Abstract
Objective
A cohort prospective study was conducted to assess the three-dimensional positioning accuracy of the implant between pre-surgical and the final implant position using a static fully guided approach in the posterior area of the jaws.
Materials and methods
A total of 60 implants (30 patients) were digitally analyzed after superimposing the Digital Imaging and Communications in Medicine (DICOM) files obtained from the Cone Beam Computed Tomography (CBCT) pre- and post-implant placement. The software calculations included deviations at the implant shoulder and at the implant apex, global deviation (3D offset), and angle deviation. Statistical analysis was performed with α = 0.05.
Results
Considering the total number of implants, mesiodistal, buccolingual, and apicocoronal mean deviations at the shoulder and implant apex were equal or below 0.21 ± 0.69 mm, and only the buccolingual mean deviation at the apex reached up to 0.67 ± 1.06 mm. The mesiodistal and apicocoronal deviations were not statistically significant at both the shoulder and apex levels of the implant. The mean total angular deviation was 5.62° ± 4.09. The main limitation of this surgical approach was the requirement for a wide mouth opening.
Conclusions
Static fully guided surgery for dental implant placement exhibits minimum deviations respect to presurgical planning. The main limitation in the posterior areas is the requirement for a wide mouth opening.
Clinical relevance
Even with minimum deviations clinically acceptable, precautions and safety margins must be respected when using static full-guided surgery to place dental implants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The future of dental implant surgery appears to be shifting to navigation surgery, static or dynamic, to enhance implant positioning in relation to presurgical planning. Static navigation surgery involving the modalities of fully or partially guided surgery, also known as static computer-aided implant surgery (s-CAIS), is widespread, and its superiority over free-hand surgery in terms of implant positioning accuracy has clearly been demonstrated [1, 2]. Incorrect implant positioning can result in esthetic, prosthetic, or anatomical complications [3, 4]. Three-dimensional (3D) planning software and the different modalities of implant navigation surgery appear to minimize human errors often associated with the freehand method [5]. Furthermore, clinicians must incorporate measures to reduce implant mispositioning to minimize the risk of implant complications in elderly patients, who have more systemic conditions, a high index of periodontitis, and a reduce ability to maintain a good oral hygiene [6]. Also, patients with chronic stress are related to develop more periodontal and implant-related diseases [7]. Thus, a conservative surgical technique involving small flaps in combination with surgical guides minimizes the postoperative inflammatory healing [7], and allows to control site’s susceptibility to peri-implantitis [8].
Nevertheless, specific clinical situations such as posterior surgical sites and restricted mouth opening can reduce the effectiveness of static navigation surgery, modifying the position of the drill in relation to the surgical stent, or, most importantly, avoiding the use of static navigation surgery with severe opening limitations [9, 10]. In addition, other parameters, such as the type of guide support (mucosa, bone, or tooth/crown-supported guides) [11], surgical procedure in terms of flap reflection (open flaps versus flapless) [12], or fully or partially edentulous patients, might also affect the accuracy of guided surgery [9, 13].
Accordingly, a study that exclusively evaluates the accuracy in transmitting the presurgical information planning to the real clinical scenario during implant placement in the posterior area of the maxilla or mandible is needed. Accessibility in this area is often limiting, and a static fully guided approach with tooth supported guides, with an open flap design for direct bone visibility to allow bone regeneration when required, should be evaluated.
Therefore, a prospective cohort study was conducted to assess the 3D positioning accuracy of the implant between presurgical planned position and the final implant position using a static fully guided approach in the posterior area of the maxilla and mandible. As a secondary outcome, we evaluated the limitations of performing static fully guided surgery in the posterior zone.
Materials and methods
Patient selection
The initial research sample consisted of 40 patients and 80 implants (2 implants in each patient). Patients selected for this prospective study were recruited at the Dental University Clinic of the Universitat Internacional de Catalunya, Barcelona, Spain, between June 2017 and November 2020. The study protocol was approved by the Research Medical Ethical Committee (registration number CIR-ECL-2016–03), and all patients provided written informed consent before participating in the study.
