zhang X, Miron RJ, Zhang Y (2022) The effect of resting and compression time post-centrifugation on the characteristics of platelet rich fibrin (PRF) membranes. Clin Oral Investig 26(8):5281–5288. https://doi.org/10.1007/s00784-022-04496-9
Kan JYK, Morimoto T, Rungcharassaeng K, Roe P, Smith DH (2010) Gingival biotype assessment in the esthetic zone: visual versus direct measurement. Int J Periodontics Restorative Dent 30(3):237–243
DohanEhrenfest DM, Pinto NR, Pereda A et al (2018) The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 29(2):171–184. https://doi.org/10.1080/09537104.2017.1293812
Zucchelli G, Marzadori M, Mounssif I, Mazzotti C, Stefanini M (2014) Coronally advanced flap + connective tissue graft techniques for the treatment of deep gingival recession in the lower incisors. A controlled randomized clinical trial. J Clin Periodontol 41(8):806–813. https://doi.org/10.1111/jcpe.12269
Zucchelli G, Marzadori M, Mele M, Stefanini M, Montebugnoli L (2012) Root coverage in molar teeth: a comparative controlled randomized clinical trial. J Clin Periodontol 39(11):1082–1088. https://doi.org/10.1111/jcpe.12002
Stefanini M, Jepsen K, de Sanctis M et al (2016) Patient-reported outcomes and aesthetic evaluation of root coverage procedures: a 12-month follow-up of a randomized controlled clinical trial. J Clin Periodontol 43(12):1132–1141. https://doi.org/10.1111/jcpe.12626
Padma R, Shilpa A, Kumar PA, Nagasri M, Kumar C, Sreedhar A (2013) A split mouth randomized controlled study to evaluate the adjunctive effect of platelet-rich fibrin to coronally advanced flap in Miller’s class-I and II recession defects. J Indian Soc Periodontol 17(5):631–636. https://doi.org/10.4103/0972-124X.119281
Eren G, Kantarcı A, Sculean A, Atilla G (2016) Vascularization after treatment of gingival recession defects with platelet-rich fibrin or connective tissue graft. Clin Oral Investig 20(8):2045–2053. https://doi.org/10.1007/s00784-015-1697-8
Gordon HP, Sullivan HC, Atkins JH (1968) Free autogenous gingival grafts. II. Supplemental findings--histology of the graft site. Periodontics 6(3):130–133
Öncü E (2017) The use of platelet-rich fibrin versus subepithelial connective tissue graft in treatment of multiple gingival recessions: a randomized clinical trial. Int J Periodontics Restorative Dent 37(2):265–271. https://doi.org/10.11607/prd.2741
Pini Prato G, Tinti C, Vincenzi G, Magnani C, Cortellini P, Clauser C (1992) Guided tissue regeneration versus mucogingival surgery in the treatment of human buccal gingival recession. J Periodontol 63(11):919–928. https://doi.org/10.1902/jop.1992.63.11.919
Jankovic S, Aleksic Z, Milinkovic I, Dimitrijevic B (2010) The coronally advanced flap in combination with platelet-rich fibrin (PRF) and enamel matrix derivative in the treatment of gingival recession: a comparative study. Eur J Esthet Dent Off J Eur Acad Esthet Dent 5(3):260–273
Del Corso M, Sammartino G, Dohan Ehrenfest DM (2009) Re: “Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: a 6-month study.” J Periodontol 80(11):1694–1697; author reply 1697–1699. https://doi.org/10.1902/jop.2009.090253
Kattlove HE, Alexander B (1971) The effect of cold on platelets. I. Cold-induced platelet aggregation. Blood 38(1):39–48
Aldana CA, Ruiz AS, Messina DR, Quirynen M, Carrasco NP (2021) Leukocyte- and platelet-rich fibrin versus connective tissue graft for a coronally advanced flap in the treatment of Miller class I and II localized gingival recessions: a randomized controlled clinical trial. Int J Periodontics Restorative Dent 41(6):e287–e296. https://doi.org/10.11607/prd.5093
Cairo F, Cortellini P, Nieri M et al (2020) Coronally advanced flap and composite restoration of the enamel with or without connective tissue graft for the treatment of single maxillary gingival recession with non-carious cervical lesion. A randomized controlled clinical trial. J Clin Periodontol 47(3):362–371. https://doi.org/10.1111/jcpe.13229