Patient screening included a clinical and radiographic examination to evaluate the following inclusion criteria: (1) overall healthy subjects; (2) females and males of age at least 18 years; (3) requiring two implants in molar and/or premolar area for two or three missing teeth (maxilla or mandible); (4) adequate oral hygiene with less than 15% Full Mouth Plaque Score (FMPS); (5) ability to follow instructions and availability to attend for regular compliance during the entire study; (6) sufficient bone availability to place a 3.3-, 4.1-, or 4.8-mm diameter implant with a minimum length of 8 mm, without bone augmentation; and (7) completely healed ridge (extraction sites older than two months). Likewise, patients with the following criteria were excluded: (1) acute local infection, (2) untreated periodontal disease, (3) smokers having more than 10 cigarettes per day, (4) drug and/or alcoholic dependencies, (5) medical conditions contraindicating implant surgery, (6) history of head and/or neck radiation, and (7) bisphosphonate therapy. Patients were all treated within the cleared indication of the study device.
Preoperative procedure
After assessing the fulfillment of the inclusion criteria, preoperative cone beam computed tomography (CBCT) was performed for each patient (Planmeca ProMax® 3D Classic, Helsinki, Finland). In addition, intraoral digital impressions were obtained (3shape TRIOS MOVE, Copenhagen, Denmark) to achieve a digital wax-up and presurgical plan for the exact position of the implants.
Digital imaging and communications in medicine (DICOM) files obtained from CBCT were imported to the guided-surgery planning software (coDiagnostix®; Dental Wings® Inc., Montreal, Canada). A single operator (D. P.) performed all preoperative planning. The first step was to segment the DICOM file to clear all the artifacts and the excess undesired soft tissue to facilitate posterior superimposition with stereolithography (STL) models. Subsequently, CBCT was correctly positioned in the software according to the midline and occlusal plane. The two STL files were then imported, and the STL file of the initial position of the patient was superimposed with the DICOM file by selecting at least three matching points between them. Automatic superimposition was performed using the same software based on these three selected points. After precise alignment, the second STL file was imported with the digital wax-up information. This file was aligned to the first STL file by selecting the function “copy alignment,” positioning the second digital model in the same correct geometric position. Thus, it could be assured that the two STL files were perfectly aligned with CBCT.
Once both STL files were perfectly aligned with the CBCT, implants selected from the digital library were settled in the planning software, maintaining a safety distance of 2 mm above the inferior alveolar canal, in a favorable prosthetic position according to the digital wax-up STL file.
Surgical guides were designed with a full-arch support on the remaining teeth. In distal extension cases, the guides were supported in teeth and in the distal mucosa area, always ensuring to have an extended area of tooth support involving all the remaining teeth to achieve excellent guide stability. Based on a good tooth support, flap design did not interfere with the guide stability. No support in the hard palate or in the buccal or lingual flanges were used. The offset assigned to all the guides was of 0.1 mm, previous calibration with the laboratory, to ensure correct fitting.
The surgical guide plan was then exported as an STL file and sent to the laboratory (Odontecnic®, Barcelona, Spain) for printing directly from the software planning. After post process and printing using a monomer based on acrylic esters for manufacturing of 3D-printed surgical guides (NextDent™ SG, Soesterberg, Netherland), metallic sleeves with an internal diameter of 5 mm (Institute Straumann AG, Basel, Switzerland) were inserted, and sterilized at 121 °C for 15 min (Autoclave Line B from W&H, Bürmoos, Austria), according to the acrylic certificate recommendations and company’s instructions for use.
Surgical procedure
Before starting the surgery, accurate fitting of the guide was confirmed. Under local anesthesia (Ultracain® 4% with epinephrine 1:100.000; Laboratorios Normon®, Madrid, Spain), a small full-thickness envelope flap was elevated in order not to interfere with the fitting and stabilization of the tooth-supported surgical guide (Fig. 1). Then, the drilling sequence was executed using the Straumann® Guided Surgery Kit (Institute Straumann AG, Basel, Switzerland) following the fully guided approach, using as a reference the drilling sequence, provided automatically from the digital planning software (coDiagnostix®; Dental Wings® Inc., Montreal, Canada). Two Straumann® Bone Level Tapered—guided implants (BLT guided, Roxolid®, SLActive®, Institute Straumann AG, Basel, Switzerland) were used in each patient. Implant diameters of 3.3 mm, 4.1 mm, or 4.8 mm and implant lengths of 8 mm, 10 mm, or 12 mm were used. Monofilament 5–0 sutures were used to close the flap. Antibiotic medication (Amoxicillin 500 mg every 8 h for 7 days; or clindamycin 300 mg every 8 h in cases of penicillin allergy) and anti-inflammatory medication (ibuprofen 400 mg every 8 h for 3 days) were prescribed in all the patients.
Follow-up
Postoperative CBCT was performed 1 week after the surgery, with the same scanning machine (Planmeca ProMax® 3D Classic, Helsinki, Finland) to analyze the positioning accuracy of the implant between pre-surgical planned position and the final implant position.
Postoperative evaluation
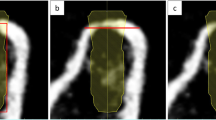
Two investigators (J. M. and M. J. Z.) performed the postoperative measurements. A new case was created using the coDiagnostix® planning software (coDiagnostix®; Dental Wings® Inc., Montreal, Canada), and the postoperative CBCT was imported. Using the “Treatment Evaluation Tool” feature of the software, preoperative planning was imported as an STL file. First, pre-operative images were loaded in a new project and were superimposed with the post-operative CBCT through 4 stable anatomical points: in mandibular cases, both mental foraminas of each side were selected and two contralateral teeth; in maxillary cases, the anterior nasal spine and two contra-lateral teeth were chosen. Confirmation of a correct superimposition was checked manually. This was corroborated to be precise in the sagittal, axial, and coronal planes. Then, the outline of the preoperative planned implant was manually positioned to be centered in the radiopaque postoperative implant image in the CBCT in order to standardize the posterior measurements. Subsequently, the software automatically calculated the pre- and post-implant positions based on point and image matching of the anatomical structures. Figure 2 illustrates the final image after superimposing the DICOM files obtained from CBCT of pre- and post-implant placement. These calculations included the following: (1) angle: angle deviation measured in degrees; (2) base: deviation at implant shoulder in millimeters; (3) tip: deviation at implant apex in millimeters; (4) 3D offset: global deviation in 3D directions in millimeters; (5) mesiodistal deviation in millimeters: ( +) deviated to the distal direction and ( −) deviated to the mesial direction; (6) buccolingual deviation in millimeters: direction: ( +) deviated to lingual direction and ( −) deviated to buccal direction; and (7) apicocoronal deviation in millimeters: direction: ( +) deviated in the apical direction and ( −) deviated in the coronal direction.
In summary, the deviation in the most coronal part (platform) and the apex of the implant were calculated in millimeters, and the overall angle deviation was measured in degrees. For a better description, Fig. 3 shows all the measurements used to perform the statistical analysis, and they are described as follows:
-
1.
Angular deviation (AD)
-
2.
Deviation at implant shoulder (3D)
-
3.
Deviation at implant shoulder-mesiodistal (MD)
-
4.
Deviation at implant shoulder-buccolingual (BL)
-
5.
Deviation at implant shoulder-apicocoronal (AC)
-
6.
Deviation at implant apex (3D)
-
7.
Deviation at implant apex-mesiodistal
-
8.
Deviation at implant apex-buccolingual
-
9.
Deviation at implant apex-apicocoronal
In addition, intraoperative complications were reported to further analyze the possible limitations of using guided surgery in the posterior area.