Gil S, de la Rosa M, Mancini E et al (2021) Coronally advanced flap achieved higher esthetic outcomes without a connective tissue graft for the treatment of single gingival recessions: a 4-year randomized clinical trial. Clin Oral Investig 25(5):2727–2735. https://doi.org/10.1007/s00784-020-03587-9
Müller HP, Eger T (2002) Masticatory mucosa and periodontal phenotype: a review. Int J Periodontics Restorative Dent 22(2):172–183
Barootchi S, Tavelli L, Zucchelli G, Giannobile WV, Wang HL (2020) Gingival phenotype modification therapies on natural teeth: a network meta-analysis. J Periodontol 91(11):1386–1399. https://doi.org/10.1002/JPER.19-0715
Rasperini G, Acunzo R, Pellegrini G et al (2018) Predictor factors for long-term outcomes stability of coronally advanced flap with or without connective tissue graft in the treatment of single maxillary gingival recessions: 9 years results of a randomized controlled clinical trial. J Clin Periodontol 45(9):1107–1117. https://doi.org/10.1111/jcpe.12932
Pini Prato G, Rotundo R, Franceschi D, Cairo F, Cortellini P, Nieri M (2011) Fourteen-year outcomes of coronally advanced flap for root coverage: follow-up from a randomized trial. J Clin Periodontol 38(8):715–720. https://doi.org/10.1111/j.1600-051X.2011.01744.x
Pini Prato GP, Franceschi D, Cortellini P, Chambrone L (2018) Long-term evaluation (20 years) of the outcomes of subepithelial connective tissue graft plus coronally advanced flap in the treatment of maxillary single recession-type defects. J Periodontol 89(11):1290–1299. https://doi.org/10.1002/JPER.17-0619
Cheng YF, Chen JW, Lin SJ, Lu HK (2007) Is coronally positioned flap procedure adjunct with enamel matrix derivative or root conditioning a relevant predictor for achieving root coverage? A systemic review. J Periodontal Res 42(5):474–485. https://doi.org/10.1111/j.1600-0765.2007.00971.x
Zucchelli G, Mele M, Mazzotti C, Marzadori M, Montebugnoli L, De Sanctis M (2009) Coronally advanced flap with and without vertical releasing incisions for the treatment of multiple gingival recessions: a comparative controlled randomized clinical trial. J Periodontol 80(7):1083–1094. https://doi.org/10.1902/jop.2009.090041
Tatakis DN, Chambrone L (2016) The effect of suturing protocols on coronally advanced flap root-coverage outcomes: a meta-analysis. J Periodontol 87(2):148–155. https://doi.org/10.1902/jop.2015.150394
Acknowledgements
Authors report no conflicts of interest related to this study. The trial director was Michele Paolantonio, Department of Innovative Technologies in Medicine & Dentistry, “G. D’Annunzio” University, Chieti-Pescara, Italy.
Author information
Authors and Affiliations
Contributions
PS, PdN, MP and BF are co-first Authors having designed, written, revised and edited the work; LS and GP collaborated in designing, writing and editing the study; MS and IR cooperated in the study design, article editing and revising; LR collaborated in interpretation of data, article editing, and data collection with IR and BF. PdN did the statistical analysis.
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study protocol was registered at ClinicalTrials.gov as NCT03712852 after the approval obtained by the G. D’Annunzio University ethic committee. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained according to the protocol from all participants included in the study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santamaria, P., Paolantonio, M., Romano, L. et al. Gingival phenotype changes after different periodontal plastic surgical techniques: a single-masked randomized controlled clinical trial. Clin Oral Invest 27, 3423–3435 (2023). https://doi.org/10.1007/s00784-023-04944-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-04944-0