Statistical analysis
First, a descriptive analysis was performed by analyzing the mean, standard deviation, minimum, maximum values, and median for each outcome. The normality of each measurement was analyzed using the Kolmogorov–Smirnov test.
The interferential analysis included the estimation of confidence intervals at 95%, to narrow the degree of imprecision of the measurements. In addition, a Student’s t-test of one sample was performed to contrast the deviation of the null hypothesis. A Student’s t-test of independent variables was performed to compare the deviation of each implant in mesial and distal positions.
The Friedman test was used to compare the absolute deviation of the three different measurements, and specific differences were assessed using the Wilcoxon test with Bonferroni correction. In addition, because of the small sample size of maxillary implants, the Mann–Whitney test was used to compare the implants in maxilla and mandible. The overall significance applied was 5% (α = 0.05).
Results
Descriptive analysis
Ten patients (20 implants) were excluded from the study prior to perform any further measurements, indeed not affecting the significance analysis, due to limited mouth opening (7 cases), incorrect postoperative CBCT acquisition (2 cases), and one lost preoperative planification. A total of 30 patients and 60 implants were considered for further analysis. Thus, there were 30 implants in the mesial position and another 30 in the distal position; 8 implants were located in the maxilla (4 patients), and 52 implants were located in the mandible (26 patients), all in molar and/or premolar positions. The mean age of the patients was 63.4 years, corresponding to 22 men and 8 women. All dental implants corresponded to BLT Straumann implants (Straumann Holding AG, Basel, Switzerland), 6 implants of 4.8 mm in diameter and 10 mm in height, 1 of 4.8 mm in diameter and 8 mm in height, 22 of 4.1 mm in diameter and 10 mm in height, 28 of 4.1 mm in diameter and 8 mm in height, and 3 of 3.3 mm in diameter and 10 mm in height.
Neither nervous disturbances nor surgical complications were encountered during the entire study.
Mesial implant positioning accuracy
The MD, BL, and AC mean deviations at the shoulder of the mesial implant were equal or below 0.31 mm, and only the BL mean deviation at the apex reached up to 0.68 mm, while the mean deviation angle was 5.24 ± 3.60°. All information about mean deviation data accuracy of mesial implant is described in Table 1.
The MD deviations at the implant shoulder and apex were considered to be null (p > 0.05). Friedman’s test and multiple comparisons with Wilcoxon test and Bonferroni correction showed that there was no sufficient statistical evidence of greater deviation in some directions compared to others at the shoulder and apex of the mesial implant. However, at the apex, the absolute deviation median in BL direction was 0.98 mm compared to 0.48 mm in AC direction, revealing a certain trend of greater accuracy in AC than in BL direction (p = 0.057). Accuracy median absolute deviation data of mesial implant is detailed in Table 2.
In addition, no statistical differences were found when considering the upper or lower arches in relation to the mesial implant (p > 0.05).
Distal implant positioning accuracy
The results obtained for the distal implant were similar to those for the mesial implant, including the BL and AC shoulder deviations, which were also considered null. MD, BL, or AC mean deviations at the shoulder of the distal implant were equal or below 0.17 mm, and only the BL mean deviation at the apex reached up to 0.67 mm, while the mean deviation angle was 5.99 ± 4.56° (Table 1).
The Friedman test and multiple comparisons with the Wilcoxon test and Bonferroni correction showed that there was insufficient statistical evidence of greater deviation in some directions compared to others at the shoulder of the distal implant; however, there were significant differences in the absolute magnitude of the deviation at the apex (p = 0.001): a median deviation of 0.95 mm and 0.79 mm for the MD and BL directions, respectively, which was significantly above the median 0.45 mm in AC, indicated less imprecision in AC than in MD and BL directions (Table 2).
Additionally, no statistical differences were found when considering the upper or lower arches in relation to the distal implant (p > 0.05).
Mesial and distal implant comparison
Figure 4 shows the mean deviations (± SD) of all the measurements evaluated for both implants. The t-test of related samples (paired t-test) revealed that only deviations in the AC direction were significantly different at the implant shoulder (p = 0.011) and at the implant apex (p = 0.009). In the mesial implant, the deviations were towards the coronal direction, while in the distal implant, they were towards the apical direction.
Implant deviation analysis
When considering the total number of implants, without differentiating the mesial from the distal, the MD, BL, and AC mean deviations at the shoulder and implant apex were equal or below 0.21 mm, and only the BL mean deviation at the apex reached up to 0.67 mm, which was significantly above the mean of 0.04 mm in AC direction. The AD and 3D offset were significant (p < 0.001), including the measurement in BL direction referring to the implant apex (p < 0.001). However, a strong trend without statistical significance of deviation was observed with respect to the implant shoulder (p = 0.059). The MD and AC deviations were statistically irrelevant at both the shoulder and apex levels of the implant. All information about mean deviation data accuracy of total number of implants is described in Table 1.
The Friedman test and multiple comparisons with the Wilcoxon test and Bonferroni correction showed that there were similar absolute deviations in all directions at the implant shoulder; however, there was more precision in the AC direction than in the MD and BL directions for the implant apex. Thus, and in correlation with the mesial and distal implant analysis, median deviations considering the total number of implants were 0.85 mm and 0.81 mm for the MD and BL directions, respectively, which was significantly above the median 0.47 mm in AC, indicating less imprecision in AC than in MD and BL directions (Table 2).
Additionally, according to the Mann–Whitney test, there were no differences attributable to the upper or lower arch location (p > 0.05).
Complications and limitations observed during analysis
The evaluators (J. M. and M. J. Z.) encountered two main complications in the data analysis. First, limited mouth opening included seven cases (17.5% of the entire sample), in which at least one implant had to be drilled or positioned free-handed because of the inability to fit the guide and the surgical sleeve during implant drilling or implant placement in the corresponding implant site. Second, two of the postoperative CBCT explorations displayed errors in its acquisition, which made it impossible to superimpose with the preoperative planning. Finally, in one of the recruited patients, the preoperative planification was lost due to computer affairs, making it impossible to study the precision of implant positioning. This resulted in a total of 10 patients being excluded from the study, reducing the sample from 40 patients (80 implants) to 30 patients (60 implants), without affecting the statistical analysis.
Discussion
The comparison between the preoperative planification and the postoperative implant positioning in this prospective cohort study did not show statistically significant differences when considering the total of the implants, except for the apical 3D deviation (mean of 1.19 mm) and the AD (mean of 5.62°). The MD and AC deviations were statistically irrelevant, both at the shoulder and apex level of the implant, and a strong trend without statistical significance of deviation was observed with respect BL deviation to the implant shoulder. In addition, there was no difference between performing this technique in the maxilla or mandible. Therefore, computer-guided surgery in the posterior jaw appears to be a reliable technique allowing accurate implant placement according to the presurgical plan.
Our results are in agreement with those of other recently published studies with similar outcomes. Derksen et al. [14] found a 3D overall deviation mean of 0.75 mm ± 0.34 (range: 0.13–1.95 mm) at the implant shoulder, and a mean of 1.06 mm ± 0.44 (range: 0.25–2.40 mm) at the implant’s apex. These results match the findings of this study, where deviation at the implant apex was higher than at the implant shoulder, although the observed deviation was small. They found an overall mean AC deviation of 0.72 mm which was in contrast to the mean overall AC deviation of 0.47 mm observed in this prospective study. Despite considering the mesial and distal implants separately, the distal implant was found to be more precise in the AC position compared to the mesial implant.
This is in agreement with the results obtained by Andreini et al. [15] in an in vitro study where differences in the AC position of the mesial and distal implants were found to be statistically different. The distal implant was found to be placed more apical than the mesial implant. Therefore, even when performing a fully guided surgery to place dental implants in the mandible, a safety distance of 1.5–2 mm is recommended to avoid possible damage to the inferior alveolar nerve, as has been mentioned in other published studies [16]. In addition, the final AC position of the implants with the Straumann® fully guided system relies on the surgeon’s precision. In this system, the operator has to stop the motor when it reaches one of the three marked positions on the guided implant transfer (H2, H4, or H6), unlike the rest of the drills that are used until reaching the final stop. It is important to consider this point when performing the final implant positioning, especially when referring to the vertical dimension.
The results of this study which demonstrate that there were no differences found between the measurements of implants in the maxilla and mandible agree with those of Smitkarn et al. [17] who compared free-handed versus guided implant surgery. Lower precision was observed in implants placed in the maxilla; however, there were no statistically significant differences between the two arches. The lack of significant differences found in this study in relation to maxillary or mandible maybe influenced by the surgical guide design employed, based on an excellent tooth support involving all the remaining teeth of the arch, and without mucosa support in the hard palate or in the buccal or lingual flanges. In contrast, Sun et al. [18] revealed statistically significant differences between implants placed in the maxilla and mandible; the mandible displayed greater accuracy. This may be attributable to the denser cortical bone in the mandible and therefore lesser possibility of deviation, especially at the implant apex.
Other factors have been known to affect the accuracy of implant positioning implant accuracy when using the fully guided approach [19]. In the present study, tooth-supported guides were employed for better standardization, exhibiting a definite influence on the good results achieved. Gallardo et al. [11] reported that the highest accuracy was observed for tooth-supported guides in comparison to bone-supported and muco-supported guides. Bone-supported guides require precise segmentation of CBCT, and good quality imaging is necessary to avoid intraoperative adjustment problems. In the case of muco-supported guides, soft tissue alterations by elevating surgical flaps may also interfere with the adjustment of the guides. In addition, the surgeon’s experience may also influence to the accuracy implant positioning in relation to the presurgical planning even using navigation surgery, although considering that fully guided surgery requires less surgical experience to overcome the limitations of free-hand surgery [5, 20]. This study was performed by postgraduate students with limited or no surgical experience in this type of procedures. Thus, the mean angular deviation found in this study was larger than mean angular deviation reported by Varga et al. [21] (5.62° versus 3.04°, respectively), in which the fully guided surgeries were performed by experienced surgeons.
Concerning the surgical guide’s sterilization process, chlorhexidine solutions and 80% of alcohol solutions are the most common disinfectant processes employed in the dental clinic, although resulting ineffective for sterilization purposes [22]. On the contrary, Marei et al. [23] and Török et al. [24] recommend the sterilization surface of the surgical guides via autoclave, applying the conventional program up to 121 °C between 15 or 20 min, ascertaining that the steam heat sterilization has no significant dimensional changes over the guides when employing an adequate acrylic material. Therefore, a material with a CE certificate with autoclave recommendations has been employed in our cases to achieve adequate sterilization, attending the instructions for use of the company’s product manual (NextDent™ SG, Soesterberg, Netherland), and being recommended to be used with the same sterilization protocol in a previous investigation [23].
Regarding the limitations observed in this study, the presence of a comparison group, such as free-handed, partially guided, or dynamic navigation surgery would be ideal to determine the exact difference between different surgical approaches. In addition, the fact that the mouth opening was not standardized did not allow us to make any conclusions or to determine any minimum opening to avoid the impossibility of bone drilling or implant placement through the surgical guide. In guided surgery for dental implant placement, longer drills are needed to achieve the desired implant-drilling length, and the volume of the guides considerably limits proper positioning in the posterior areas of the maxilla or mandible. Furthermore, even though these surgeries were performed by second- and third-year post graduate residents of the departments of Oral Surgery and Periodontics of the University, it is important to mention that they were not performed by experienced surgeons. Another factor that requires consideration is the minimum MD space, because for most guided-surgery systems, the drilling process is across a metallic sleeve, which requires an adequate MD space to be inserted. The standard sleeve of the Straumann®-guided system has an internal diameter of 5 mm, which is a factor to consider, especially in narrow MD spaces. Hence, the entire fully guided approach requires systematic processing in terms of high-quality intra-oral scanning, CBCT imaging, and a highly precise printing protocol in order to achieve an adequate guide fitting and, therefore, an accurate final treatment.
In conclusion, static fully guided surgery for dental implant placement exhibited minimum deviations. Considering the total number of implants, the MD, BL, and AC mean deviations at the shoulder and implant apex were equal or below 0.21 mm, and only the BL mean deviation at the apex reached up to 0.67 mm. The mean total AD was 5.62°. Even if the deviations are clinically acceptable, precautions and safety margins must be respected at all times. In addition, the main limitation of this surgical approach in the posterior areas is the requirement for wide mouth opening. Further clinical studies are needed to compare static fully guided dental implant surgery with dynamic navigation surgery and sleeve-free guide approaches to potentially overcome the limitation of mouth opening.
References
Younes F, Eghbali A, De Bruyckere T, Cleymaet R, Cosyn J (2019) A randomized controlled trial on the efficiency of free-handed, pilot-drill guided and fully guided implant surgery in partially edentulous patients. Clin Oral implants Res 30:131–138. https://doi.org/10.1111/clr.13399
Kühl S, Zurcher S, Mahid T, Muller-Gerbl M, Filippi A, Cattin P (2013) Accuracy of full guided vs. Half-guided implant surgery. Clin. Oral Implants Res 24:763–769
Colombo M, Mangano C, Mijiritsky E, Krebs M, Hauschild U, Fortin T (2017) Clinical applications and effectiveness of guided implant surgery: a critical review based on randomized controlled trials. BMC Oral Health 17:150. https://doi.org/10.1186/s12903-017-0441-y
D’Haese J, Van De Velde T, Komiyama A, Hultin M, De Bruyn H (2012) Accuracy and complications using computer-designed stereolithographic surgical guides for oral rehabilitation by means of dental implants: a review of the literature. Clin Implant Dent Rel Res 14:321–335. https://doi.org/10.1111/j.1708-8208.2010.00275.x
Younes F, Cosyn J, De Bruyckere T, Cleymaet R, Bouckaert E, Eghbali A (2018) A randomized controlled study on the accuracy of free-handed, pilot-drill guided and fully guided implant surgery in partially edentulous patients. J Clin Periodontol 45:721–732
Curtis DA, Lin GH, Rajendran Y, Gessese T, Suryadevara J (2000) Kapila YL (2021) Treatment planning considerations in the older adult with periodontal disease. Periodontol 87:157–165. https://doi.org/10.1111/prd.12383
Decker AM, Kapila YL (2000) Wang HL (2021) The psychobiological links between chronic stress-related diseases, periodontal/peri-implant diseases, and wound healing. Periodontol 87:94–106. https://doi.org/10.1111/prd.12381
Fu JH, Wang HL (2020) Breaking the wave of peri-implantitis Periodontol 2000(84):145–160. https://doi.org/10.1111/prd.12335
Gargallo-Albiol J, Barootchi S, Salomo-Coll O, Wang HL (2019) Advantages and disadvantages of implant navigation surgery. A systematic review. Annals of anatomy = Anatomischer Anzeiger: official organ of the Anatomische Gesellschaft 225:1–10. https://doi.org/10.1016/j.aanat.2019.04.005
Koop R, Vercruyssen M, Vermeulen K, Quirynen M (2013) Tolerance within the sleeve inserts of different surgical guides for guided implant surgery. Clin Oral implants Res 24:630–634. https://doi.org/10.1111/j.1600-0501.2012.02436.x
Raico Gallardo YN, da Silva-Olivio IRT, Mukai E, Morimoto S, Sesma N, Cordaro L (2017) Accuracy comparison of guided surgery for dental implants according to the tissue of support: a systematic review and meta-analysis. Clin Oral implants Res 28:602–612. https://doi.org/10.1111/clr.12841
Arisan V, Karabuda CZ, Ozdemir T (2010) Implant surgery using bone- and mucosa-supported stereolithographic guides in totally edentulous jaws: surgical and post-operative outcomes of computer-aided vs. standard techniques. Clin Oral implants Res 21:980–988. https://doi.org/10.1111/j.1600-0501.2010.01957.x
Gargallo-Albiol J, Barootchi S, Marques-Guasch J, Wang HL (2020) Fully guided versus half-guided and freehand implant placement: systematic review and meta-analysis. Int J Oral Maxillofac Implants 35:1159–1169. https://doi.org/10.11607/jomi.7942
Derksen W, Wismeijer D, Flügge T, Hassan B, Tahmaseb A (2019) The accuracy of computer-guided implant surgery with tooth-supported, digitally designed drill guides based on CBCT and intraoral scanning. A prospective cohort study. Clin Oral Implants Res 30:1005–15
Andreini NI (2018) Assessment of surgical guide accuracy utilizing a digital workflow. Graduate Theses, Dissertations, and Problem Reports. 5107. https://researchrepository.wvu.edu/etd/5107
Chmielewski K, Ryncarz W, Yüksel O, Goncalves P, Baek K-W, Cok S et al (2019) Image analysis of immediate full-arch prosthetic rehabilitations guided by a digital workflow: assessment of the discrepancy between planning and execution. Int J Implant Dent 5:26
Smitkarn P, Subbalekha K, Mattheos N, Pimkhaokham A (2019) The accuracy of single-tooth implants placed using fully digital-guided surgery and freehand implant surgery. J Clin Periodontol 46:949–957
Sun T-M, Lee H-E, Lan T-H (2020) Comparing accuracy of implant installation with a navigation system (NS), a laboratory guide (LG), NS with LG, and freehand drilling. Int J Environ Res Public Health 17:2107. https://doi.org/10.3390/ijerph17062107
Zhou W, Liu Z, Song L, Kuo C-L, Shafer DM (2018) Clinical factors affecting the accuracy of guided implant surgery—a systematic review and meta-analysis. J Evid Based Dent Pract 18:28–40
Vermeulen J (2017) The accuracy of implant placement by experienced surgeons: guided vs freehand approach in a simulated plastic model. Int J Oral Maxillofac Implants 32(3):617–624
Varga E Jr, Antal M, Major L, Kiscsatári R, Braunitzer G, Piffkó J (2020) Guidance means accuracy: a randomized clinical trial on freehand versus guided dental implantation. Clin Oral Implants Res 31:417–430
Sennhenn-Kirchner S, Weustermann S, Mergeryan H, Jacobs HG, Borg-von Zepelin M, Kirchner B (2008) Preoperative sterilization and disinfection of drillguide templates. Clin Oral Investig 12:179–187
Marei HF, Alshaia A, Alarifi S, Almasoud N, Abdelhady A (2019) Effect of steam heat sterilization on the accuracy of 3D printed surgical guides. Implant Dent 28:372–377
Török G, Gombocz P, Bognár E, Nagy P, Dinya E, Kispélyi B, Hermann P (2020) Effects of disinfection and sterilization on the dimensional changes and mechanical properties of 3D printed surgical guides for implant therapy — pilot study. BMC Oral Health 20:19. https://doi.org/10.1186/s12903-020-1005-0
Funding
The work was partially supported by the Department of Oral and Maxillofacial Surgery of the Universitat Internacional de Catalunya (Barcelona, Spain), and Institute Straumann AG, Basel, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the Research Medical Ethical Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gargallo-Albiol, J., Zilleruelo-Pozo, M.J., Lucas-Taulé, E. et al. Accuracy of static fully guided implant placement in the posterior area of partially edentulous jaws: a cohort prospective study. Clin Oral Invest 26, 2783–2791 (2022). https://doi.org/10.1007/s00784-021-04254-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-04254-3